Abstract
The zinc-finger transcription factor Insulinoma-associated 1 (Insm1, previously IA-1) is expressed in the developing nervous and neuroendocrine systems, and is required for cell type specific differentiation. Expression of Insm1 is largely absent in the adult, although it is present in neurogenic regions of the adult brain and zebrafish retina. While expression of Insm1 has also been observed in the embryonic retina of numerous vertebrate species, its function during retinal development has remained unexplored. Here, we demonstrate that in the developing zebrafish retina, insm1a is required for photoreceptor differentiation. Insm1a-deficient embryos were microphthalmic and displayed defects in rod and cone photoreceptor differentiation. Rod photoreceptor cells were more sensitive to loss of insm1a expression than were cone photoreceptor cells. Additionally, we provide evidence that insm1a regulates cell cycle progression of retinoblasts, and functions upstream of the bHLH transcription factors ath5/atoh7 and neurod, and the photoreceptor specification genes crx and nr2e3. Finally, we show that insm1a is negatively regulated by Notch-Delta signaling. Taken together, our data demonstrate that Insm1 influences neuronal subtype differentiation during retinal development.
Keywords: Insm1, photoreceptor, retinal development, cell cycle, zebrafish
INTRODUCTION
The vertebrate retina is an excellent model for studies of cellular differentiation during nervous system development, because all seven retinal cell types (6 neuronal and 1 glial), develop from a single pool of multipotent retinal progenitor cells (RPC's), demonstrate a precise laminar arrangement, and are generated in a conserved order of histogenesis (Andreazzoli, 2009; Turner et al., 1990). In order for the diverse array of retinal cell types to be generated in the proper numbers and at the appropriate times, RPC proliferation, migration and differentiation are tightly controlled by cell-intrinsic factors and cell-extrinsic signals (Baye and Link, 2007; Bilitou and Ohnuma, 2010; Ohsawa and Kageyama, 2008). Much of the intrinsic regulation is accomplished by spatio-temporally controlled expression of cascades of transcription factors (TFs). These TF's in turn regulate expression of other TF's, cell cycle genes, cell-fate specification genes and other genes of unknown function (Hatakeyama and Kageyama, 2004). Although several TF's essential for these processes have been identified, our knowledge of the molecular determinants of cell type differentiation in the vertebrate retina remains incomplete.
Insulinoma-associated 1 (Insm1, formerly IA-1) is an evolutionarily conserved zinc finger transcription factor, which was first identified from a tumor subtraction library (Goto et al., 1992). Insm1 expression is restricted to the developing nervous and neuroendocrine systems (Lan and Breslin, 2009), neurogenic regions in the adult brain (Duggan et al., 2008), and tumors of neuroendocrine origin (Lan and Breslin, 2009). Although very few direct target genes have been identified, Insm1 has been shown to regulate transcription of insulin and NeuroD in the developing pancreas, where Insm1 is essential for beta cell development (Liu et al., 2006; Mellitzer et al., 2006; Wang et al., 2008).
Insm1 has been implicated both in the regulation of cell cycle progression (Candal et al., 2007; Farkas et al., 2008; Wildner et al., 2008; Zhang et al., 2009) as well as in cell fate specification (Gierl et al., 2006; Jacob et al., 2009; Liu et al., 2006). Additionally, recent studies have identified a role for Insm1 in acinar cell trans-differentiation in vivo (Zhang et al., 2012a) and in Müller glia de-differentiation in the adult zebrafish retina following injury (Ramachandran et al., 2012). In a previous study, we demonstrated that a zebrafish ortholog of Insm1, insm1a, is expressed in the developing retina in a spatiotemporal pattern that mirrors the progression of neurogenesis, and that it is also expressed in rod photoreceptor progenitor cells in the adult zebrafish retina (Morris et al., 2011). Together, these data suggest that Insm1 regulates subtype differentiation, especially of photoreceptors in the developing retina. However, the precise function of Insm1 in retinal development has not yet been directly examined, and the embryonic lethality of Insm1 null mice (Gierl et al., 2006) has precluded more detailed studies of the role of on Insm1 during murine retinogenesis.
In this study, we have used morpholino-mediated gene knockdown in zebrafish to examine the role of insm1a during retinal development. We show that insm1a is required for the proper differentiation of rod and cone photoreceptors, and that insm1a regulates RPC cell cycle kinetics. Additionally, we establish that insm1a lies upstream of the bHLH transcription factors ath5/atoh7 and neurod, as well as the photoreceptor specification genes crx and nr2e3, whereas it lies downstream of and is negatively regulated by Notch-Delta signaling. Taken together, our results identify insm1a as a novel regulator of neuronal subtype differentiation in the developing vertebrate retina.
METHODS
Zebrafish lines and maintenance
Zebrafish were bred, raised and maintained in accordance with established protocols for zebrafish husbandry (Westerfield, 1995). Embryos and larvae were housed at 28°C, on a 14 h light:10 h dark cycle. Fish were anaesthetized with Ethyl 3-aminobenzoate methanesulfonate salt (MS-222, Tricaine, Sigma-Aldrich, St. Louis, MO). Embryos were staged as previously described (Kimmel et al., 1995). Wild type strains included the Ekwill strain (Ekwill Fish Farm, Gibsonton, FL), the AB strain obtained from the Zebrafish International Research Center (ZIRC, Eugene, OR) and hybrids produced by crossing the Ekwill and AB strains. The Tg (XRho: gap43-mCFP) q13 transgenic line, hereafter called XOPS-mCFP, has been previously described (Morris et al., 2011; Morris et al., 2008a). This line harbors a fluorescent mCFP reporter transgene under the control of a 5.5 kb Xenopus rhodopsin promoter. Expression of this transgene results in selective degeneration of the rod photoreceptor cells (Morris et al., 2011; Morris et al., 2005). The Tg (3.2TαC-EGFP) transgenic line, hereafter called TαC-EGFP, has been previously described (Kennedy et al., 2007), and was generously provided by Susan Brockerhoff (University of Washington, Seattle WA). The Tg (nyx:GAL4-VP16)q16a/(UAS:gap43-YFP)q16b transgenic line, hereafter referred to as nyx::YFP, and the Tg (XlRho:EGFP)fl1 transgenic line (hereafter called XOPS-GFP) have both been previously described (Fadool, 2003; Schroeter et al., 2006), and were obtained from James Fadool (Florida State University, Tallahassee, FL). The Tg (gfap:GFP)mi2001 transgenic line (hereafter called gfap:GFP) has been previously described (Bernardos and Raymond, 2006), and was obtained from ZIRC. All animal procedures were carried out in accordance with guidelines established by the University of Kentucky Institutional Animal Care and Use Committee.
Microinjections of morpholinos and mRNA
A translation-blocking antisense morpholino (MO) designed against insm1a was injected into fertilized embryos prior to the second cell division. Two non-overlapping morpholino sequences were used: MO1 (5'-GGTTGAAATCAGAGGCACACCT-3') and MO2 (5'-CGCCAGCTGAAAGGCACTTCA-3'). Both produced similar phenotypes; unless otherwise indicated, MO1 was used for all analyses described in this study. The insm1a MO1 was injected at 6.0–7.2 ng/embryo and the insm1a MO2 was injected at 7.2ng/embryo. Since injection of MO1 caused some toxicity to the embryos, an antisense tp53 morpholino (p53MO) was co-injected to suppress cell death (Bill et al., 2009b). The p53MO (5'-GCGCCATTGCTTTGCAAGAATTG-3') was injected at 1.5-fold the amount of the insm1a MO. A standard control MO, targeting a mutant variant of the human β-globin gene (5'-CCTCTTACCTCAGTTACAATTTATA-3'), was injected similarly to the insm1a MO. All morpholinos were synthesized by GeneTools, LLC (Philomath, OR).
Capped mRNA was synthesized from a cloned insm1a coding sequence lacking the morpholino binding site using the mMessage (T7 or Sp6) Kit (Ambion, Austin, TX) according to the manufacturer's instructions. mRNA was cleaned by column purification (RNeasy kit, Qiagen, Valencia, CA), followed by phenol-chloroform extraction and ethanol precipitation. All injected embryos were transferred to fish water containing 0.003% 1-phenyl-2-thiourea (PTU) at 24 hours post fertilization (hpf) to inhibit pigmentation. Embryos were immobilized in an acrylic mold for morpholino injection, and in depression slides at 48 and 72 hpf for live imaging.
Testing morpholino effectiveness
A pair of complimentary oligonucleotides corresponding to the insm1a morpholino target sequence (Table 1) were synthesized and purified by HPLC (Biosynthesis, Lewisville, TX). The oligos were designed to produce overhangs complimentary to the ends produced by enzyme digestion of the pEF1α:GFP plasmid (Addgene plasmid 11154). The oligos were resuspended in oligo annealing buffer (10mM Tris pH 7.52, 50mM NaCl, 1mM EDTA) at 100ng/μl, and 1μg of each oligo was combined into a 50μl annealing reaction. The annealing reaction was heated at 95°C for 2 minutes and cooled to 25°C over 45 minutes. The annealed oligos were diluted 10-fold, and 1μl was ligated with 100 ng of double-digested pEF1α:GFP plasmid.
Table 1.
Primer sequences used in this study.
| Gene | Forward | Reverse | |
|---|---|---|---|
| Insm1a MOBS | AATTCAGGTGTGCCTCTGATTTCAACCCGAGGTAC | CTCGGGTTGAAATCAGAGGCACACCTG | cloning |
| ath5 | CCGGAGAAGTTTGAGAGTGC | GCTCAGAGCCATCTGTAGGG | qPCR |
| crx | ATGCTGTGAACGGGTTAAC | AAGCTTCCAGAATGTCCAG | qPCR |
| insm1a | GGCACCACAGTAACCACCA | CGCTGGAAGTCTCCTCTTTCT | probe |
| neurod | ATACAGCGAGGAAAGCATGA | CCGTTCGTGATGCGAGTG | qPCR |
| nr2e3 | CCAGCAGTGGGAAACACTAT | ATGGGCTTTATCCACAGGAC | qPCR |
Wild type embryos at the one-cell stage were co-injected with 100pg/embryo of pEF1α-GFP containing the insm1a MO1 binding site (pEF1α-MOBS:GFP) and either the control or the insm1a morpholino. Embryos were then screened each day from 1 through 5 dpf for expression of GFP.
RNA isolation
RNA was isolated from adult retinas or whole embryos at selected developmental time points. Adults were euthanized by rapid cooling as previously described (Wilson et al., 2009). The eyes were dissected; the sclera, choroid and lens were removed. Retinas were transferred to tubes containing an RNA Stabilizer (RNALater, Ambion/Applied Biosystems). RNA was collected from pooled retinas using TRIzol Reagent (Life Technologies, Invitrogen), following the manufacturer's protocol, then treated with RNAse-Free DNAse I (Roche, Indianapolis, IN) to remove genomic DNA. RNA was collected from pooled embryos following a similar purification protocol.
Reverse transcription and quantitative PCR
Approximately 500ng of RNA was reverse transcribed into cDNA (GoScript Reverse Transcriptase System, Promega, Madison, WI) according to the manufacturer's instructions. Quantitative PCR (qPCR) was performed on an iCycler iQ Real Time PCR Detection System (Bio-Rad, Hercules, CA) using detection of SYBR Green incorporation, according to the manufacturer's instructions. Primer sequences used in qPCR are given in Table 1. Statistical analysis of the qPCR data was completed using SAS 9.3 software.
Preparation of digoxigenin labeled riboprobes
Digoxigenin (DIG)-labeled antisense and sense riboprobes were prepared from a linearized plasmid containing a portion of the coding region of the gene of interest by in vitro transcription with T7 or SP6 polymerase using the an RNA labeling kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. The insm1a and neurod plasmids were prepared by cloning PCR products into the pGEM T-easy vector (Promega) and pCR-TOPO (Invitrogen), and have been previously described (Morris et al., 2011; Morris et al., 2008b). The nr2e3 and crx plasmids were generously provided by Yuk Fai Leung (Purdue University, West Lafayette. IN), and have been previously described (Chen et al., 2005; Ochocinska and Hitchcock, 2007; Zhang et al., 2012b). The ath5/atoh7 plasmid was a generous gift from Brian Link (Medical College of Wisconsin, Milwaukee, WI). The ath5/atoh7 probe sequence was subcloned into pGEM-T easy vector for probe synthesis, and the expression pattern matched previously published data (Stenkamp and Frey, 2003).
