Abstract
Objective
We hypothesized that ablation of recently described stable AF sources, either directly by Focal Impulse and Rotor Modulation (FIRM) or coincidentally when anatomical ablation passes through AF sources, may explain long term freedom of AF.
Background
It is unclear why conventional anatomical AF ablation can be very effective in some patients yet ineffective in others with similar profiles.
Methods
The CONFIRM trial prospectively revealed stable AF rotors or focal sources in 98/101 subjects with AF at 107 consecutive ablation cases. In 1:2 fashion, subjects received targeted source ablation (FIRM) then conventional ablation, or conventional ablation alone. We determined if ablation lesions on electroanatomic maps passed through AF sources on FIRM maps.
Results
Subjects who completed followup (n=94, 71.2% persistent AF) each showed 2.3±1.1 concurrent AF rotors or focal sources, that lay near pulmonary veins (22.8%), left atrial roof (16.0%), and elsewhere in left (28.2%) and right (33.0%) atria. AF sources were ablated directly in 100% FIRM cases and coincidentally (e.g. left atrial roof) in 45% conventional cases (p<0.05). During 273 days (median, IQR 138–636) after 1 procedure, AF was absent in 80.3% of patients if sources were ablated but recurred in 81.8% of patients if sources were missed (p<0.001). Freedom from AF was highest if all sources were ablated, intermediate if some sources were ablated, and lowest if no sources were ablated (p<0.001).
CONCLUSIONS
Elimination of stable AF rotors and focal sources may explain freedom from AF after diverse approaches to ablation. Patient-specific AF source distributions are consistent with the reported success of specific anatomical lesion sets, and of widespread ablation. These results support targeting AF sources to reduce unnecessary ablation, and motivate studies on FIRM-only ablation.
Keywords: Atrial fibrillation, Treatment, Ablation, Rotor, Focal source, FIRM
Atrial fibrillation (AF) is a major cause of morbidity and mortality, for which pharmacologic approaches for rate (1) or rhythm control (2) remain suboptimal. Catheter ablation is a non-pharmacological therapy with the potential to eliminate AF yet, while it is more effective than medications (3–5), has had suboptimal success for paroxysmal (6) and persistent (7,8) AF.
Mechanistic uncertainty for AF may contribute to these limitations of ablation. When mechanisms are well-defined, such as for Wolff-Parkinson-White syndrome (9) or atrioventricular nodal reentry (10), ablation provides >85–90% one procedure success. Conversely, the reentry circuits or focal sources that sustain AF are undefined after AF has been triggered by ectopy (from pulmonary veins, PVs or elsewhere). It is thus unexplained how paroxysmal or persistent AF may be eliminated by ablation that does (5–7,11), but also does not (12–14), isolate the PVs.
We hypothesized that freedom from AF should be higher if lesions pass through stable rotors or focal sources, mechanisms recently shown to sustain AF in the CONFIRM trial (CONventional ablation with or without Focal Impulse and Rotor Modulation) (15) and independent reports (16,17), than if they do not. These studies used a novel computational approach to map stable sources in >97% of patients with paroxysmal or persistent AF, in whom source ablation (FIRM) improved AF freedom compared to conventional ablation alone on intention-to-treat analysis (15). However, while sources were seen in patients in both limbs of CONFIRM, it is unclear whether conventional ablation was more successful if passed through rather than bypassed AF sources.
We tested our hypothesis by defining the locations of ablation lesions and AF sources in each patient in each limb of the CONFIRM trial (FIRM-guided and -blinded). We determined whether lesions that passed through AF sources, directly by FIRM-mapping guidance or coincidentally by conventional anatomic ablation, conferred higher long-term freedom from AF in a prespecified on-treatment analysis of the CONFIRM trial.
Methods
Study Design and Enrollment
CONFIRM (15) prospectively enrolled 92 subjects at 107 consecutive AF ablation procedures for standard-of-care indications. Subjects were ≥21 years of age, with AF despite one or more class I or III anti-arrhythmic drugs. To study AF sources and their response to ablation across a broad spectrum of AF presentations, we included patients with paroxysmal, persistent and longstanding persistent AF (8), and AF despite prior conventional ablation. The only exclusion was inability or refusal to provide specific written informed consent (NCT01008722).
Consecutive cases were prospectively mapped then assigned 2:1 to FIRM-blinded (conventional) ablation alone or, after real-time FIRM mapping had been developed, to FIRM-guided ablation comprising targeted source ablation (FIRM) followed by conventional ablation.
Electrophysiology Study
Electrophysiology study was performed after discontinuing anti-arrhythmic medications for 5 half lives (for amiodarone, >60 days, median 230 days; table 1). Intravenous heparin was infused to maintain activated clotting time > 350 seconds. A 64-pole basket catheter (48 mm or 60 mm diameter, 4–6 mm electrode spacing; Constellation™, Boston Scientific, Natick, MA) was advanced trans-septally to map the left atrium in all patients, and also to map the right atrium in n=73 patients (including all FIRM-guided cases).
Table I.
