Abstract
Differences in neural activation during performance on an attentionally demanding Stroop task were examined between 23 young adults with ADHD carefully selected to not be co-morbid for other psychiatric disorders and 23 matched controls. A hybrid blocked/single-trial design allowed for examination of more sustained vs. more transient aspects of attentional control. Our results indicated neural dysregulation across a wide range of brain regions including those involved in overall arousal, top-down attentional control, late-stage and response selection and inhibition. Furthermore, this dysregulation was most notable in lateral regions of DLPFC for sustained attentional control and in medial areas for transient aspects of attentional control. Because of the careful selection and matching of our two groups, these results provide strong evidence that the neural systems of attentional control are dysregulated in young adults with ADHD and are similar to dysregulations seen in children and adolescents with ADHD.
Although a variety of studies have examined the neural systems dysfunctional in children with ADHD (for a review Bush, Valera, & Seidman, 2005), fewer have carefully examined the functional and anatomical abnormalities associated with ADHD in adulthood. While early positron emission tomography (PET) studies suggested the possibility of a general reduction in neural responsiveness (e.g., Zametkin et al., 1990), more recent research has implicated a variety of regions. Bush and colleagues (1999) suggested that underactivation of the dorsal anterior cingulate cortex (ACC), in the face of otherwise intact functioning of a fronto-striatal-insular network, underlies the inattention and impulsivity in adults with ADHD. Other research based on neuroanatomical comparisons between adults with ADHD and controls (Bush, Valera & Seidman, 2005; Makris et al., 2007), as well as functional imaging suggests disruption in prefrontal and parietal regions involved in executive control (Hale et al., 2007; Valera et al., 2005; Wolf et al. 2009). Both prefrontal regions involved in “cold” executive function as well as those involved also “hot” emotional functions (Castellanos et al., 2006) have been implicated. And still other recent research suggests potential dysfunction of the “default” brain network (Buckner, Hanna-Andrews & Schacter, 2008) both in terms of reduced coherence of activity (Uddin et al. 2008) as well as specific disruptions of this network with regions involved in cognitive control such as the anterior cingulate (Castellanos et al., 2008). Given these inconsistencies, and the overall dearth of functional neuroimaging studies in adults with ADHD, we believe that additional studies are warranted.
The focus of the current study was to compare brain activation during performance of an attentionally demanding task for a well-characterized college-aged sample of young adults with ADHD who were not comorbid for other psychiatric disorders or learning disabilities and controls matched for IQ and age. Such careful sample selection was designed to maximize the possibility that any group differences were likely attributable to ADHD rather than other factors, such as co-morbid psychiatric disorders or overall level of intelligence. We utilized the Stroop task, not only because it is considered the “gold standard” of attentional tasks (MacLeod, 1991) but also because our prior neuroimaging studies in neurologically-normal young adults performing this task (Banich et al., 2000a, b; Liu et al., 2004, 2006; Milham & Banich, 2005; Milham, Banich, & Barad, 2003; Milham, Banich, Claus, & Cohen, 2003; Milham et al., 2001) provides a strong empirical and theoretical base from which to interpret any observed group differences..
Because our prior research suggests distinct roles for medial versus lateral prefrontal regions involved in attentional control, we wished to investigate whether one or both of these systems are affected in young adults with ADHD. Our prior neuroimaging work indicates that the dorsolateral prefrontal cortex (DLPFC) is very important in implemeting a top-down attentional bias or “goal state”, especially one that must be maintained over time in a sustained manner (e.g. Banich et al., 2000a; Milham, Banich, Claus & Cohen, 2003). Because a major symptom of ADHD is distractibility and an inability to stay on task, we hypothesized that this region might function atypically in young adults with ADHD. Our prior results also suggest that caudal regions of the dorsal anterior cingulate (BA 32) are more involved in response-related aspects of attentional control that varies in a more transient (i.e. trial-by-trial) manner and that such control is dissociable from that exerted by DLPFC (e.g., Milham, Banich, Claus, & Cohen, 2003; Milham et al. 2001; Milham et al., 2002). Given prior research that suggests moment-to-moment fluctuations in cognitive control, at least in children with ADHD (van Meel, Hesienfeld, Ossteriaan, & Sergeant, 2007), we also wished to investigate the integrity of functioning of these medial prefrontal regions in adults with ADHD.