Whole mount in situ hybridizations
Embryos and larvae were collected as described above. Tissues were fixed overnight at 4°C in freshly prepared 4% paraformaldehyde (PFA) prepared in PBS pH 7.0. Samples were dehydrated and stored in 100% methanol at −20°C for a minimum of 24 hours. Tissues were rehydrated through a graded PBST series (PBS, 0.1% Tween-20) and permeabilized in proteinase K (20μg/ml in PBST). After washing in triethanolamine (0.1 M TEA), tissues were acetylated using acetic anhydride, washed in TEA and PBST and refixed in 4% PFA in PBS. After additional PBST washes, samples were prehybridized in hybridization buffer (50% formamide, 5X Saline Sodium Citrate Buffer (SSC), 5mg/ml torula (yeast) RNA, 50μg/ml heparin sulfate, 0.1% Tween-20) for a minimum of 2 hours at 60°C. Riboprobes were hybridized to the tissue overnight at 60°C at a final concentration of 2ng/ul in hybridization buffer. Samples were washed through a graded SSC series at 60°C and 70°C, and a graded PBST series at room temperature, before blocking for a minimum of 2 hours at 4°C in PBST containing 2% BSA and 2% sheep serum. Samples were incubated overnight at 4°C with an anti-DIG-AP antibody (Roche) diluted 1:2500 in blocking buffer. The following day, samples were washed for 2 hours with multiple changes of blocking buffer, and equilibrated in NTMT buffer (0.1M Tris pH 9.5, 0.05M MgCl2, 0.1M NaCl, 0.1% Tween-20) before coloration with 4-Nitro blue tetrazolium (NBT; Roche) and 5-bromo-4-chloro-3-indolyl-phosphate, 4-toluidine salt (BCIP; Roche) in NTMT. Coloration was halted by washing with a stop solution (PBS pH5.5, 1mM EDTA). Whole embryos or dissected eyes were imaged on an inverted microscope (Eclipse Ti-U; Nikon Instruments, Melville, NY), using a 40X objective.
Histology and immunohistochemistry
Adult retinas or whole embryos were collected as described above. Tissues were fixed overnight at 4°C in freshly prepared 4% PFA. For immunolabeling, fixed embryos were cryoprotected in 10% sucrose in PBS for at least 3 hours and in 30% sucrose overnight at 4°C. Samples were mounted in OCT Medium (Ted Pella, Redding, CA) and frozen on dry ice. Ten to 12μm sections were cut on a cryostat (Leica CM 1850, Leica Biosystems, Buffalo Grove, IL), mounted on gelatin-coated slides, and dried overnight at room temperature. Before immunolabeling, sections were rehydrated and postfixed in 1% PFA for 10 minutes at room temperature. After 2 washes in PBS, and 2 washes in PBST, sections were blocked in PBST containing 1% BSA for at least 30 minutes at room temperature. Slides were incubated with primary antibody in PBST/BSA with 5% Normal Goat serum, overnight at 4°C in a humidified chamber. The following day, slides were washed 3 times in PBST, and incubated with secondary antibody in PBST/BSA for 1 hour at room temperature in the dark. Slides were washed 2 times with PBST, counterstained with DAPI (4', 6-diamidino-2-phenylindole, 1:10,000 dilution; Sigma-Aldrich) in PBS, and mounted in 40% glycerol in PBS. Images were obtained on an inverted fluorescent microscope (Eclipse Ti-U; Nikon Instruments), using a 40X objective. The following antibodies were used: Zpr-1 (1:20 dilution), a monoclonal antibody that recognizes red and green cones (ZIRC); 4C12 (1:100 dilution), a monoclonal antibody that recognizes an unknown epitope on rods (Fadool J, Linser, P unpublished; a generous gift of James Fadool, FSU, Tallahassee, FL); Zn-8 (1:10 dilution), a monoclonal antibody that recognizes retinal ganglion cells (ZIRC); 5E11 (1:10 dilution), a monoclonal antibody that labels amacrine cells (generously provided by James Fadool, FSU, Tallahassee FL), HuC/D (1:20 dilution), a monoclonal antibody that recognizes retinal ganglion cells and amacrine cells (Invitrogen, Grand Island, NY); anti-PKCα (H300), a polyclonal antibody that recognizes bipolar cells (Santa Cruz Biotechnology, Santa Cruz, CA); anti-Prox1 (1:2000 dilution) a polyclonal antibody that recognizes horizontal cells (Millipore, Billerica, MA); anti-BrdU (clone B-33; 1:500 dilution), a mouse monoclonal antibody that marks cells in S phase of the cell cycle (Sigma-Aldrich); and anti-phospho-Histone H3 (Ser10;1:500 dilution), a polyclonal antibody that recognizes cells in late G2/M-phase (Millipore). Alexa Fluor 488 goat anti-mouse, 488 goat anti-rabbit, 546 goat anti-rabbit, 546 goat anti-mouse (Molecular Probes, Invitrogen), 647 goat anti-rabbit and Cy5 conjugated goat anti-mouse (Jackson ImmunoResearch, West Grove, PA) secondary antibodies were all used at 1:200 dilution.
Morphometric analysis
Control and insm1a morphant embryos were positioned in depression slides and imaged on an inverted microscope (Eclipse Ti-U; Nikon Instruments), using 4X and 20X objectives. Measurements were taken using the Nikon Elements software. The 4X images were used to measure total body length from the otic vesicle to the tip of the tail following the line of the spinal column The 20X images were used to measure the area of the eye by outlining the entire eye and the lens. The exclusion or inclusion of lenses was statistically irrelevant (p<0.0001 both with and without inclusion of the lens area), and all statistics reported here include lens measurements. All measurements were taken in triplicate. Additional measurements were taken if the variation was greater than 5% across the three measurements. SAS 9.3 was used for all statistical analyses.
Cell counts
Immunolabeled control and insm1a morphant cryosections containing three distinct cell layers and a lens were used for cell counts. Cells were counted a minimum of 3 times, and counts were averaged for statistical analysis. SAS 9.3 was used for all statistical analyses.
BrdU injection and immunohistochemistry
For cell cycle analysis, 30 hpf control and insm1a morphant embryos were dechorionated, anesthetized with MS-222, and transferred to an agarose microinjection plate. A 10mM solution of BrdU (Sigma-Aldrich) was injected into the yolk. The embryos were returned to 28°C, and collected at 0.5 hours post injection (hpi), 2 hpi, 4 hpi and 6 hpi. These correspond to 30.5, 32, 34 and 36 hpf, respectively. The embryos were fixed and cryoprotected as described above, and 10μm transverse cryosections were taken through the head.
Sections were immunolabeled as described above, with the addition of incubation in 2N HCl for 30–40 minutes in at 37°C prior to blocking. Following incubation with the secondary antibody, slides were washed 2 times with PBST then equilibrated twice in 2X SSC buffer. The slides were treated with 100μg/ml RNAse A (Qiagen) in 2X SSC, counterstained with propidium iodide (1μg/ml; Invitrogen), washed 2 times in 1X PBS and mounted in 40% glycerol in PBS. Images were obtained on an inverted fluorescent microscope (Eclipse Ti-U; Nikon Instruments), using a 40X objective.
Dual luciferase assays
HEK293 cells were transfected with varying amounts of the pcDNA3 mammalian expression vector (Invitrogen) containing the zebrafish her4 cDNA, the pGL3 Firefly Luciferase reporter vector (Promega) containing an insm1a promoter cloned upstream of the luciferase gene, and the pRL-TK vector (Promega), containing the Renilla luciferase gene driven by a ubiquitous tyrosine kinase promoter (to control for transfection efficiency) using Fugene 6 (Promega), following the manufacturer's instructions. The total amount of transfected DNA was kept constant across transfections, and transfection experiments were repeated a minimum of 3 times. Between 24 and 36 hours after transfection, when cells were at least 80% confluent, Firefly and Renilla luciferase activity were measured using the Dual Glo Luciferase Assay System (Promega). Data was analyzed as follows: Firefly luciferase (FFLuc) was baselined using an untransfected control (UTC) sample (=FFLuc – UTC) and normalized using the Renilla luciferase (RLuc). The Relative Luciferase Activity (RLA) was calculated as (FFLuc-UTC)/RLuc). The RLA was compared between experimental and control transfections, and statistical significance was determined by ANOVA using SAS 9.3 software.
DAPT treatment
Pharmacological inhibition of Notch-Delta signaling in embryonic zebrafish was accomplished using N-[N- (3, 5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a γ-secretase inhibitor that prevents proteolytic cleavage of the Notch intracellular domain from the cell membrane. Embryos were partially dechorionated and transferred to pre-warmed embryo medium containing 100 μM DAPT in 1% DMSO (carrier). Time-matched sibling embryos in embryo medium with 1% DMSO served as carrier controls. Embryos were treated from 10.5 hpf until 28 hpf, when embryos were collected and processed for whole mount in situ hybridization as described above.
RESULTS
Insm1a is expressed in adult rod progenitor cells and in the developing retina
We previously demonstrated that in the wild type adult zebrafish, insm1a expression is very low or absent in the central retina, although it is present in the persistently neurogenic ciliary marginal zone (CMZ; Morris et al., 2011). In contrast, expression of insm1a is strongly upregulated in presumptive rod progenitor cells in a zebrafish model of chronic rod photoreceptor degeneration and regeneration (XOPS-mCFP). Rod progenitor cells can be identified by their location at the base of the outer nuclear layer (ONL) in the central retina, and by their expression of markers of cell proliferation and rod photoreceptor cell fate commitment (Morris et al., 2011; Morris et al., 2008a; Morris et al., 2005). To confirm that insm1a is expressed in proliferating rod progenitor cells, in situ hybridization with an insm1a probe was performed on retinal sections from adult XOPS-mCFP zebrafish that had been exposed to BrdU for four hours, followed by immunohistochemistry to detect BrdU incorporation. In XOPS-mCFP retinas, insm1a-positive cells were observed at the base of the ONL, and most (but not all) of the insm1a-positive cells were also BrdU-positive (Fig. 1A–C). These data confirm our previously published results showing that in XOPS-mCFP retinas insm1a expression co-localizes with the S-phase marker pcna (Morris et al., 2011), and demonstrate that insm1a is expressed in rod progenitor cells in the adult zebrafish retina.
Figure 1. Insm1a is expressed in rod progenitor cells and in the developing retina.