Clinical Characteristics
| Characteristic | Any Source Ablation (n=61) | No Source Ablation (n=33) | P |
|---|---|---|---|
| AF Presentation | 0.63 | ||
| Paroxysmal | 19 (31.1%) | 8 (24.2%) | |
| Persistent | 42 (68.9%) | 25 (75.8%) | |
| Age (years) | 61.6±8.3 | 61.0±8.8 | 0.77 |
| Gender (Male/Female) | 58/3 | 31/2 | 1.00 |
| Non-white race | 9 (14.8%) | 4 (12.1%) | 1.00 |
| History of AF/months | 47 (27–107) | 47 (21–110) | 0.81 |
| Left Atrial diameter/mm | 46±7 | 45±7 | 0.35 |
| LVEF (%) | 54±13 | 55±11 | 0.48 |
| CHADS2 score | |||
| 0 or 1 | 26 (42.6%) | 21 (63.6%) | 0.08 |
| 2 or more | 35 (57.4%) | 12 (36.4%) | |
| NYHA Class | 0.32 | ||
| 0–I | 52 (85.2%) | 31 (93.9%) | |
| II–III | 9 (14.8%) | 2 (6.1%) | |
| Comorbid Conditions | |||
| Hypertension | 50 (82.0%) | 23 (69.7%) | 0.20 |
| Diabetes | 21 (34.4%) | 7 (21.1%) | 0.24 |
| Prior Stroke/TIA | 11 (18.0%) | 4 (12.1%) | 0.56 |
| Coronary Disease | 26 (42.6%) | 5 (15.2%) | 0.01 |
| Hypercholesterolemia | 47 (77.0%) | 20 (60.6%) | 0.10 |
| Prior Conventional | 22 (36.1%) | 10 (30.3%) | 0.65 |
| Ablation | |||
| Previously Failed >1 Anti- | 21 (34.4%) | 12 (36.4%) | 1.00 |
| Arrhythmic Drug | |||
| Class I | 15 (24.6%) | 8 (24.2%) | 1.00 |
| Sotalol | 30 (49.2%) | 19 (57.6%) | 0.52 |
| Dofetilide | 13 (21.3%) | 5 (15.2%) | 0.59 |
| Amiodarone | 29 (47.5%) | 15 (45.5%) | 1.00 |
| Days since Amiodarone discontinued | 365 (60–365) | 97 (60–365) | 0.36 |
| Concomitant Drug Therapy | |||
| ACEI/ARB | 36 (59.0%) | 22 (66.7%) | 0.51 |
| Beta Adrenoceptor | 43 (70.5%) | 22 (66.7%) | 0.82 |
| Antagonists | |||
| Calcium Channel Blockers | 15 (24.6%) | 9 (27.3%) | 0.81 |
| Statins | 35 (57.4%) | 14 (42.2%) | 0.20 |
Values are number (%), Mean±SD, or median (interquartile range) evaluated with Fisher exact test, Student's t-test, or Mann-Whitney U test, respectively.
Figure 1A shows a patient in whom simultaneous bi-atrial baskets were used (recent studies have used one basket for both atria (16,17)). AF was observed in 101 cases (including FIRM-guided cases) (15). When required, AF was induced (rapid pacing, n=26; isoproterenol, n=2) since several studies show similar frequency (18) and spatial activation (19) for induced versus spontaneous AF. Unipolar and bipolar AF electrograms were filtered at 0.05 – 500 Hz and exported at 1kHz sampling frequency.
Figure 1. Focal Impulse and Rotor Mapping (FIRM) of AF Sources.
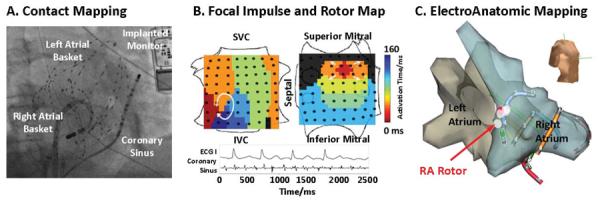
(A) Fluoroscopy of Biatrial 128 electrode wide-field of view mapping in AF (Right Atrial Oblique projection). An implantable continuous ECG recorder, diagnostic catheter in the coronary sinus, ablation catheter in the left atrium and esophageal temperature probe are also seen. (B) FIRM Maps Show Two Independent Sources: an AF Rotor in an unconventional lateral right atrial location and a concurrent left atrial focal source. Orientation right atrium: Top, superior vena cava; Left, lateral tricuspid. Left atrium: Top, superior mitral annulus; Left, septal. (C) AF Rotor in Lateral RA on Patient-Specific Electroanatomic shell (same patient as figure 1B).
FIRM Mapping of AF Sources
FIRM mapping for AF has been described(15,19). Briefly, AF electrograms were recorded in a wide field-of-view then analyzed by RhythmView™ (Topera, San Diego, California) to produce maps of AF propagation. Figure 1B shows a FIRM map of an AF rotor (i.e. red-to-blue, early-meets-late spiral wave). As shown in figure 1C, this rotor lay at a lateral right atrial site that would not be ablated conventionally.
AF propagation (FIRM) maps were analyzed intra-procedurally in FIRM-Guided patients to guide ablation, and post-procedurally in FIRM-Blinded patients. Electrical rotors (figure 1B, 2) were defined as sustained clockwise or counterclockwise activation around a center of rotation, while focal impulses (figure 2) were defined by centrifugal activation from an origin. Rotors and focal impulses were considered AF sources only if stable in repeated samples over 30–120 minutes (i.e. thousands of cycles), distinct from transient fibrillatory activity (20,21). The AF focal source origin or rotor center of rotation was located by its electrode coordinates, `shadowed' digitally within each patient-specific anatomic shell (NavX, St Jude Medical, MN) at the time of each FIRM map, to eliminate errors from subsequent possible movement. Each digital shell was created, in turn, by carefully sampling atrial and venous points with reference to (or fused with) pre-procedural patient-specific computed tomographic (CT) scans when available.
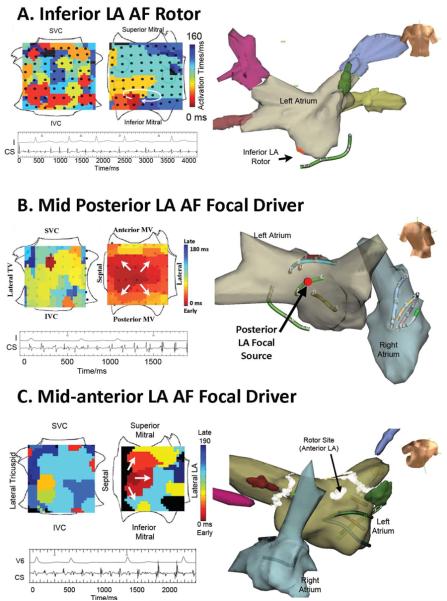
Figure 2. AF Sources Remote from Conventional Ablation Sites.
(A) AF rotor (clockwise, 3.2 cm2) in Inferior Left atrium in an 85 year old with paroxysmal AF. (B) AF focal source (2.0 cm2) in mid-posterior left atrium in a 64 year old with PAF. (C) AF focal source (1.8 cm2) in anterior left atrium (anterior to typical roof line) in a 48 year old with persistent AF. All cases received FIRM-guided ablation, and are AF-free on implanted cardiac monitoring at > 1 year. Orientations for FIRM maps as in figure 1B.
Ablation Approach
Radiofrequency energy was delivered with a 3.5 mm tip irrigated catheter (Thermocool, Biosense-Webster, Diamond Bar, CA) at 25–35 W or, in heart failure subjects, an 8 mm tip catheter (Blazer, Boston Scientific, Natick, MA) at 40–50 W, target 52°C. Each lesion was registered digitally on each patient's digital NavX anatomy. Ablation in FIRM-guided subjects always commenced with FIRM. Energy was applied at adjacent sites for 15–30 seconds to cover the AF source (rotational center or focal source origin) (15). The endpoint was AF termination, coverage of the typical≈2 cm2 source area (≈5 minutes ablation; <2–5% of atrial area (22)) or 10 minutes' energy application, whichever came first. If AF terminated, vigorous attempts were made to reinitiate AF. FIRM was repeated for ≤ 3 sources (≤30 minutes permitted(15)), followed by conventional ablation.
Conventional ablation (8), performed after FIRM in FIRM-guided patients and as sole therapy in FIRM-blinded patients, comprised wide area circumferential ablation to isolate left and right PV pairs, with verification of PV entrance block using a circular mapping catheter. In persistent AF, a left atrial roof line with confirmation of block was also performed. Cardioversion was performed if necessary.
On-Treatment Analysis: Did Ablation Pass Through Sources?
Electroanatomic and FIRM maps were analyzed per patient blinded to demographics, outcomes or assignment into FIRM-guided or conventional ablation limbs. `Source ablation' was assigned if 5 mm lesion markers on NavX (figures 1,2,4) lay within ± half the inter-electrode spacing (on the digital shell) to electrodes recording the rotor core or focal origin. Two analyses were performed. First, `any source ablation' was assigned if ≥1 source was ablated, and `no source ablation' was assigned otherwise. Conventional ablation was left atrial, so that this analysis could be performed in all patients (FIRM-blinded and -guided) who all received left atrial FIRM maps. Second, we measured FIRM dose response by assigning `all source ablation' (e.g. 3 of 3 concurrent sources), `some source' ablation (e.g. 2 of 3 sources ablated) and `no source' ablation. Because of potential right atrial AF sources, this secondary analysis was performed only in patients with bi-atrial FIRM maps. Assignments were performed independently by SMN, DEK, KS, JMM and disputes were resolved by consensus.
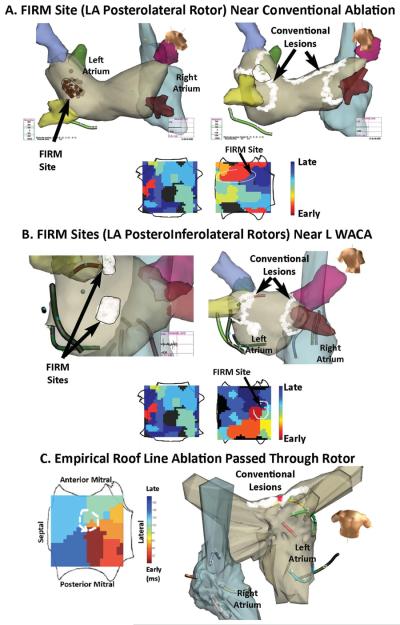
Figure 4. AF Rotors Near Conventional Ablation Targets.
(A) Left atrial AF rotor successfully ablated by FIRM, that was then incorporated into left wide-area circumferential PV ablation. (B) Two left atrial AF rotors, each successfully ablated by FIRM. The superior rotor was incorporated into the left wide-area circumferential PV ablation, while the inferior rotor (isochrones shown) was nearby. (C) Left atrial roof AF rotor ablated coincidentally by empirical roof ablation in a man with persistent AF. AF terminated at the point indicated by a red dot. Offline FIRM analysis later revealed an AF rotor at the precise point of AF termination. All patients are AF-free. FIRM atrial orientations are as in figure 1B.