(A–C) Expression of insm1a in XOPS-mCFP retinas. (A) In situ hybridization with an antisense probe for insm1a was performed on retinal cryosections of XOPS-mCFP retinas after a 4 hour exposure to BrdU. (B) Immunolabeling with anti-BrdU identified rod progenitor cells at the base of the ONL. (C) Overlay of in situ and immunolabeling demonstrates that many, but not all insm1a+ cells are also BrdU+. Arrows indicate insm1a+/BrdU+ cells; asterisks indicate insm1a+/BrdU− cells. (D–G) Whole mount in situ hybridization of wild type embryos during development. (D) Insm1a expression was observed in the ventronasal patch between 24 and 28 hpf (arrow), (E) and insm1a expression expanded counterclockwise to the dorso-nasal retina at 36 hpf (arrows). (F) By 48 hpf, expression of insm1a had progressed to the dorso-temporal quadrant. (G) At 72 hpf, insm1a expression was only observed adjacent to the proliferative marginal zone at the retinal periphery. (A scale bar = 25μm; D scale bar = 50μm; D, dorsal; V, ventral; A, anterior; P, posterior; ONL, outer nuclear layer; INL, inner nuclear layer: L, lens; hpf, hours post fertilization)
Using whole mount in situ hybridization (WISH), the onset of insm1a expression was first detected in the ventro-nasal region of the developing retina between 24 and 28 hpf (Fig. 1D), which coincides with the initiation of the retinal neurogenic wave in zebrafish (Hu and Easter, 1999; Masai et al., 2000). The expression of insm1a continued to track the progression of neurogenesis, expanding in a counterclockwise fashion to the dorso-nasal retina at 36 hpf (Fig. 1E), and then to the dorso-temporal quadrant of the retina by 48 hpf. This counterclockwise progression of insm1a expression in the sagittal plane was accompanied by a shift in expression from the central to peripheral retina in the transverse plane, which we have described previously (Morris et al., 2011). By 72 hpf, when retinal neurogenesis is largely complete, expression of insm1a was absent from the central differentiated retina and was only observed adjacent to the proliferative marginal zone at the retinal periphery (Fig. 1G and (Morris et al., 2011)). Zebrafish possess two co-orthologs of the mammalian Insm1 gene, insm1a and insm1b. We did not observe expression of insm1b in the developing retina at any stage (data not shown), which agrees with a previous developmental study (Lukowski et al., 2006). Taken together, the developmental expression pattern of insm1a in the retina suggests that it functions during the window when retinal progenitor cells (RPC's) are exiting the cell cycle to begin differentiation.
Insm1a morphants display a specific reduction in eye size
To examine the function of insm1a in retinal development, two different translation-blocking morpholinos (MOs) were designed to specifically knock down insm1a expression. Splice-blocking morpholinos could not be used against insm1a because it is a single-exon gene. Both MOs produced similar phenotypes when injected into 1-cell-stage embryos (data not shown). MO1 was used for all subsequent analysis unless otherwise stated. A standard control morpholino was used to control for non-specific phenotypes resulting from the microinjection procedure. The effectiveness of the insm1a MO at blocking translation was evaluated by co-injecting the morpholino along with an EF1α-GFP plasmid containing the insm1a morpholino binding site (pEF1α-MOBS:GFP). We compared the numbers of GFP-positive embryos injected with pEF1α-MOBS:GFP and the standard control morpholino to those injected with pEF1α-MOBS:GFP and the insm1a morpholino (Supplementary Fig. 1). This experiment showed that the insm1a morpholino was highly efficient and its translation-blocking activity lasted through 4 dpf. Because some non-specific cell death was observed in embryos injected with either of the insm1a MOs, in all subsequent analyses we co-injected the insm1a MO with a morpholino for tp53, which has been shown to block apoptosis (Bill et al., 2009). Survival of morpholino-injected and uninjected embryos between 8 and 24 hpf was not significantly different (one way ANOVA Pr > F = 0.44) across insm1a morphants (76.7±10.7%), control morphants (84.6±17.5%) and uninjected embryos (81.9±21.3%).
Control and insm1a morpholino-injected embryos were categorized based upon their morphology and developmental stage at 24 hpf using standard staging criteria (Kimmel et al., 1995). At 24 hpf, morphological markers (including the presence or absence of a beating heart) were scored to determine developmental stage, and any individuals with developmental delay were categorized as “severe”. Nearly all embryos injected with the standard control morpholino (Fig. 2A, B) were at the correct developmental stage at 24 hpf (98%) and showed no overt morphological changes (93%). Any injected embryos that displayed pericardial effusion or other defects (5%) were not used for further analysis. Among the insm1a morphants without developmental delay (90%), the body shape, head shape, and presence/absence of pericardial effusion were scored at 48 hpf, and the embryos were categorized as mild, moderate or severe. Insm1a morphants in the “mild” category displayed no developmental delay or overt morphological changes when compared to control morphants. This category represented a very small number of insm1a morphants (2%). Insm1a morphants in the “moderate” category (Fig. 2C, D) were also at the correct developmental stage, but displayed mild body torqueing, with a spinal curvature. Moderate category insm1a morphants displayed no other malformations of the body, and had no pericardial effusion. This category represented the majority of the insm1a morphants (74%). Insm1a morphants categorized as “severe” displayed significant body torque, with malformations of the tail. Insm1a morphants with significant pericardial effusion or any global delay in development were also categorized as severe (24%). For all subsequent analyses, only insm1a morphants in the moderate phenotypic category were examined.
Figure 2. Insm1a-deficient embryos are microphthalmic.

Compared to embryos injected with a control morpholino (A, B), insm1a morphants (C, D) displayed mild body torqueing and smaller eyes at 48 hpf. Whereas the eye area was significantly smaller in insm1a morphants (E), body lengths were not significantly different (F), and eye areas remained significantly smaller after controlling for body length (G). (A, C scale bar =500μm; B, D scale bar = 100μm; E–G ◊=mean; *p<0.0001, t-test; n > 12; L, lens; hpf, hours post fertilization; MO, morpholino)
Measurements of eye size at 48 hpf revealed a significant reduction in eye area in the insm1a morphants compared to controls (Fig. 2E). To determine whether this reduction in eye area was the result of an overall reduction in the body size of insm1a morphant embryos, the ratio of eye area to body length was compared in control and insm1a morphants. This analysis demonstrated that eye area was significantly reduced in insm1a morphants even when expressed as a ratio of eye area to body length (Fig. 2G). To rule out off-target effects as a cause of the decrease in eye area in insm1a morphants, we co-injected the insm1a morpholino with an in vitro transcribed insm1a mRNA lacking the morpholino binding site. Co-injection of insm1a mRNA significantly increased the eye area at 48 hpf compared to insm1a morpholino injection alone (88% of the control eye area versus 66–70%, data not shown) demonstrating that the eye size reduction observed in insm1a morphants was due to a specific knockdown of insm1a.
We did not observe an increase in TUNEL-positive cells in insm1a morphant retinas (data not shown), suggesting that the reduced eye size is unlikely to be the result of increased cell death. We also attempted to investigate whether over-expression of insm1a alone resulted in larger eyes than controls. We did not detect an increase in eye size at 48 hpf using a dosage of insm1a mRNA similar to that used for the morpholino rescue. However, we were unable to test higher amounts of insm1a mRNA, because these were toxic to the embryos.
Knockdown of insm1a significantly impairs photoreceptor differentiation
Because insm1a was previously shown to be expressed in rod progenitor cells in the adult retina we next investigated whether knockdown of insm1a altered rod photoreceptor development in the embryonic retina. Retinal cryosections were prepared from control and insm1a morphants at 3 dpf, and the number of rod photoreceptors per section was counted using either immunohistochemistry for a rod photoreceptor-specific antibody (4C12; Fig. 3), or by counting GFP-positive cells in a rod photoreceptor-specific transgenic reporter line (Fadool, 2003; not shown). Using either method, insm1a morphants displayed greatly reduced numbers of rod photoreceptor cells at 3 dpf (Fig. 3B), with an average of 2.55 rods per section, compared with control morphants, which contained an average of 41.13 rods per section. This represents a 93.7% decrease in rod photoreceptors in insm1a morphants compared with controls. Additionally, several retinal sections from 3 dpf insm1a morphants contained no detectable rods. Similar to our observation of eye size described above, co-injection of insm1a mRNA along with the insm1a MO significantly increased the rod photoreceptor number to an average of 32.98 rods per section, demonstrating that the lack of rod photoreceptors in insm1a morphants is due to a specific knockdown of insm1a.
Figure 3. Photoreceptor cell differentiation is impaired in insm1a morphants.
(A) At 3 dpf cone (green) and rod (red) photoreceptor cells were severely reduced in insm1a morphants. (B) The reduction in rods was significant and could be partially rescued by co-injection of in vitro transcribed insm1a mRNA with the insm1a morpholino. (control: n= 31 eyes from 22 embryos; insm1a MO: n=24 eyes from 17 embryos; insm1a MO + insm1a mRNA: n=18 eyes from 10 embryos.) (C) The reduction in cones was significant and could be rescued by co-injection of in vitro transcribed insm1a mRNA with the insm1a morpholino. (control: n= 26 eyes from 17 embryos; insm1a MO: n=24 eyes from 17 embryos; insm1a MO+ insm1a mRNA n=18 eyes from 10 embryos.) (D) At 4 dpf, the rod photoreceptors remain significantly reduced. However, cone photoreceptors (green) significantly recovered, but were less dense and contained visible gaps (dotted box). (D') In regions lacking cones INL cells breached the OPL and extended into the ONL. Both small (arrowheads) and large (arrows) gaps were observed, accompanying the breached OPL. (B, D: ◊=mean, *p<0.0001; scale bars = 50μm; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; L, lens; ON, optic nerve; dpf, days post fertilization; MO, morpholino)
Because rod photoreceptors display a protracted period of differentiation relative to cone photoreceptors and other retinal neurons (Cepko et al., 1996; Stenkamp, 2007), we sought to determine whether rod photoreceptor number in insm1a morphants remained significantly reduced one day later in development. Retinal cryosections from 4 dpf control larvae contained an average of 55.74 rods per section; in contrast, rod photoreceptor number remained significantly lower in 4 dpf insm1a morphant retinal sections (Fig. 3D), with an average of 14.54 rods per section. Because the number of rod photoreceptors increased from 3 to 4 dpf in insm1a morphant retinas, it is possible that knockdown of insm1a does not cause a complete arrest in rod photoreceptor differentiation. Alternatively, the small increase in rods may be due to incomplete knockdown of insm1a, although our data indicate that the insm1a morpholino is highly efficient through 4 dpf (Supplementary Fig. 1). In any case, our observation that the number of rod photoreceptors in insm1a morphants was significantly lower than controls at both 3 and 4 dpf, suggests that differentiation of proper numbers of rod photoreceptors requires insm1a expression. We did not count rod photoreceptor number at later developmental stages in insm1a morphants, because of the concern that at 5 dpf and beyond the morpholino may no longer be effective at blocking translation (Supplementary Fig. 1).
Next, we investigated whether cone photoreceptor cell number was affected by the knockdown of insm1a. Cone number was scored on retinal cryosections from control and insm1a morphants either by counting GFP-positive cones in a cone photoreceptor-specific transgenic reporter line (TαC-EGFP; Kennedy et al., 2007; Fig. 3), or by immunolabeling with the red/green cone antibody Zpr-1 (not shown). Using either method, we observed that differentiated cone photoreceptors were significantly reduced in insm1a morphants (average of 12.0 Zpr1-positive cones/section) compared to controls (average 73.17 Zpr1-positive cones/section) at 3 dpf (Fig. 3C). This phenotype was specifically caused by knockdown of insm1a, because co-injection of the insm1a morpholino with insm1a mRNA lacking the morpholino binding site was able to significantly rescue cone photoreceptor number (average 67.39 Zpr1-positive cones/section).
Interestingly, when this experiment was repeated at 4 dpf, we observed that unlike the rods, cone photoreceptor differentiation had substantially recovered in insm1a morphant retinas (Fig. 3D). However, we noticed that while the cone photoreceptors appeared continuous and well packed in control retinas, the cone photoreceptor layer in 4 dpf insm1a morphant retinas contained visible gaps in some regions. The gaps in cone photoreceptors were categorized as small if they were only 1–2 cells wide (Fig. 3D' arrowhead) and large if they were >5 cells wide (Fig. 3D' arrow); these gaps were observed in nearly all insm1a morphant retinal sections examined at 4 dpf (23/25), whereas they were never observed in control retinal sections (0/28). The number of cone photoreceptor gaps observed in individual insm1a morphant retinal sections ranged from zero to fifteen, with an average of 3.86 gaps per section. The absence of cones in these gaps was accompanied by the presence of cells from the inner nuclear layer (INL) that had “breached” the outer plexiform layer (OPL). Overall, these data suggest that insm1a knockdown delays cone photoreceptor differentiation in addition to causing impaired rod photoreceptor differentiation. The cones appear to be less sensitive to the lack of insm1a than the rods, because they are able to recover to near control levels by 4 dpf. Moreover, insm1a knockdown (and the resulting reduction in differentiated photoreceptors at 3 dpf) may affect the integrity of the OPL, allowing cells from the INL to migrate into the ONL as a consequence.
Effect of insm1a knockdown on other retinal cell types
Photoreceptor cells are relatively late-born neurons in the zebrafish (Stenkamp, 2007). Therefore, it is possible that the delay in rod and cone photoreceptor differentiation observed in insm1a morphants reflects a more general delay in the differentiation of later born retinal cell types. To determine whether this is the case, we evaluated differentiation of the Müller glia, another late-differentiating cell type (Peterson et al., 2001; Scheer et al., 2001) in the zebrafish retina. We counted the number of GFP-positive Müller cells in control and insm1a morphant retinal sections at 3 dpf, using the gfap:GFP transgenic zebrafish line, which specifically labels Müller glia in the retina (Bernardos et al., 2007; Bernardos and Raymond, 2006; Qin et al., 2009). Although the Müller cell bodies in insm1a morphant retinas were slightly less well aligned in the INL compared to controls (data not shown), the number of Müller cells was not significantly different in the insm1a morphants compared to controls at 3 dpf (Fig. 4). This result suggests that the reduced photoreceptor cell number in insm1a morphants is not the consequence of an overall delay in the differentiation of late-appearing retinal cell types.
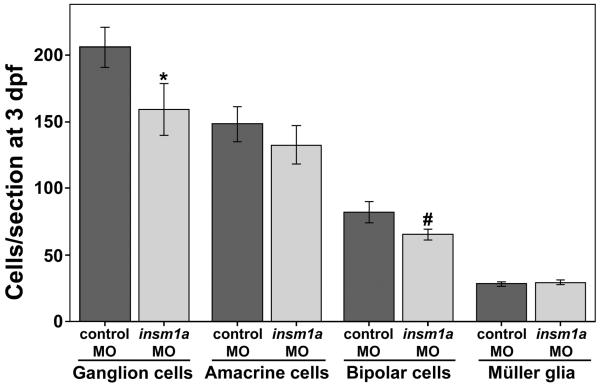
Figure 4. Knockdown of insm1a causes modest changes in cell number for some non-photoreceptor cell types.
At 3 dpf the numbers of ganglion cells and bipolar cells were slightly reduced in insm1a morphant retinas, whereas the numbers of amacrine cells and Müller glia were not significantly affected. (*p<0.005; #p<0.003; mean±stdev for all; n=6 embryos each for ganglion and amacrine cells, 5 each for bipolar cells and 18 eyes from 10 embryos each for Müller glia).
While Müller glia cell number was not reduced in insm1a morphants relative to controls, it is possible that knockdown of insm1a causes a neuronal-specific delay in differentiation. To determine if this was the case we evaluated differentiation of the other classes of retinal neurons, starting with the bipolar cells. Bipolar cells in control and insm1a morphant retinal sections at 3 dpf were identified using the nyx∷YFP transgenic zebrafish line, which labels ON-bipolar cells, in combination with immunolabeling for the bipolar cell marker PKCα. Bipolar cell counts revealed a modest reduction (by approximately 22%) in insm1a morphants compared to controls (Fig. 4). Furthermore, we observed that the bipolar cell terminal boutons in the inner plexiform layer (IPL) were less mature in insm1a morphants when compared to controls, appearing thinner and less intensely stained (data not shown). We repeated this experiment at 4 dpf to determine if the bipolar cell numbers would recover in the insm1a morphants, as we had observed with the cone photoreceptors. Although we observed an increase in the number of bipolar cells in insm1a morphants from 3 to 4 dpf, we found that the number of bipolar cells remained modestly reduced relative to controls. For the most part, at 4 dpf the morphology of the bipolar cells in insm1a morphants appeared very similar to the controls, with well-stratified terminal boutons in the IPL and nicely staining dendritic terminals in the OPL (Fig. 5). However, there were some regions of the OPL where the bipolar processes were disrupted. These regions corresponded to the areas in which cells from the INL had breached the OPL. Bipolar cell bodies were sometimes observed in the breaches, at the level of the OPL (Fig. 5B, arrowhead). Staining with fluor-conjugated phalloidin revealed that these OPL breaches lacked all neuronal processes (Fig. 5B). Overall, these data suggest that a loss of insm1a causes a modest reduction in bipolar cell number, a delay in bipolar cell maturation, and compromises the integrity of the OPL.
Figure 5. Bipolar and horizontal cell maturation is delayed in insm1a morphants.
(A) At 4 dpf, bipolar cell morphology and labeling intensity in insm1a morphants appeared similar to controls. However, some breaches in the OPL were observed (B) and these regions (shown by an absence of phalloidin staining) occasionally contained bipolar cell bodies (white arrowhead). (C) At 4 dpf, Prox1+ cells were present in the INL adjacent to the OPL in insm1a morphant retinas; however, they were rounder and more widely spaced than in controls. (D) In regions of 4 dpf insm1a morphant retinas that contained cones, the adjacent Prox1+ horizontal cells displayed a more mature morphology (white arrows); however, in regions devoid of cones, the Prox1+ cells were absent or appeared less mature (white arrowheads). Prox1+ cells were also observed in the regions where cells from the INL had breached the ONL (white arrowheads). Scale bars = 50μm; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; IPL, inner plexiform layer; OPL, outer plexiform layer; L, lens; ON, optic nerve; dpf, days post fertilization; MO, morpholino.
Because the OPL was disrupted in some regions of the insm1a morphant retinas, we wondered whether this would affect the differentiation of horizontal cells, which are located directly adjacent to the OPL. To evaluate horizontal cell differentiation we immunolabeled retinal sections with the Prox1 antibody, which recognizes horizontal cells and precursors of horizontal, amacrine and bipolar cells. Mature horizontal cells could be distinguished from other Prox1-positive cells by their location (directly abutting the OPL) and the elongated, flattened morphology of their cell bodies. We observed that in 4 dpf insm1a morphant retinal sections, Prox1-positive cells were either absent at the OPL or lacked a mature horizontal cell morphology. In insm1a morphants, the Prox1-positive cells that were found near the OPL were rounder and more widely spaced than in the control embryos (Fig. 5C, D). Interestingly, in regions of retinal sections where cone photoreceptors were present, we observed some Prox1-positive cells with a more mature horizontal cell morphology located directly adjacent to the cones (Fig. 5D, arrows). However, even these cells were more widely spaced, rounder, and less well aligned with the border of the OPL than horizontal cells in sections from control retinas. Moreover, similar to our observation of bipolar cells, we found that at 4 dpf, some of the regions of the ONL where the OPL was disrupted contained Prox1-positive cells, indicating that cells from the INL had extended into the ONL (Fig. 5D, arrowheads). Taken together, these data suggest that the knockdown of insm1a causes a delay in the maturation of the horizontal cells, perhaps as an indirect consequence of the delay in photoreceptor differentiation.
Although the OPL of insm1a morphants was disrupted in some areas, the rest of the insm1a morphant retina appeared nicely laminated and well organized. By DAPI staining, both the INL and the ganglion cell layer (GCL) in insm1a morphants appeared qualitatively similar to control retinas at 3 and 4 dpf. To determine if ganglion and amacrine cell differentiation was quantitatively altered after insm1a knockdown, we immunolabeled control and insm1a morphant retinal sections with the HuC/D antibody, which labels the cell bodies of amacrine and ganglion cells. At both 3 and 4 dpf HuC/D-positive amacrine cells in the inner half of the INL appeared qualitatively similar in control and insm1a morphant retinal sections (data not shown). When we counted the number of HuC/D-positive cells in the INL, no significant difference was observed between control and insm1a morphants at 3 dpf (Fig. 4). However, when HuC/D-positive cells in the GCL (which may include displaced amacrine cells as well as ganglion cells), were counted, we observed a 32.8% reduction in insm1a morphants compared to controls at 3 dpf (Fig. 4). Interestingly, the number of HuC/D-positive cells in insm1a morphants did not significantly increase between 3 and 4 dpf (data not shown), which suggests that the reduced number of HuC/D-positive cells in insm1a morphants is not caused by a delay in differentiation.
Insm1a knockdown increases cell cycle length
Previous studies have shown that Insm1 physically interacts with cell cycle regulatory proteins (Liu et al., 2006; Zhang et al., 2009). Because apoptotic cell death remains very low in the insm1a morphant retinas, one alternative explanation for the reduced eye area and decrease in differentiated cell numbers in insm1a morphants is that loss of Insm1a results in a change in retinoblast cell cycle kinetics. To determine if cell cycle length was altered in insm1a morphants, the thymidine analog BrdU was injected into the yolk of 30 hpf control and morphant embryos. Embryos were collected at 0.5, 2, 4 and 6 hours post BrdU injection (hpi). Retinal cryosections were immunolabeled with anti-BrdU to label cells that were in S phase during the BrdU exposure window, and with anti-phosphohistone H3 (PH3), to mark cells that were in late G2/M phase at the time of collection.
At all time points, there were fewer BrdU-positive cells in insm1a morphant retinas compared to controls (Fig. 6B). At 0.5 hpi (30.5 hpf), control sections contained numerous areas of BrdU-positive cells spanning the retina from the basal to apical surface; in contrast, the insm1a morphants had fewer BrdU-positive cells at the apical side of the retina (Fig. 6A). Additionally, at 2 hpi (32 hpf), insm1a morphants contained fewer BrdU-negative cells at the basal surface when compared with controls (Fig. 6A), indicating a delay in cell cycle exit of retinal progenitors at this time. At 2, 4, and 6 hpi the number of PH3-positive cells was also reduced in insm1a morphants compared to controls (Fig. 6C). Expressing the BrdU and PH3 cell counts from insm1a morphant retinal sections at 6 hpi as a percentage of the equivalent cell counts from control retinal sections revealed an approximate 50% and 65% reduction in the number of BrdU-positive and PH3-positive cells, respectively (Fig. 6E). We noted that PH3-positive cells were observed only at the apical surface in both insm1a morphants and controls, indicating that knockdown of insm1a did not affect the location of mitoses in morphant retinas.
Figure 6. Cell cycle progression is delayed after insm1a knockdown.
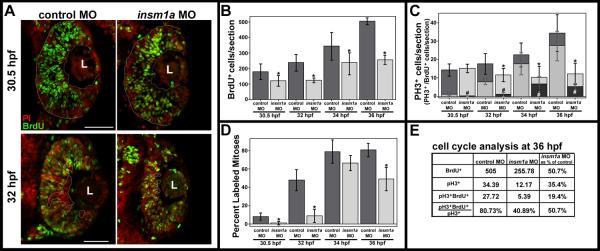
(A) At 30.5 hpf (0.5 hpi, top panels), BrdU+ cells (green) spanned the retina from basal to apical (dotted lines) in control retinas. However, in insm1a morphant retinas, the BrdU+ cells did not extend to the apical edge of the retina. At 32 hpf (2 hpi, bottom panels), fewer post-mitotic BrdU- cells were observed at the basal surface (dotted circles) in insm1a morphants compared with controls, indicating a delay in cell cycle exit in insm1a morphant retinas. (B) Quantitation of BrdU+ cells revealed a decrease in insm1a morphant retinas at all time points (*p<0.05). (C) The numbers of PH3+ cells were reduced at 2, 4 and 6 hpi in insm1a morphant retinas (*p<0.04). The number of cells double-positive for PH3 and BrdU was also reduced at all time points (#p<0.007). (D) The percent labeled mitoses (see Results for explanation) was significantly reduced in insm1a morphants at 0.5, 2 and 6 hpi, indicating that insm1a morphants took longer to progress from S phase into late G2/M phase compared with controls. (E) Summary of cell count analysis at 36 hpf (6 hpi); cell counts for insm1a morphants are expressed as a percentage of controls in the last column (for both controls and morphants: n=6 at 30.5, 32, and 34 hpf and n=3 at 36 hpf; mean ± st.dev). Scale bars = 50μm; L, lens; PI, Propidium iodide; hpf, hours post fertilization; hpi, hours post BrdU injection; MO, morpholino.