Post-Procedure Clinical Management
Follow up for arrhythmia recurrence met or exceeded guidelines (23). In the first 3 months post-ablation, anti-arrhythmic medications were continued and cardioversion performed if indicated, but neither repeat ablation nor cross-overs were permitted. Subjects were then evaluated quarterly for up to 24 months. Arrhythmia recurrence was detected using continuous implanted ECG monitors when consent was obtained, i.e. Reveal XT™ (Medtronic, Minneapolis, MN) after U.S. approval in 2009 (figure 1A), or clinical pacemaker/defibrillators. Remaining subjects received external Holter or event monitors each quarter and also at the time of any symptoms.
Study Endpoints
The primary long-term efficacy endpoint was freedom from AF after the FIRM-mapping procedure, defined as <1% burden on continuous implanted ECG monitors (actual burden 0.1±0.2%, as presented in CONFIRM (15)), or AF <30 seconds on intermittent monitors (8). Secondary efficacy measures included freedom from all atrial arrhythmias, and outcomes in patients at first ablation. Four patients in the CONFIRM trial were lost to followup(15).
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate. Normality was evaluated using the Kolmogorov-Smirnov test. Comparisons between 2 groups were made with Student's t-tests and summarized with means and standard deviations for independent samples if normally distributed or, if not normally distributed, evaluated with the Mann-Whitney U test and summarized with medians and quartiles. Nominal values are expressed as n (%) and compared with the Fisher exact test. Associations between continuous variables were evaluated with Spearman's correlation. Inter-observer agreement in assigning source ablation (SMN, DEK, KS, JMM) was measured by the Kappa score. Long-term outcome was assessed and reported after the single FIRM-map procedure, and raw event rates were compared with chi-square tests and event-free survival plots were made by the Kaplan-Meier method and compared with log-rank tests. A probability of <0.05 was considered statistically significant throughout. Statistical analyses were completed in SPSS v.19.
Results
Procedural AF was observed in 101 patients in the CONFIRM trial, of whom this on-treatment analysis reports the n=94 with identified sources and followup. Table 1 describes this study population.
Characteristics of AF Rotors and Focal Sources
FIRM mapping revealed 2.3±1.1 concurrent AF sources per patient (median 2, IQR 1–3) in diverse bi-atrial locations.
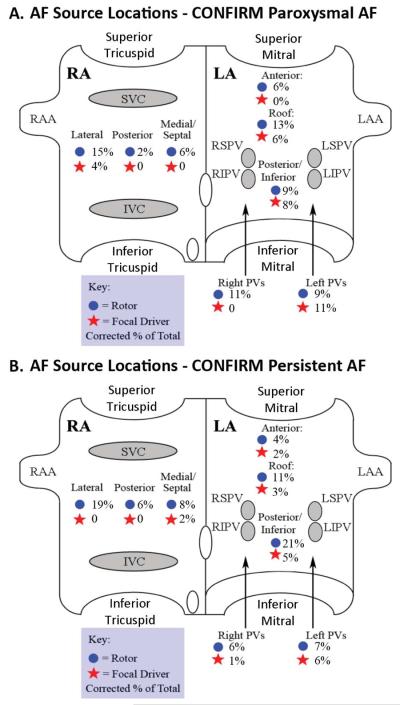
Figure 1BC and 2A–C shows stable AF sources at atrial sites not conventionally ablated. After FIRM-guided ablation, these patients remain AF free at 15, 15, 15 and 14 months, respectively. Figure 3 summarizes source locations in the CONFIRM trial for paroxysmal AF (fig 3A) and persistent AF (fig 3B) patients. Notably, no sources lay inside PV sleeves, as would have been indicated by activation emanating from electrodes spanning PV ostia. However, 50.9% of sources lay near the PVs and LA roof in paroxysmal AF and 33.5% in persistent AF (p<0.05).
Figure 3. AF Focal Impulse and Rotor Locations in the CONFIRM trial, as a % of all sources for each AF type: (A) Paroxysmal AF; (B) Persistent AF.
Focal impulses indicated by stars, rotors by circles. Key: IVC, SVC = inferior, superior vena cavae; LAA, RAA = left, right atrial appendages; LSPV=Left Superior Pulmonary vein; LIPV = Left Inferior Pulmonary vein; RSPV=right superior pulmonary vein; RIPV=right inferior pulmonary vein.
Source Ablated and Non-Source Ablated Groups
Ablation lesions passed through one or more stable AF sources in 64.9% (61/94) of cases, comprising 45% (27/60) of FIRM-blinded patients and 100% (34/34) of FIRM-guided cases (Table 1). The interobserver kappa score for assignment of source- versus no-source lesion delivery was 0.898.
Sources comprised rotors in 79.1% and focal impulses in 20.9%, that did not differ statistically between source ablated and no-source ablated groups. The ratio of right-to-left atrial sources trended higher in patients with no-source versus source ablation (p=0.13; table 2).
Table 2.
Results of FIRM Mapping
| Characteristic | Any Source Ablation (n=61) | No Source Ablation (n=33) | P |
|---|---|---|---|
| No. Concurrent Sources/patient | 2.3±1.1 | 1.8±0.6 | 0.02 |
| Left Atrial Sources | 107 (69.9%) | 31 (58.5%) | 0.13 |
| Right Atrial Sources | 46 (30.1%) | 22 (41.5%) | |
| Left Atrial Sources | |||
| Rotors | 73 (47.7%) | 27 (50.9%) | 0.03 |
| Focal Sources | 34 (22.2%) | 4 (7.5%) | |
| Near Pulmonary Veins | 37/153 (24.2%) | 10/53 (18.9%) | 0.28 |
| Left Atrial Roof | 28/153 (18.3%) | 5/53 (9.4%) | 0.08 |
| Right Atrial Sources | |||
| Rotors | 42 (27.5%) | 21 (39.6%) | 1.00 |
| Focal Sources | 4 (2.6%) | 1 (1.9%) | |
| Lateral/Posterior | 34/153 (22.2%) | 15/53 (28.3%) | 0.77 |
| Medial/septal | 12/153 (7.8%) | 7/53 (13.2%) |
Values are mean ± SD or n(%) compared with t-tests or Fisher exact, respectively.
Coincidental Ablation of AF sources
Figure 4 illustrates AF sources lying near conventional ablation sites. Figure 4A shows an AF rotor septal to the left superior PV ostium in persistent AF. Its FIRM lesion area (2.1 cm2) fell naturally along the subsequent left WACA, indicating that this AF rotor may have been ablated `coincidentally' by conventional ablation alone. Figure 4B shows 2 FIRM sites near the left PVs sustaining persistent AF, of which the superior FIRM set (2.2 cm2) also lay naturally along the subsequent left WACA. In this case, the inferior FIRM site (1.9 cm2) may have been missed by conventional ablation although with FIRM-guidance it was ablated and joined to the left WACA.
Figure 4C illustrates an AF rotor in persistent AF that was coincidentally ablated during an empirical LA roof line. Bilateral PVI had been performed, with no measurable impact on AF. Shortly after starting the LA roof line, before it was completed, AF terminated abruptly to sinus rhythm by ablation at the red dot site. The FIRM map was generated after the procedure, and showed a rotor at this precise site. Each patient remains AF free on followup.
AF Source Ablation and Long-Term Efficacy
Over followup of 273 days (median; IQR 138–636), freedom from AF was higher in patients in whom ablation passed through sources versus no sources (49/61, 80.3% vs 6/33, 18.2 %; p<0.001). Results were unchanged when examining only first ablation cases (33/42, 78.6% vs 4/22, 18.2%; p<0.001). Figure 5A shows the Kaplan-Meier plot. Freedom from AF was similar whether sources were ablated directly (FIRM-guided) or coincidentally (FIRM-blinded; figure 5B). Freedom from any atrial tachyarrhythmias was also higher when ablation did versus did not pass through sources (43/61, 70.5 % vs 5/33, 15.2 %; p<0.001). In FIRM-guided patients, this included 1 cavotricuspid isthmus dependent flutter and 2 left atrial tachycardias; in FIRM-blinded patients this included 4 left atrial tachycardias. Finally, figure 5C presents a Kaplan-Meier plot showing that freedom from AF was highest when all sources were ablated (29/33, 87.9%), intermediate when some sources were ablated (8/12, 66.7%), and lowest when no sources were ablated (4/24, 16.7%).
Figure 5.
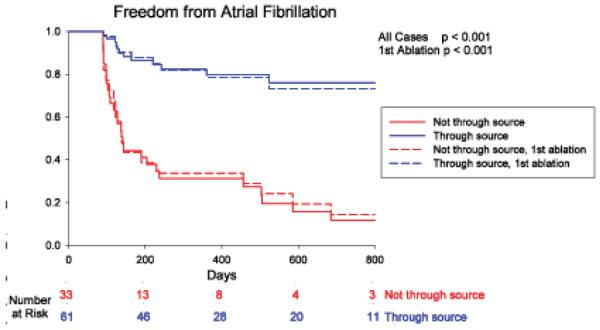
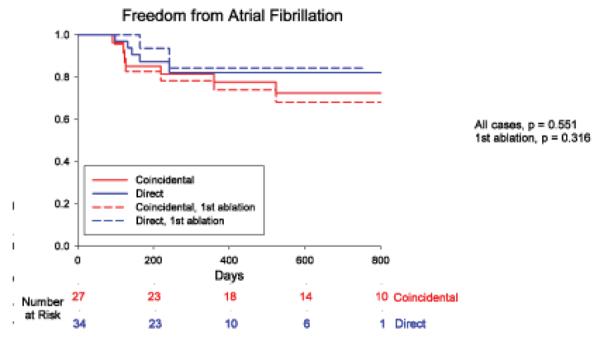
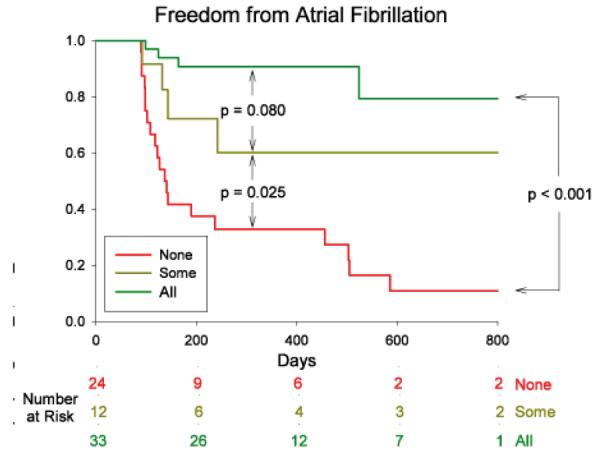
Cumulative freedom from Atrial Fibrillation Based on (A) Whether Ablation Did (Blue) or Did Not (Red) Pass Through AF Sources. Entire Population (solid lines) and First Ablation patients only (dashed lines). (B) Direct (FIRM-guided, Blue) or Coincidental (FIRM-blinded, Red) Source Ablation. (C) Elimination of All (Green), Some (Yellow) or No (Red) Stable AF Sources, in patients with bi-atrial baskets. p-values reflect the complete followup periods.