Although the altered pattern of BrdU incorporation in insm1a morphant retinas suggested a change in cell cycle kinetics, an alternative explanation is that knockdown of insm1a resulted in a reduction in the size of the optic primordia, which could explain the smaller eye and the reduced numbers of S- and G2/M-phase cells observed in insm1a morphant retinas compared to controls. To determine whether cell cycle length was altered in insm1a morphants, we tracked the proportion of cells that had traversed S phase and progressed into G2/M during the period of BrdU exposure. This metric, referred to as “percent labeled mitoses” (Quastler and Sherman, 1959) was calculated as follows:
The %LM should rise over time, as more cells complete S phase and progress to G2/M. Indeed, we observed that in both control and insm1a morphant retinas, the %LM increased over the time course of the BrdU pulse, demonstrating that knockdown of insm1a did not cause a complete arrest in progression from S to G2/M phase. However, the %LM was significantly reduced in insm1a morphants when compared to controls at 0.5, 2, and 6 hpi (Fig. 6D). This suggests that insm1a morphant retinal progenitors made the transition from S to G2/M more slowly than in controls. Taken together, these data indicate that the knockdown of insm1a causes a delay in cell cycle exit, due at least in part to an increase in the transition time from S-phase to G2/M-phase.
Insm1a acts upstream of the bHLH TFs Ath5/Atoh7 and Neurod
In the retina, as in other regions of the central nervous system, the precise coordination of cell cycle exit with cell fate specification is essential for generating the correct proportions of different cell types in the appropriate order. Because we observed changes in both cell cycle progression and neuronal differentiation in insm1a morphants, we next examined whether knockdown of insm1a altered expression of transcription factors known to regulate cell fate specification in the retina. Ath5/atoh7 is a basic helix-loop-helix (bHLH) transcription factor expressed during or after the terminal division of a subset of retinal progenitor cells (Kay et al., 2001; Poggi et al., 2005; Yang et al., 2003). In both zebrafish and mice mutant for ath5/atoh7, retinal ganglion cells (RGCs) fail to differentiate (Brown et al., 2001; Kay et al., 2001). Interestingly, overexpression studies in the chick and lineage tracing studies in mouse suggest that ath5/atoh7 also contributes to the photoreceptor cell lineage (Brzezinski et al., 2012; Ma et al., 2004). We examined the expression of ath5/atoh7 in control and insm1a morphants by WISH. At 33 hpf, control retinas displayed the expected circular fan-like pattern of strong ath5/atoh7 expression; in contrast, in insm1a morphant retinas expression of ath5/atoh7 was observed in a small patch of cells in the ventro-nasal retina, the location of initiation of ath5/atoh7 expression (Fig. 7A). At 48 hpf, the peak of ath5/atoh7 expression had passed in control retinas and was confined to a subset of cells within the GCL. However, in insm1a morphant retinas ath5/atoh7 expression had spread throughout the inner retina, in a pattern that closely resembled the control retinas at 33 hpf (Fig. 7A). Although the ath5/atoh7 probe signal appeared more intense in 48 hpf insm1a morphants than in controls, no significant difference in transcript abundance was detected by quantitative RT-PCR (data not shown). This could be due to inter-embryo variability in ath5/atoh7 expression following insm1a knockdown. Overall, these data indicate that insm1a is required for proper developmental timing, but not the maintenance or patterning of ath5/atoh7 expression.
Figure 7. Insm1a acts upstream of pro-neural TF's ath5/atoh7 and neurod.

(A) At 33 hpf ath5/atoh7 was expressed throughout the developing retina in controls; in contrast ath5/atoh7 expression was detectable only in the ventro-nasal patch of insm1a morphants (arrowhead). At 48 hpf, ath5/atoh7 expression in control retinas was restricted to the GCL; in insm1a morphant retinas, expression had expanded throughout the retina, in a pattern that closely resembled that of 33 hpf controls. (B) Neurod expression at 48 hpf was observed strongly in the developing photoreceptors and in the adjacent INL of control retinas. In insm1a morphants, however, neurod expression was mostly confined to the ventro-nasal retina (black arrowhead), with some expression throughout the central retina (gray arrowheads). In contrast, strong expression of neurod was observed in the olfactory epithelium (arrows) of insm1a morphants, which was absent in controls. By 72 hpf, neurod expression in the ONL (black bracket) and inner portion of the INL (blue bracket) was qualitatively similar in both controls and insm1a morphants. Scale bars = 50μm; D, dorsal; V, ventral; A, anterior; P, posterior; GCL, ganglion cell layer; oe, olfactory epithelium; ONL, outer nuclear layer INL, inner nuclear layer; hpf, hours post fertilization.
Neurod is another bHLH-transcription factor with known roles in cell cycle regulation, cell fate determination, and cellular differentiation (Morrow et al., 1999). In the developing zebrafish retina, Neurod promotes photoreceptor progenitor cell exit from the cell cycle, and may regulate early cone maturation (Ochocinska and Hitchcock, 2009). In the differentiated zebrafish retina, neurod is expressed in amacrine cells, nascent cone photoreceptors near the retinal margin, and in progenitors of the rod lineage (Ochocinska and Hitchcock, 2007). Additionally, Insm1 has been shown to regulate and be regulated by Neurod during mammalian pancreatic development (Liu et al., 2006). To determine if Insm1a acts upstream of Neurod in the retina, we examined neurod expression using WISH. In control retinas at 48 hpf we observed a strong band of neurod expression in the developing photoreceptors in the ONL, and less intense neurod expression throughout the INL. In contrast, in insm1a morphant retinas expression was observed in a small patch of cells in the ventro-nasal retina, and diffusely in groups of cells scattered throughout the rest of the retina (Fig. 7B). Interestingly, while neurod expression was decreased in the retinas of insm1a morphants, expression was strongly induced in the olfactory epithelium of insm1a morphants (Fig. 7B, arrowheads). At 72 hpf, neurod expression in insm1a morphants appeared to have recovered such that it was qualitatively similar to controls, with expression observed in the ONL (Fig. 7B, black bracket) and in the inner portion of the INL (Fig. 7B, blue bracket). Taken together, these results suggest that insm1a is required for the proper timing, but not maintenance, of neurod expression in the developing retina. Moreover, since our results indicate that insm1a inhibits neurod expression in the developing olfactory system but not in the retina, the genetic interaction between insm1a and neurod is likely to be context dependent.
Insm1a is required for proper temporal expression of photoreceptor-specific TFs
Because insm1a knockdown produced the most significant effects on the differentiation of rod and cone photoreceptors, we next sought to determine whether insm1a is required for the proper expression of TF's that specify photoreceptor cell fate. We first focused on the homeobox transcription factor Crx. Crx is expressed in late stage retinal progenitors just prior to differentiation, as well as in post-mitotic differentiated photoreceptor cells (both rods and cones) and in a subset of cells in the INL (Liu et al., 2001; Shen and Raymond, 2004). Crx has been shown to directly bind and transactivate photoreceptor-specific genes in vitro (Chen et al., 1997; Furukawa et al., 1997), as well as to regulate photoreceptor differentiation in vivo (Furukawa et al., 1999). Knockdown of crx results in a dramatic decrease in photoreceptor-specific gene expression (Blackshaw et al., 2001; Furukawa et al., 1999; Livesey et al., 2000), and delayed cell cycle exit (Shen and Raymond, 2004).
In control retinas at 48 hpf, crx was very strongly expressed in the developing ONL, and weakly in some cells of the INL. In 48 hpf insm1a morphant retinas however, crx was only strongly expressed in the ventro-nasal retina, with expression decreasing in a counterclockwise fashion across the rest of the retina (Fig. 8A). Quantitative RT-PCR at 48 hpf confirmed that crx expression was reduced in insm1a morphants (by 2.7-fold) relative to controls (Fig. 8C). By 72 hpf, crx expression in insm1a morphants and controls appeared qualitatively similar by WISH, with expression primarily in the ONL (Fig. 8A). Thus, we conclude that the very low expression of crx at 48 hpf at least partially contributes to the reduced numbers of differentiated rod and cone photoreceptors in 3 dpf insm1a morphant retinas. Likewise, the recovery of cone photoreceptor differentiation we observed at 4 dpf in the insm1a morphants may be a consequence of the rebound in crx expression at 72 hpf.
Figure 8. Expression of crx and nr2e3 is reduced at 48 hpf in insm1a morphants.
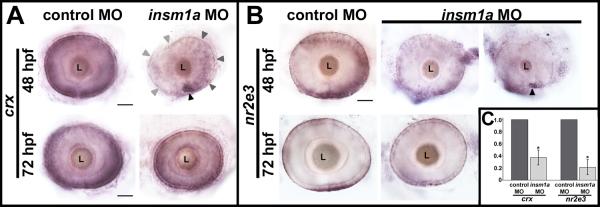
(A) At 48 hpf, crx was expressed strongly in the developing ONL and weakly in some cells of the INL in control retinas. In insm1a morphant retinas crx was expressed in the ventro-nasal retina (black arrowhead), and expression decreased in a counterclockwise fashion across the retina (gray arrowheads). At 72 hpf, crx expression appeared similar between insm1a morphants and controls, and was observed primarily in the ONL. (B) At 48 hpf, nr2e3 was expressed strongly in nascent photoreceptor cells in the ONL, and in scattered cells in the distal half of INL in control retinas. Insm1a morphants displayed varying degrees of reduction in nr2e3 expression. In approximately half of the insm1a morphant retinas the nr2e3 expression pattern was only slightly less intense than controls (left panel), whereas the remaining half expressed nr2e3 only in the ventro-nasal retina (right, black arrowhead). At 72 hpf, nr2e3 expression had recovered greatly in insm1a morphants; however, nr2e3 staining did appear less dense and more discontinuous in insm1a morphants than in controls. (C) qPCR confirmed a 2.7-fold reduction in crx transcripts (*p<0.0128), and a 4.6-fold reduction in nr2e3 (*p<0.0084) at 48 hpf. Scale bar = 50μm; ONL, outer nuclear layer; INL, inner nuclear layer; L, lens; hpf, hours post fertilization; MO, morpholino.
To determine whether knockdown of insm1a alters rod photoreceptor specification, we examined the expression pattern of the rod-specific factor Nr2e3 in control and insm1a morphant retinas by WISH and quantitative RT-PCR. Nr2e3 is an orphan nuclear receptor expressed transiently in all developing photoreceptors in zebrafish, later becoming restricted to rod precursors and rod photoreceptors (Alvarez-Delfin et al., 2009; Chen et al., 2005; Morris et al., 2008b). Nr2e3 binding sites have been shown to mediate both activation of rod-specific genes (Cheng et al., 2004) and repression of cone-specific genes (Chen et al., 2005) in rod photoreceptor cells. Mutation of nr2e3 results in accumulation of cone-specific transcripts in S-cones, and progressive photoreceptor degeneration in the rd7 mouse (Akhmedov et al., 2000; Cheng et al., 2004). In control retinas at 48 hpf, nr2e3 was expressed in nascent photoreceptors in the developing ONL, as well as in scattered cells in the distal half of the INL, consistent with its previously described expression pattern (Fig. 8B; (Alvarez-Delfin et al., 2009; Chen et al., 2005; Nelson et al., 2008)). In contrast, nr2e3 expression was reduced to varying degrees in insm1a morphants at 48 hpf relative to controls. Approximately half of the insm1a morphant retinas displayed scattered nr2e3 expression in the outer half of the INL and in a few cells of the ONL. However, in the remaining insm1a morphant retinas, expression of nr2e3 was observed only in the ventro-nasal patch region of the retina (Fig. 8B). Quantitative RT-PCR analysis confirmed that nr2e3 expression was reduced (by 4.6-fold) in insm1a morphants relative to controls at 48 hpf (Fig. 8C). By 72 hpf, nr2e3 expression was confined to the photoreceptors in the ONL of controls retinas, as has been described previously (Alvarez-Delfin et al., 2009; Chen et al., 2005; Nelson et al., 2008). Interestingly, nr2e3 expression was also detectable in the ONL of insm1a morphant retinas at 72 hpf, although the expression pattern was less contiguous and not as intense as in controls (Fig. 8B). Therefore, these data suggest that the loss of insm1a delays rod photoreceptor specification during retinal development. The partial recovery of nr2e3 expression in insm1a morphants at 72 hpf could explain the small increase in rod photoreceptor number we observed at 4 dpf compared to 3 dpf. However, given that rod photoreceptors remain significantly reduced relative to controls in 4 dpf insm1a morphants, it is likely that the increase in nr2e3 expression is not sufficient to completely rescue rod photoreceptor development. Alternatively, the partial reduction in nr2e3 expression at 72 hpf may be compounded by reductions in nr2e3 interaction partners (Cheng et al., 2004), producing an additive effect on rod photoreceptor differentiation in insm1a morphants.