Overall, 44 % of patients had implantable ECG monitors (88.2% FIRM-guided, 26.1% FIRM-blinded), comprising 33/61 (54.1%) in the source ablation versus 10/33 (30.3%, p=0.03) in the no source ablation groups.
Discussion
The major finding of this study is that ablation of stable AF sources at diverse patient-specific locations provides a potential unifying explanation for why diverse AF lesion sets eliminate AF on followup in some patients but not others. Stable AF sources occurred in 97% of patients in the CONFIRM trial, lying both near and remote from conventional lesion sets including in the right atrium. Notably, bi-atrial source distributions were consistent with the single-procedure success for PV focused ablation in paroxysmal AF, the higher success achieved when extensive lesions are delivered, and the ≈70% success ceiling for persistent AF after multiple left atrial procedures (since ≈30% of sources were right atrial). These data further support patient-tailored ablation, suggesting that ablation of stable AF sources may improve outcomes and reduce the extent of ablation, and motivating future studies on FIRM-only ablation.
Stable AF Sources
Although direct evidence for stable sources for human AF is recent (15,16), a large indirect body of literature exists showing that localized ablation at sites of high dominant frequency, fractionated electrograms and other `substrates' may eliminate AF (24–26), that persistent AF can terminate at the first ablation steps (25), and that AF patients show consistent gradients in frequency (27,28) and organization over time (29,30).
Human AF rotors show many characteristics similar to rotors in some animal models (31–34), including limited precession (22,35) in conserved areas that define FIRM lesion sets. The role of stable sources in sustaining human AF is shown by the ability of FIRM ablation alone (35,36) to terminate and render AF non-inducible (16,17,22), and by higher freedom from AF in patients receiving FIRM-guided versus conventional ablation (82.4 versus 44.9% in the CONFIRM trial) (15). Other recent reports have used contact mapping to reveal stable human AF rotors by Shannon entropy (37), wavelet similarity (38) and isochronal (39) analyses. Collectively, these studies contradict earlier reports that human AF rotors did not exist (20) or were not stable (21).
The CONFIRM trial (15) and a preliminary 12-center experience of >200 patients (17) suggest that FIRM guided ablation may substantially improve AF freedom over conventional ablation. Ongoing studies are investigating why coincidental source ablation terminates AF less often than direct FIRM ablation (15). The most likely explanation is that FIRM ablation targets all ≈2–3 concurrent rotors or focal sources (15), while this is inconsistent for conventional ablation. Elimination of all sources (figure 5C) was actually more effective than reported in the CONFIRM trial by intention-to-treat FIRM-guided ablation (15), that was not completed at all sources in some patients. Elimination of all sources (figure 5C) was more effective than elimination of some or none, while ablation of some sources yielded similar results to that reported for conventional ablation (3–5).
Patient-Specific Distributions of Stable AF Sources
We show that stable AF sources lie in patient-specific locations with ≈40–50% near PVs and left atrial roof, and ≈20–30% in right atrium. AF source locations were more widely distributed for persistent than paroxysmal AF.
AF source distributions may reflect fiber architecture (40), fibrosis or scar (41), electrical (42) remodeling or altered innervation (43). The precise determinants of AF source location awaits definition, that will require detailed translational studies in model systems (32,34) that recapitulate human AF. However, the similar source numbers and FIRM ablation success in patients with and without prior ablation (15) argues that FIRM-identified sources are not created by prior lesions, and are thus distinct from studies of ablation line gaps (44).
Elimination of Stable AF Sources May Explain AF Freedom After Diverse Ablation Strategies
It is clear that AF can be triggered by ectopic beats from the PVs. However, while PV isolation strategies can eliminate triggers acutely, it is often difficult to achieve robust PV isolation and additional triggers may lie at undefined sites outside the PVs (3–6,8).
The present study provides an alternative potential explanation for why ablation treats AF in some patients and not others. Elimination of AF sources may explain why wide-area ablation is more effective than ostial PV isolation (45), why AF may not recur in patients whose PVs have reconnected (46), why non-PV encircling lines (47) or fractionated electrogram ablation (12) may be effective and, potentially, why ablation success correlates with the extent of ablated tissue (25) in persistent AF.
Limitations
The CONFIRM study was limited by its non-randomized design, although subjects were enrolled, mapped and treated prospectively. Its strengths included an active control group (conventional ablation) rather than previously ineffective drugs as in prior studies, and the use of implanted monitors in 88% of FIRM-guided patients. We accept that the lower use of implanted monitors in conventional ablation patients (and in prior AF trials) reduces the accuracy of followup by potentially missing intermittent arrhythmia recurrences. Future studies may improve upon current definitions of `source-ablation' by using contact force sensing and/or magnetic resonance imaging to ensure and verify effective ablation at each site. The relatively small numbers of at-risk patients at longer followup periods is also a limitation. Multicenter studies will address the limitations introduced by this predominantly male VA population, and will test the benefits of FIRM-guided ablation in a much larger population.