Notch-Delta signaling negatively regulates insm1a
Finally, we sought to identify genetic pathways that lie upstream of Insm1a activity in the retina. The results of our knockdown experiments provide strong evidence that insm1a is required for proper differentiation of retinal neurons. Moreover, the developmental expression pattern of insm1a in the retina matches the pattern of retinal progenitor cell (RPC) exit from the cell cycle and the onset of neurogenesis. This suggests that Notch-Delta signaling may function as an upstream negative regulator of insm1a expression, since one well-known role of this pathway is to preserve a pool of undifferentiated proliferative RPC's during retinal development (Bernardos et al., 2005; Richard et al., 1995; Scheer et al., 2001). This hypothesis is supported by previous work demonstrating that inactivation of Notch-Delta signaling in mouse retinal explants caused an increase in Insm1 expression (Nelson et al., 2007). Therefore, we examined whether expression of insm1a was similarly affected by reducing Notch activity in vivo in the zebrafish.
We blocked all Notch signaling by exposing zebrafish embryos to the γ-secretase inhibitor DAPT from 10.5 to 28 hpf. This period of DAPT treatment allowed specification of the eye field to occur normally, but inhibited Notch activity during retinal neurogenesis. We then evaluated insm1a expression in control (DMSO-treated) and DAPT-treated embryos at 28 hpf by WISH. Whereas very little expression of insm1a was observed in the retinas of control embryos at this time (Fig. 9A), strong expression of insm1a was observed throughout the retina and lens of DAPT-treated embryos (Fig. 9B). Expression was also increased in other tissues, including the brain. This result indicates that Notch-Delta signaling is an early negative regulator of insm1a expression.
Figure 9. Notch-Delta and Her4 negatively regulate insm1a expression.

(A, B) Pharmacologic inhibition of Notch signaling with DAPT resulted in an upregulation of insm1a expression in the developing eye and brain (B), compared to control (DMSO) treated embryos (A). (C) Co-transfection of HEK293 cells with a her4 cDNA expression vector repressed a luciferase reporter gene driven by either 300bp (left) or 2.5kb (right) of the insm1a promoter. D, dorsal; V, ventral; A, anterior; P, posterior; L, lens; hpf, hours post fertilization.
Her4 interacts directly with the insm1a promoter in vitro
To further explore how Notch signaling regulates insm1a expression, we next investigated whether insm1a is a direct target of Notch effector genes. The transcriptional repressor Her4 is a Notch target gene that is expressed throughout the developing nervous system (Clark et al., 2012; Yeo et al., 2007). To determine whether Her4 directly interacts with the insm1a promoter, we carried out in vitro reporter assays using her4 cDNA and a luciferase reporter driven by two different lengths of the insm1a promoter. Co-transfection of HEK293 cells using the her4 expression vector and either a short insm1a regulatory sequence (−282 to +59) or long insm1a regulatory sequence (−2440/+59) demonstrated that Her4 is able to negatively regulate the insm1a promoter in a dose-dependent fashion (Fig. 9C). In the absence of Her4, the 2.5kb insm1a promoter drove significantly greater luciferase activity than the 300bp promoter (t-test p < 0.001), indicating that important regulatory elements exist in both the distal and proximal promoter regions. Regardless of which promoter was used, co-expression of Her4 significantly decreased the luciferase activity, demonstrating that Her4 is a negative regulator of the insm1a gene (Figure 10 C). Taken together, these data suggest that Notch-Delta signaling inhibits insm1a expression, potentially via its effector Her4.
DISCUSSION
The zinc-finger transcriptional regulator Insm1 has been primarily studied in the context of its roles in regulating neuroendocrine development and neurogenesis in various regions of the brain and spinal cord (Duggan et al., 2008; Farkas et al., 2008; Jacob et al., 2009; Lan and Breslin, 2009; Wildner et al., 2008). Recently, we and others have demonstrated that Insm1 is expressed in embryonic retinal progenitor cells as well as adult rod progenitor cells (Morris et al., 2011; Nelson et al., 2007), suggesting that cellular differentiation in the retina is also subject to Insm1 regulatory control. In this study, we have directly tested this hypothesis, and indeed our results reveal a requirement for Insm1a in the differentiation of rod and cone photoreceptors. Thus, this study, in combination with a recent study showing that Insm1a is required for retinal regeneration following acute damage in the adult zebrafish (Ramachandran et al., 2012), conclusively demonstrate that Insm1 is among the genes important for generating the diverse neuronal subtypes in the vertebrate retina.
In insm1a-deficient zebrafish embryos, we observed a decrease in retinal area, and a severe reduction of differentiated rod and cone photoreceptors at 3 dpf. Interestingly, cone photoreceptor cell number in insm1a morphants recovered significantly by 4 dpf, but rod photoreceptor cells did not. These data suggest that rod photoreceptors are more sensitive to perturbations in insm1a expression than cone progenitors or other retinal cell types, either inherently or as a consequence of their extended period of differentiation.
We also observed small reductions in the numbers of bipolar and ganglion cells and delayed maturation of bipolar and horizontal cell morphology in insm1a morphants. Thus, although loss of insm1a produced the most significant effects on the photoreceptors, it also resulted in changes to inner retinal neurons. The reduction in bipolar and ganglion cell number is unlikely to be the result of increased cell death, because the insm1a morpholino was co-injected with a morpholino targeting tp53, which is known to inhibit apoptotic cell death in morpholino injected embryos (Bill et al., 2009a), and we did not observe an increase in TUNEL-positive cells in insm1a morphant retinas (data not shown). Instead, we propose that the smaller eye and reduced numbers of differentiated cells in the insm1a morphants are the result of delayed cell cycle exit of at least a subset of RPCs, in combination with impaired terminal differentiation of photoreceptor precursors (and perhaps other subtypes as well). Additionally, we hypothesize that the delayed maturation observed for the bipolar and horizontal cells (as well as the disruptions in the OPL) are a secondary consequence of the lack of differentiated photoreceptors in the ONL, as these two classes of retinal neurons make direct synaptic contacts with photoreceptors and may require this interaction to complete their maturation. Interestingly, knockdown of insm1a did not affect either the number or maturation of the Müller glia, suggesting that this subtype does not require insm1a to properly differentiate.
The “breaching” phenotype observed in insm1a morphants, in which cells from the INL were observed in the ONL, is very interesting. The presence of these ectopic INL cells at 4 dpf was only observed in regions of insm1a-deficient retinas that also displayed gaps in the cone photoreceptors. This could suggest that the presence of cone photoreceptors (or their precursors) is needed to establish a physical boundary between the INL and ONL. A related possibility is that the loss of integrity in the OPL of insm1a morphants is caused by a lack of synaptic contacts between the missing cones and second order neurons in the INL. Interestingly, in mice, mosaic deletion of Rb (a tumor suppressor gene known to have cell cycle regulatory function) results in both a loss of rod photoreceptor cells and a breaching phenotype very similar to that observed following insm1a knockdown (Johnson et al., 2006).
At 3 dpf, the ONL of insm1a morphants did contain cells visible by DAPI staining, but these cells did not immunolabel with markers of differentiated rod or cone photoreceptors. Therefore, it is possible that in the absence of insm1a, ONL cells are correctly specified as photoreceptor cells, but are either arrested or delayed in the final stages of differentiation. This hypothesis is supported by our WISH data showing that the photoreceptor specification genes crx and nr2e3 are expressed similarly to controls in 3 dpf insm1a morphant retinas. In the murine neuroendocrine pancreas, Insm1 is expressed in precursors of all pancreatic cell types. When Insm1 is absent, pancreatic precursor cells are specified, but terminal differentiation is impaired in α, δ and β-cells, as suggested by delayed onset of differentiation markers and reduced expression of genes involved in hormone secretion. Pancreatic β-cells are the most severely affected cell type in the Insm1-null pancreas, with β-cells precursors arresting completely prior to terminal differentiation (Gierl et al., 2006). In the mouse sympatho-adrenal lineage, differentiation of sympathetic neurons is delayed in the absence of Insm1, while chromaffin cell precursors are made in normal numbers, but fail to properly differentiate (Wildner et al., 2008). Additional studies are needed to determine if the loss of insm1a in the retina causes rod photoreceptor cell differentiation to be delayed, similar to sympathetic neurons and pancreatic α- and δ-cells, or if the rod photoreceptor progenitors are arresting prior to terminal differentiation, as is seen in chromaffin cells and pancreatic β-cells. The small increase in rod photoreceptor number from 3 to 4 dpf in insm1a morphants may indicate the former, but the latter has not been conclusively excluded.
In insm1a morphants, we observed a significant delay in the developmental expression of bHLH and other transcription factors that specify cell fate. For example, ath5/atoh7 expression in insm1a morphants at 33 hpf resembled controls at 25 hpf, and ath5/atoh7 expression in insm1a morphants at 48 hpf resembled the 33 hpf control expression pattern. Although ath5/atoh7 is known to be required for RGC differentiation (Brown et al., 2001; Kay et al., 2005; Yang et al., 2003), a recent study in the mouse retina demonstrated that math5-expressing progenitors also contribute significantly to the photoreceptor lineage (Brzezinski et al., 2012). Therefore, the delayed onset and progression of ath5/atoh7 expression in insm1a morphants may presage the delay in photoreceptor differentiation observed later in development. At this point it is unclear whether the delayed timing of ath5/atoh7 expression in insm1a morphants is due to altered transcriptional regulation of ath5/atoh7 by insm1a (or an insm1a target gene, since insm1a is thought to function as a transcriptional repressor), or whether it is a secondary consequence of the delay in cell cycle progression (discussed below) that was also observed in insm1a morphants.
The bHLH transcription factor neurod, also displayed delayed expression in insm1a morphants. Neurod can be directly induced by ath5/atoh7 (Ma et al., 2004) and has been shown to be repressed by human INSM1 in vitro (Liu et al., 2006). Neurod expression promotes photoreceptor differentiation, and regulates proliferation of RPCs (Morrow et al., 1999; Ochocinska and Hitchcock, 2007; Ochocinska and Hitchcock, 2009). Interestingly, although we observed delayed onset and progression of neurod expression in the retina of insm1a-deficient embryos, neurod expression was strongly induced in the developing olfactory epithelium. Therefore, the decreased retinal expression of neurod may be an indirect effect of the changes in ath5/atoh7 expression, whereas in the developing olfactory epithelium and elsewhere, neurod may be directly repressed by insm1a.
Similar to ath5/atoh7 and neurod, expression of the photoreceptor-specific transcription factors crx and nr2e3 was also delayed in insm1a morphants. While expression of both genes was much lower than controls at 48 hpf, they recovered to near control levels by 72 hpf. However, recovery of nr2e3 was less complete than crx, with patchier expression even at 72 hpf. The rebound of crx expression at 72 hpf may explain the recovery in cone photoreceptors observed between 3 and 4 dpf. However, the increase in crx and nr2e3 expression is apparently not sufficient to permit recovery of rod photoreceptors in a similar window. It may be that the rods require higher expression of nr2e3, or that other factors required for rod photoreceptor differentiation remain reduced below a critical threshold in insm1a morphants.
Additionally, we have presented evidence that expression of insm1a is negatively regulated by Notch-Delta signaling, and have identified Her4 as a possible effector of Notch-mediated repression. Pharmacologic inhibition of Notch signaling resulted in increased expression of insm1a in the retina and the brain. Her4, a known effector of Notch signaling (Clark et al., 2012; Yeo et al., 2007), repressed expression of a luciferase reporter gene driven by the insm1a promoter. While this evidence supports a role for Notch-Delta signaling in control of insm1a expression in the retina, other signaling cascades are likely to be involved. For example, in murine sympatho-adrenal development, BMPs have been implicated in regulating Insm1 expression (Lan and Breslin, 2009).
In several Insm1-expressing tissues, including the hindbrain (Jacob et al., 2009), spinal cord (Duggan et al., 2008) and endocrine cells of the pancreas and intestines (Wildner et al., 2008), Insm1 expression is observed mainly in postmitotic or terminally dividing cells which are undergoing differentiation, and no cell cycle regulatory role has been identified. In contrast, Insm1 has been shown to regulate cell cycle progression in the mouse neocortex (Farkas et al., 2008) and sympathetic nervous system, where it also regulates differentiation (Wildner et al., 2008), and insm1a regulates expression of cell cycle genes in the regenerating zebrafish retina (Ramachandran et al., 2012). In this study, we have shown that knockdown of insm1a caused a delay in cell cycle progression in the developing zebrafish retina. From 30 to 36 hpf, progression through S-phase and entry into late G2/M phase occurred more slowly in insm1a morphant retinas than in controls. In control retinal sections, nearly half of cells in late G2/M phase had been in S-phase in the preceding two hours, whereas in insm1a morphants, less than 10% of late G2/M phase cells had progressed from S-phase. While insm1a may directly regulate the transcription of cell cycle genes, previous studies suggest that Insm1 can also regulate the cell cycle independently from its transcriptional regulatory activity. For example, in Medaka, insm1b was shown to alter cell cycle progression without being localized to the nucleus (Candal et al., 2007), and murine Insm1 directly binds to CyclinD in cultured cells, interrupting CyclinD-CDK4 interaction and causing hypophosphorylation of the retinoblastoma protein (Rb), thereby inducing cell cycle arrest (Zhang et al., 2009). The interaction of Insm1 with Rb is particularly intriguing given that in the mouse Rb-deficient retinas have defects in rod photoreceptor differentiation, RPC proliferation, OPL integrity, and horizontal cell maturation, phenotypes strikingly similar to those we observed in insm1a morphants (Johnson et al., 2006; Zhang et al., 2004). Therefore, it will be important to determine whether both the photoreceptor differentiation and cell cycle defects observed in insm1a morphants result from a downstream dysregulation of Rb.
What is the precise mechanism whereby Insm1a regulates photoreceptor differentiation? Several non-mutually exclusive functions can be imagined. One possibility is that Insm1a directly regulates the transcription of photoreceptor specification genes. This hypothesis seems less likely, because the expression pattern of insm1a does not match that of other known photoreceptor determination genes such as neurod, crx and nr2e3 at later stages of retinal development (this study and (Nelson et al., 2008)). However, we did observe that expression of all three genes initiated at approximately the same time and location, i.e. 28 hpf in the ventro-nasal patch (Fig. 1D and data not shown). Therefore, it is possible that Insm1a protein is very stable and is retained in RPC lineages that produce photoreceptor progenitors. In contrast to neurod, crx, and nr2e3, we observed that the developmental expression pattern of insm1a was very similar spatially to that of ath5/atoh7 (this study, (Masai et al., 2000; Morris et al., 2011)). As math5-expressing cells have been shown to contribute significantly to the photoreceptor lineage in the mouse retina (Brzezinski et al., 2012), it is possible that expression of insm1a in ath5/atoh7-positive RPCs influences photoreceptor competency. Yet another possibility is that Insm1a functions non-cell autonomously to promote photoreceptor differentiation by regulating expression of secreted regulatory factors. Finally, Insm1a may influence the timing of photoreceptor differentiation through its regulation of cell-cycle kinetics.
In summary, our results demonstrate that insm1a is required for photoreceptor differentiation, regulates cell cycle progression during retinal development, functions upstream of pro-neural bHLH transcription factors neurod and ath5/atoh7, and is negatively regulated by Notch-Delta signaling. In addition to addressing gaps in our knowledge of the function of insm1a during retinal development, our data provide another genetic link between cell cycle progression and progenitor differentiation in the vertebrate retina. Future studies will address the mechanisms of how Insm1 regulates cell cycle progression and transcription of retinal genes, as well as the identification of direct targets of Insm1 transcriptional control.
Supplementary Material
Supplemental Figure 1. The insm1a MO is highly effective through 4 dpf. Embryos were co-injected with either standard control or insm1a MO and an eF1α-GFP plasmid containing the insm1a MO binding site (pEF1α-MOBS:GFP; n>40 embryos for each group). (A) At 1 dpf, control MO injected embryos showed robust, ubiquitous expression of GFP (top panels), whereas insm1a MO injected embryos showed no expression of GFP (bottom panels). Tetramethylrhodamine dextran (Dex Red) was used as an internal injection control. (B) Quantitation of the numbers of injected embryos showing GFP expression from 1 dpf through 5 dpf. GFP expression similar to that shown in (A) (top panel) was categorized as “strong GFP”. GFP expression that was limited to just a few cells in the embryo was categorized as “weak GFP” expression. “No GFP” required the complete absence of any identifiable GFP signal. From 1 dpf through 4 dpf, no insm1a morphants exhibited any GFP expression. In contrast, nearly all embryos co-injected with the standard control MO were GFP-positive, with over 90% strongly expressing GFP. By 5 dpf, nearly half of the insm1a morphants showed low levels of GFP expression, indicating a decline in the effectiveness of the insm1a MO. Scale bar = 200μm MOBS, insm1a morpholino binding site; MO, morpholino; dpf, days post fertilization.
HIGHLIGHTS
Insm1a is expressed during zebrafish retinal development and in adult rod photoreceptor progenitors
Insm1a is required for photoreceptor differentiation
Insm1a regulates cell cycle progression of retinoblasts
Insm1a functions upstream of subtype specification genes and downstream of Notch signaling
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (R01-EY021769), the Pew Biomedical Scholar Program, and the Fight for Sight Foundation to A.C.M, and by a graduate fellowship from the University of Kentucky Department of Biology and the Gertrude Flora Ribble Endowment to M.A.F.O. The authors are grateful to Sara Perkins for zebrafish care and laboratory assistance. Some fish lines and antisera were obtained from ZIRC, supported by NIH-NCRR grant P40 RR012546.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akhmedov N, Piriev N, Chang B, Rapoport A, Hawes N, Nishina P, Nusinowitz S, Heckenlively J, Roderick T, Kozak C, Danciger M, Davisson M, Farber D. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc. Nat. Acad. Sci. U.S.A. 2000;97:5551–5556. doi: 10.1073/pnas.97.10.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Delfin K, Morris AC, Snelson CD, Gamse JT, Gupta T, Marlow FL, Mullins MC, Burgess HA, Granato M, Fadool JM. Tbx2b is required for ultraviolet photoreceptor cell specification during zebrafish retinal development. Proc. Nat. Acad. Sci. U.S.A. 2009;106:2023–2028. doi: 10.1073/pnas.0809439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreazzoli M. Molecular regulation of vertebrate retina cell fate. Birth Defects Res. C Embryo Today. 2009;87:284–295. doi: 10.1002/bdrc.20161. [DOI] [PubMed] [Google Scholar]
- Baye L, Link B. Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J. Neurosci. 2007;27:10143–10152. doi: 10.1523/JNEUROSCI.2754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-Stage Neuronal Progenitors in the Retina Are Radial Müller Glia That Function as Retinal Stem Cells. J. Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardos RL, Lentz SI, Wolfe MS, Raymond PA. Notch-Delta signaling is required for spatial patterning and Müller glia differentiation in the zebrafish retina. Dev. Biol. 2005;278:381–395. doi: 10.1016/j.ydbio.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr. Patterns. 2006;6:1007–1013. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Bilitou A, Ohnuma S.-i. The role of cell cycle in retinal development: Cyclin-dependent kinase inhibitors co-ordinate cell-cycle inhibition, cell-fate determination and differentiation in the developing retina†. Dev. Dyn. 2010;239:727–736. doi: 10.1002/dvdy.22223. [DOI] [PubMed] [Google Scholar]
- Bill BR, Petzold AM, Clark KJ, Schimmenti LA, Ekker SC. A primer for morpholino use in zebrafish. Zebrafish. 2009;6:69–77. doi: 10.1089/zeb.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S, Fraioli R, Furukawa T, Cepko C. Comprehensive analysis of photoreceptor gene expression and the identification of candidate retinal disease genes. Cell. 2001;107:579–589. doi: 10.1016/s0092-8674(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Brown N, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinski J, Prasov L, Glaser T. Math5 defines the ganglion cell competence state in a subpopulation of retinal progenitor cells exiting the cell cycle. Dev. Biol. 2012;365:395–413. doi: 10.1016/j.ydbio.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candal E, Alunni A, Thermes V, Jamen F, Joly J-S, Bourrat F. Ol-insm1b, a SNAG family transcription factor involved in cell cycle arrest during medaka development. Dev. Biol. 2007;309:1–17. doi: 10.1016/j.ydbio.2007.04.038. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc. Nat. Acad. Sci. U.S.A. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Rattner A, Nathans J. The Rod Photoreceptor-Specific Nuclear Receptor Nr2e3 Represses Transcription of Multiple Cone-Specific Genes. J. Neurosci. 2005;25:118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wang Q, Nie Z, Sun H, Lennon G, Copeland N, Gilbert D, Jenkins N, Zack D. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19:1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- Cheng H, Khanna H, Oh ECT, Hicks D, Mitton KP, Swaroop A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum. Mol. Genet. 2004;13:1563–1575. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- Clark B, Cui S, Miesfeld J, Klezovitch O, Vasioukhin V, Link B. Loss of Llgl1 in retinal neuroepithelia reveals links between apical domain size, Notch activity and neurogenesis. Development. 2012;139:1599–1610. doi: 10.1242/dev.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan A, Madathany T, de Castro S, Gerrelli D, Guddati K, García-Añoveros J. Transient expression of the conserved zinc finger gene INSM1 in progenitors and nascent neurons throughout embryonic and adult neurogenesis. J. Comp. Neurol. 2008;507:1497–1520. doi: 10.1002/cne.21629. [DOI] [PubMed] [Google Scholar]
- Fadool JM. Development of a rod photoreceptor mosaic revealed in transgenic zebrafish. Dev. Biol. 2003;258:277–290. doi: 10.1016/s0012-1606(03)00125-8. [DOI] [PubMed] [Google Scholar]
- Farkas LM, Haffner C, Giger T, Khaitovich P, Nowick K, Birchmeier C, Pääbo S, Huttner WB. Insulinoma-Associated 1 Has a Panneurogenic Role and Promotes the Generation and Expansion of Basal Progenitors in the Developing Mouse Neocortex. Neuron. 2008;60:40–55. doi: 10.1016/j.neuron.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow E, Cepko C. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow E, Li T, Davis F, Cepko C. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat. Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Gierl M, Karoulias N, Wende H, Strehle M, Birchmeier C. The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev. 2006;20:2465–2478. doi: 10.1101/gad.381806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, De Silva M, Toscani A, Prabhakar B, Notkins A, Lan M. A novel human insulinoma-associated cDNA, IA-1, encodes a protein with “zinc-finger” DNA-binding motifs. J. Biol. Chem. 1992;267:15252–15257. [PubMed] [Google Scholar]