Conclusions
In this on-treatment analysis of the CONFIRM trial, patients in whom ablation lesions passed through stable AF sources enjoyed 80.3% freedom from AF, while patients in whom ablation missed all sources had an 81.8% rate of AF recurrence. Sources lay in widespread biatrial locations, providing a potential explanation for the success of diverse anatomically-designed lesion sets. These data further support FIRM ablation at patient-specific stable AF sources, and motivate studies on FIRM only ablation.
Acknowledgements
This work was supported by grants to SMN from the Doris Duke Foundation and National Institutes of Health (HL70529, HL83359, HL83359-S1, HL103800). We thank Antonio Moyeda, RCVT, Kenneth Hopper, RCVT, Judy Hildreth, RN, Sherie Janes, RN, Stephanie Yoakum, RNP, Elizabeth Greer, RN, Donna Cooper, RN and Kathleen Mills, BA for helping to perform the clinical study and collecting followup data.
This work was supported by grants to Dr Narayan from the NIH (HL70529, HL83359, HL83359-S1) and Doris Duke Charitable Foundation. Drs. Narayan and Rappel are authors of intellectual property owned by the University of California Regents and licensed to Topera Inc. Topera does not sponsor any research, including that presented here. Dr. Narayan holds equity in Topera, and reports having received honoraria from Medtronic, St. Jude Medical, and Biotronik. Dr. Miller reports having received honoraria from Medtronic, St. Jude Medical, Biotronik, Biosense-Webster, Boston Scientific and has received modest honoraria from Topera. Dr. Shivkumar is an unpaid scientific advisor to Topera.
Clinical trial ID: NCT01008722
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Krummen and Mr. Clopton report no conflicts.
References
- 1.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–73. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 2.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 3.Oral H, Pappone C, Chugh A, et al. Circumferential Pulmonary-Vein Ablation for Chronic Atrial Fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 4.Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 5.Morillo C, Verma A, Kuck KH, et al. Radiofrequency Ablation Vs Antiarrhythmic Drugs As First-lne Treatment Of Symptomatic Atrial Fibrillation: (RAAFT 2): A Randomized Trial (Late Breaking Abstract) Heart Rhythm. 2012;9 [Google Scholar]
- 6.Nielsen JC, Johannessen A, Raatikainen P, et al. Radiofrequency Ablation as Initial Therapy in Paroxysmal Atrial Fibrillation. New Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 7.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–6. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 8.Calkins CH. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design. Heart Rhythm. 2012;9:632–696. [Google Scholar]
- 9.Jackman WM, Wang XZ, Friday KJ, et al. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605–11. doi: 10.1056/NEJM199106063242301. [DOI] [PubMed] [Google Scholar]
- 10.Jackman WM, Beckman KJ, McClelland JH, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med. 1992;327:313–318. doi: 10.1056/NEJM199207303270504. [DOI] [PubMed] [Google Scholar]
- 11.Elayi CS, Di Biase L, Barrett C, et al. Atrial fibrillation termination as a procedural endpoint during ablation in long-standing persistent atrial fibrillation. Heart Rhythm. 2010;7:1216–23. doi: 10.1016/j.hrthm.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004a;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Tanner H, Hindricks G, Kobza R, et al. Trigger activity more than three years after left atrial linear ablation without pulmonary vein isolation in patients with atrial fibrillation. J Am Coll Cardiol. 2005;46:338–43. doi: 10.1016/j.jacc.2005.03.063. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Zheng L, Zhang S, et al. Stepwise linear approach to catheter ablation of atrial fibrillation. Heart Rhythm. 2007;4:1497–504. doi: 10.1016/j.hrthm.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller JM. Treatment of Atrial Fibrillation by the Ablation of Localized Sources: The Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation (CONFIRM) Trial. J Am Coll Cardiol. 2012d;60:628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute Termination of Human Atrial Fibrillation by Identification and Catheter Ablation of Localized Rotors and Sources: First Multicenter Experience of Focal Impulse and Rotor Modulation (FIRM) Ablation. J Cardiovasc Electrophysiol. 2012;23:1277–85. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayan SM, Day J, Ellenbogen K, et al. Elimination of Sources for Human Atrial Fibrillation (Focal Impulse and Rotor Modulation, FIRM) Organizes and Acutely Terminates AF Prior to Pulmonary Vein Isolation: A Multicenter Experience (Abstract) Circulation. 2012;126 [Google Scholar]
- 18.Calvo D, Atienza F, Jalife J, et al. High-rate pacing-induced atrial fibrillation effectively reveals properties of spontaneously occurring paroxysmal atrial fibrillation in humans. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2012 doi: 10.1093/europace/eus180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayan SM, Krummen DE, Rappel W-J. Clinical Mapping Approach To Diagnose Electrical Rotors and Focal Impulse Sources for Human Atrial Fibrillation (cover article) J Cardiovasc Electrophysiol. 2012a;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konings K, Kirchhof C, Smeets J, Wellens H, Penn O, Allessie M. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 21.Cuculich PS, Wang Y, Lindsay BD, et al. Noninvasive Characterization of Epicardial Activation in Humans With Diverse Atrial Fibrillation Patterns. Circulation. 