- Hatakeyama J, Kageyama R. Retinal cell fate determination and bHLH factors. Semin. Cell Dev. Biol. 2004;15:83–89. doi: 10.1016/j.semcdb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hu M, Easter S. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev. Biol. 1999;207:309–321. doi: 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- Jacob J, Storm R, Castro D, Milton C, Pla P, Guillemot F, Birchmeier C, Briscoe J. Insm1 (IA-1) is an essential component of the regulatory network that specifies monoaminergic neuronal phenotypes in the vertebrate hindbrain. Development. 2009;136:2477–2485. doi: 10.1242/dev.034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Donovan S, Dyer M. Mosaic deletion of Rb arrests rod differentiation and stimulates ectopic synaptogenesis in the mouse retina. J. Comp. Neurol. 2006;498:112–128. doi: 10.1002/cne.21059. [DOI] [PubMed] [Google Scholar]
- Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal Ganglion Cell Genesis Requires lakritz, a Zebrafish atonal Homolog. Neuron. 2001;30:725–736. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- Kay JN, Link BA, Baier H. Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development. 2005;132:2573–2585. doi: 10.1242/dev.01831. [DOI] [PubMed] [Google Scholar]
- Kennedy BN, Alvarez Y, Brockerhoff SE, Stearns GW, Sapetto-Rebow B, Taylor MR, Hurley JB. Identification of a Zebrafish Cone Photoreceptor–Specific Promoter and Genetic Rescue of Achromatopsia in the nof Mutant. Invest. Ophthalmol. Vis. Sci. 2007;48:522–529. doi: 10.1167/iovs.06-0975. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lan MS, Breslin MB. Structure, expression, and biological function of INSM1 transcription factor in neuroendocrine differentiation. FASEB J. 2009;23:2024–2033. doi: 10.1096/fj.08-125971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W-D, Wang H-W, Muguira M, Breslin MB, Lan MS. INSM1 functions as a transcriptional repressor of the neuroD/β2 gene through the recruitment of cyclin D1 and histone deacetylases. Biochem J. 2006;397:169–177. doi: 10.1042/BJ20051669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shen Y-C, Rest JS, Raymond PA, Zack DJ. Isolation and Characterization of a Zebrafish Homologue of the Cone Rod Homeobox Gene. Invest. Ophth. Vis. Sci. 2001;42:481–487. [PubMed] [Google Scholar]
- Livesey F, Furukawa T, Steffen M, Church G, Cepko C. Microarray analysis of the transcriptional network controlled by the photoreceptor homeobox gene Crx. Curr. Biol. 2000;10:301–310. doi: 10.1016/s0960-9822(00)00379-1. [DOI] [PubMed] [Google Scholar]
- Lukowski CM, Ritzel RG, Waskiewicz AJ. Expression of two insm1-like genes in the developing zebrafish nervous system. Gene Expr. Patterns. 2006;6:711–718. doi: 10.1016/j.modgep.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Ma W, Yan R-T, Xie W, Wang S-Z. A role of ath5 in inducing neuroD and the photoreceptor pathway. J. Neurosci. 2004;24:7150–7158. doi: 10.1523/JNEUROSCI.2266-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai I, Stemple DL, Okamoto H, Wilson SW. Midline Signals Regulate Retinal Neurogenesis in Zebrafish. Neuron. 2000;27:251–263. doi: 10.1016/s0896-6273(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Mellitzer G, Bonné S, Luco R, Van De Casteele M, Lenne-Samuel N, Collombat P, Mansouri A, Lee J, Lan M, Pipeleers D, Nielsen F, Ferrer J, Gradwohl G, Heimberg H. IA1 is NGN3-dependent and essential for differentiation of the endocrine pancreas. EMBO J. 2006;25:1344–1352. doi: 10.1038/sj.emboj.7601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Forbes-Osborne MA, Pillai LS, Fadool JM. Microarray Analysis of XOPS-mCFP Zebrafish Retina Identifies Genes Associated with Rod Photoreceptor Degeneration and Regeneration. Invest. Ophthalmol. Vis. Sci. 2011;52:2255–2266. doi: 10.1167/iovs.10-6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Scholz T, Fadool JM. In: Rod Progenitor Cells in the Mature Zebrafish Retina Recent Advances in Retinal Degeneration. Anderson RE, LaVail MM, Hollyfield JG, editors. Springer; New York: 2008a. pp. 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Scholz TL, Brockerhoff SE, Fadool JM. Genetic dissection reveals two separate pathways for rod and cone regeneration in the teleost retina. Dev. Neurobiol. 2008b;68:605–619. doi: 10.1002/dneu.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Schroeter EH, Bilotta J, Wong ROL, Fadool JM. Cone Survival Despite Rod Degeneration in XOPS-mCFP Transgenic Zebrafish. Invest. Ophthalmol. Vis. Sci. 2005;46:4762–4771. doi: 10.1167/iovs.05-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- Nelson B, Hartman B, Georgi S, Lan M, Reh T. Transient inactivation of Notch signaling synchronizes differentiation of neural progenitor cells. Dev. Biol. 2007;304:479–498. doi: 10.1016/j.ydbio.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Frey RA, Wardwell SL, Stenkamp DL. The developmental sequence of gene expression within the rod photoreceptor lineage in embryonic zebrafish. Dev. Dyn. 2008;237:2903–2917. doi: 10.1002/dvdy.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochocinska MJ, Hitchcock PF. Dynamic expression of the basic helix-loop-helix transcription factor neuroD in the rod and cone photoreceptor lineages in the retina of the embryonic and larval zebrafish. J. Comp. Neurol. 2007;501:1–12. doi: 10.1002/cne.21150. [DOI] [PubMed] [Google Scholar]
- Ochocinska MJ, Hitchcock PF. NeuroD regulates proliferation of photoreceptor progenitors in the retina of the zebrafish. Mech. Dev. 2009;126:128–141. doi: 10.1016/j.mod.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa R, Kageyama R. Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 2008;1192:90–98. doi: 10.1016/j.brainres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Fadool JM, McClintock J, Linser PJ. Müller cell differentiation in the zebrafish neural retina: Evidence of distinct early and late stages in cell maturation. J. Comp. Neurol. 2001;429:530–540. doi: 10.1002/1096-9861(20010122)429:4<530::aid-cne2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Poggi L, Vitorino M, Masai I, Harris WA. Influences on neural lineage and mode of division in the zebrafish retina in vivo. J. Cell Biol. 2005;171:991–999. doi: 10.1083/jcb.200509098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc. Nat. Acad. Sci. U.S.A. 2009;106:9310–9315. doi: 10.1073/pnas.0811186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastler H, Sherman FG. Cell population kinetics in the intestinal epithelium of the mouse. Exp. Cell Res. 1959;17:420–438. doi: 10.1016/0014-4827(59)90063-1. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Zhao X-F, Goldman D. Insm1a-mediated gene repression is essential for the formation and differentiation of Müller glia-derived progenitors in the injured retina. Nat. Cell Biol. 2012 doi: 10.1038/ncb2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard ID, David HR, William AH. Xotch inhibits cell differentiation in the xenopus retina. Neuron. 1995;14 doi: 10.1016/0896-6273(95)90305-4. [DOI] [PubMed] [Google Scholar]
- Scheer N, Groth A, Hans S, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128:1099–1107. doi: 10.1242/dev.128.7.1099. [DOI] [PubMed] [Google Scholar]
- Schroeter EH, Wong ROL, Gregg RG. In vivo development of retinal ON-bipolar cell axonal terminals visualized in nyx∷MYFP transgenic zebrafish. Vis. Neurosci. 2006;23:833–843. doi: 10.1017/S0952523806230219. [DOI] [PubMed] [Google Scholar]
- Shen Y.-c., Raymond PA. Zebrafish cone-rod (crx) homeobox gene promotes retinogenesis. Dev. Biol. 2004;269:237–251. doi: 10.1016/j.ydbio.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Stenkamp DL. Neurogenesis in the Fish Retina. In: Kwang WJ, editor. International Review of Cytology. Academic Press; 2007. pp. 173–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL, Frey RA. Extraretinal and retinal hedgehog signaling sequentially regulate retinal differentiation in zebrafish. Dev. Biol. 2003;258:349–363. doi: 10.1016/s0012-1606(03)00121-0. [DOI] [PubMed] [Google Scholar]
- Turner D, Snyder E, Cepko C. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Wang H-W, Muguira M, Liu W-D, Zhang T, Chen C, Aucoin R, Breslin M, Lan M. Identification of an INSM1-binding site in the insulin promoter: negative regulation of the insulin gene transcription. J. Endocrinol. 2008;198:29–39. doi: 10.1677/JOE-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (brachydanio rerio) University of Oregon Press; Eugene, OR: 1995. [Google Scholar]
- Wildner H, Gierl MS, Strehle M, Pla P, Birchmeier C. Insm1 (IA-1) is a crucial component of the transcriptional network that controls differentiation of the sympatho-adrenal lineage. Development. 2008;135:473–481. doi: 10.1242/dev.011783. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Bunte RM, Carty AJ. Evaluation of Rapid Cooling and Tricaine Methanesulfonate (MS222) as Methods of Euthanasia in Zebrafish (Danio rerio) J. Am. Assoc. Lab. Anim. Sci. 2009;48:785–789. [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ding K, Pan L, Deng M, Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev. Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Yeo S-Y, Kim M, Kim H-S, Huh T-L, Chitnis A. Fluorescent protein expression driven by her4 regulatory elements reveals the spatiotemporal pattern of Notch signaling in the nervous system of zebrafish embryos. Dev. Biol. 2007;301:555–567. doi: 10.1016/j.ydbio.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gray J, Wu L, Leone G, Rowan S, Cepko C, Zhu X, Craft C, Dyer M. Rb regulates proliferation and rod photoreceptor development in the mouse retina. Nat. Genet. 2004;36:351–360. doi: 10.1038/ng1318. [DOI] [PubMed] [Google Scholar]
- Zhang T, Liu W-D, Saunee NA, Breslin MB, Lan MS. Zinc Finger Transcription Factor INSM1 Interrupts Cyclin D1 and CDK4 Binding and Induces Cell Cycle Arrest. J. Biol. Chem. 2009;284:5574–5581. doi: 10.1074/jbc.M808843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Saunee NA, Breslin MB, Song K, Lan MS. Functional role of an islet transcription factor, INSM1/IA-1, on pancreatic acinar cell trans-differentiation. J. Cell.Physiol. 2012a;227:2470–2479. doi: 10.1002/jcp.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang Y, Trujillo C, Zhong W, Leung YF. The Expression of irx7 in the Inner Nuclear Layer of Zebrafish Retina Is Essential for a Proper Retinal Development and Lamination. PLoS ONE. 2012b;7:e36145. doi: 10.1371/journal.pone.0036145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The insm1a MO is highly effective through 4 dpf. Embryos were co-injected with either standard control or insm1a MO and an eF1α-GFP plasmid containing the insm1a MO binding site (pEF1α-MOBS:GFP; n>40 embryos for each group). (A) At 1 dpf, control MO injected embryos showed robust, ubiquitous expression of GFP (top panels), whereas insm1a MO injected embryos showed no expression of GFP (bottom panels). Tetramethylrhodamine dextran (Dex Red) was used as an internal injection control. (B) Quantitation of the numbers of injected embryos showing GFP expression from 1 dpf through 5 dpf. GFP expression similar to that shown in (A) (top panel) was categorized as “strong GFP”. GFP expression that was limited to just a few cells in the embryo was categorized as “weak GFP” expression. “No GFP” required the complete absence of any identifiable GFP signal. From 1 dpf through 4 dpf, no insm1a morphants exhibited any GFP expression. In contrast, nearly all embryos co-injected with the standard control MO were GFP-positive, with over 90% strongly expressing GFP. By 5 dpf, nearly half of the insm1a morphants showed low levels of GFP expression, indicating a decline in the effectiveness of the insm1a MO. Scale bar = 200μm MOBS, insm1a morpholino binding site; MO, morpholino; dpf, days post fertilization.