2010;122:1364–72. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narayan SM, Patel J, Mulpuru S, Krummen DE. Focal Impulse and Rotor Modulation (FIRM) Ablation of Sustaining Rotors Abruptly Terminates Persistent Atrial Fibrillation To Sinus Rhythm With Elimination On Followup. Heart Rhythm. 2012b;9:1436–1439. doi: 10.1016/j.hrthm.2012.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calkins H, Brugada J, Packer D, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. European Heart Rhythm Association (EHRA); European Cardiac Arrhythmia Scoiety (ECAS); American College of Cardiology (ACC); American Heart Association (AHA); Society of Thoracic Surgeons (STS) Heart Rhythm. 2007;4:816–61. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Atienza F, Almendral J, Moreno J, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2434–42. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 25.Haissaguerre M, Sanders P, Hocini M, et al. Catheter Ablation of Long-Lasting Persistent Atrial Fibrillation: Critical Structures for Termination. Journal of Cardiovascular Electrophysiology. 2005a;16:1125–1137. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 26.Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–18. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 27.Sahadevan J, Ryu K, Peltz L, et al. Epicardial Mapping of Chronic Atrial Fibrillation in Patients: Preliminary Observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 28.Wu T-J, Doshi RN, Huang H-LA, et al. Simultaneous Biatrial Computerized Mapping During Permanent Atrial Fibrillation in Patients with Organic Heart Disease. J Cardiovasc Electrophysiol. 2002;13:571–577. doi: 10.1046/j.1540-8167.2002.00571.x. [DOI] [PubMed] [Google Scholar]
- 29.Krummen DE, Peng KA, Bullinga JR, Narayan SM. Centrifugal Gradients of Rate and Organization in Human Atrial Fibrillation. Pacing Clin Electrophysiol. 2009;32:1366–1378. doi: 10.1111/j.1540-8159.2009.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazar S, Dixit S, Marchlinski FE, Callans DJ, Gerstenfeld EP. Presence of Left-to-Right Atrial Frequency Gradient in Paroxysmal but Not Persistent Atrial Fibrillation in Humans. Circulation. 2004;110:3181–3186. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 31.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846–54. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jalife J. Deja vu in the theories of atrial fibrillation dynamics. Cardiovascular research. 2011;89:766–75. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou CC, Chang PC, Wen MS, et al. Epicardial ablation of rotors suppresses inducibility of acetylcholine-induced atrial fibrillation in left pulmonary vein-left atrium preparations in a beagle heart failure model. J Am Coll Cardiol. 2011;58:158–66. doi: 10.1016/j.jacc.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 34.Comtois P, Nattel S. Impact of tissue geometry on simulated cholinergic atrial fibrillation: a modeling study. Chaos. 2011;21:013108. doi: 10.1063/1.3544470. [DOI] [PubMed] [Google Scholar]
- 35.Narayan SM, Shivkumar K, Krummen DE, Miller JM, Rappel W-J. Panoramic Electrophysiological Mapping But Not Individual Electrogram Morphology Identifies Sustaining Sites for Human Atrial Fibrillation: AF Rotors and Focal Sources Relate Poorly to Fractionated Electrograms. Circ Arrhythm Electrophysiol. 2013;6 doi: 10.1161/CIRCEP.111.977264. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayan SM, Krummen DE, Enyeart MW, Rappel W. Computational Mapping Approach Identifies Stable and Long-Lived Electrical Rotors and Focal Sources in Human Atrial Fibrillation. PLoS One. 2012;7:e46034. doi: 10.1371/journal.pone.0046034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganesan AN, Kuklik P, Lau DH, et al. Bipolar Electrogram Shannon Entropy at Sites of Rotational Activation: Implications for Ablation of Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2012 doi: 10.1161/CIRCEP.112.976654. in press. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y-J, Chang S-L, Lo L-W, et al. Small-radius-reentry Rotors in Maintaining Atrial Fibrillation: Clinical and Substrate Characteristics with Implication for Catheter Ablation (abstract) Heart Rhythm. 2012;9 [Google Scholar]
- 39.Lee G, Sanders P, Kalman JM. Catheter ablation of atrial arrhythmias: state of the art. Lancet. 2012;380:1509–19. doi: 10.1016/S0140-6736(12)61463-9. [DOI] [PubMed] [Google Scholar]
- 40.Klos M, Calvo D, Yamazaki M, et al. Atrial septopulmonary bundle of the posterior left atrium provides a substrate for atrial fibrillation initiation in a model of vagally mediated pulmonary vein tachycardia of the structurally normal heart. Circ Arrhythm Electrophysiol. 2008;1:175–83. doi: 10.1161/CIRCEP.107.760447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 43.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 44.Haissaguerre M, Hocini M, Sanders P, et al. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation. 2006;113:616–25. doi: 10.1161/CIRCULATIONAHA.105.546648. [DOI] [PubMed] [Google Scholar]
- 45.Arentz T, Weber R, Bürkle G, et al. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007;115:3057–3063. doi: 10.1161/CIRCULATIONAHA.107.690578. [DOI] [PubMed] [Google Scholar]
- 46.Pratola C, Baldo E, Notarstefano P, Toselli T, Ferrari R. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–43. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 47.Oral H, Chugh A, Good E, et al. Randomized comparison of encircling and nonencircling left atrial ablation for chronic atrial fibrillation. Heart Rhythm. 2005;2:1165–72. doi: 10.1016/j.hrthm.2005.08.003. [DOI] [PubMed] [Google Scholar]