To distinguish between sustained and transient aspects of cognitive control, we employed a hybrid blocked-event related design (Visscher et al. 2003). Blocked activity provides a rough index of the ability to sustain attentional control, while event-related activity provides an index of transient control. Participants were required to manually identify the ink color in which words were presented. Our design included three different types of block that varied by the type of word that was presented: incongruent (e.g., “red” in blue ink), congruent (e.g., “red” in red ink), and neutral (e.g., “sum” in red ink) blocks. For all three blocks, one must maintain an attentional set towards ink color identification and avoid the more automatic process of word reading. However, the blocks vary in the degree to which the stimuli themselves serve as a reminder of or reinforce the required attentional set (i.e., to pay attention to ink color). As argued by Kane & Engle (2003), the conflict inherent in the incongruent word reminds one that word reading is distracting and not task relevant (“Let’s see the ink color is red the word is blue. Now which one of these is important?”), but is lacking congruent blocks, where simply reading the word can substitute for correctly identifying ink color. Hence, if the neural substrate for maintaining a top-down set is dysfunctional in young adults with ADHD, one would predict atypical activation of DLPFC compared to controls for all three blocks (as compared to a fixation baseline). Furthermore, this effect should be greatest for the congruent blocks in which the attentional set is not reinforced by the stimuli themselves and least for the incongruent blocks where the task inherently reminds one of the attentional set to be maintained.
To examine transient changes in attentional control, we varied trial types within block (e.g., Milham et al., 2001 for a similar approach). Half of the trials within each block were neutral and identical across all blocks (these we refer to as neutral-frequent trials) while the remainder of trials were specific to that block (e.g., congruent, incongruent, neutral infrequent). Thus, within a given block, attentional demand on a trial-by-trial basis was unpredictable. In all cases, the block specific trials (incongruent, congruent, and neutral infrequent) engendered higher attentional demand than the neutral frequent trials, which provide a common baseline against which to evaluate transient aspects of attentional control. Incongruent trials are attentionally demanding because there are two conflicting sources of color information, congruent trials are demanding because they require one to differentiate whether the ink color contained in the word or that contained in the ink color should be used to guide responding (see Milham & Banich, 2005; Posner & DiGiralomo, 1998 for a longer discussion), and neutral infrequent trials because words presented less frequently capture attention (see Milham, Banich, & Barad, 2003). Our prior work indicates that caudal regions of dorsal ACC are important in processing transient aspects of attentional control that cannot be subsumed under a general attentional set for task-relevant processes (e.g., Milham, Banich, Claus, & Cohen, 2003). Hence, if this neural substrate for attentional control is also disregulated in adults with ADHD, one would predict atypical activation of medial prefrontal regions, including caudal regions of dorsal ACC.
Method
Participants and Recruitment
Participants and Recruitment Procedures
Study participants consisted of 23 adults who met criteria for DSM-IV ADHD combined subtype (9 female, 14 male) and a control group of 23 participants without ADHD (10 female, 13 male). A three-stage screening procedure was used to identify the final groups.
Initial screening of the unselected sample
An unselected sample of 3,913 undergraduates completed a battery of self-report rating scales that included the Self-Report form of the ADHD Current and Childhood Symptom Scales (Barkley & Murphy, 1998). The initial screening measures were administered to groups of 20–40 individuals as part of the research participation requirement of a large introductory psychology course. Permission was also requested to allow us to send the Other Report version of the Current and Childhood Symptom Scales (Barkley & Murphy, 1998) to the participant’s parent or other primary caregiver during childhood. Approximately 72% of the participants provided consent for the questionnaire to be sent to their parent or caregiver.
Individual assessment of groups with and without DSM-IV ADHD
As part of an ongoing study of neuropsychological functioning in young adults with ADHD, a subset of participants from the initial screening sample were invited to participate in a more extensive individual testing session that included measures of general intelligence, academic achievement, and neuropsychological functioning. This subset included participants who met symptom criteria for any DSM-IV ADHD subtype based on parent or self-report ratings on the Childhood and Current Symptom Scales. They were invited to complete the individual testing session (N = 207) and a randomly selected comparison sample without ADHD (N = 98).
Identification of groups with and without DSM-IV ADHD combined type for the current fMRI study
Diagnostic algorithm for the combined type
At the conclusion of the individual assessment session, participants who met criteria for DSM-IV ADHD - combined type and who met all inclusion criteria for the MR protocol were invited to participate in the current fMRI study (N = 23). Because the diagnosis of the combined type in adulthood is complicated by the fact that symptoms of ADHD decline with increasing age, particularly on measures of hyperactivity-impulsivity (e.g., DuPaul et al., 1998; Nolan et al., 1999; 2001), we used the follow four criteria: (1) Retrospective reports by the participant or the parent indicating that he or she met DSM-IV criteria for the combined type during childhood; (2) the participant either currently met criteria for DSM-IV ADHD (N = 20) or scored above the 90th percentile on the ADHD symptom measures while exhibiting marked functional impairment, consistent with the DSM-IV specification of ADHD in partial remission (N = 3); (3) the ADHD symptoms led to significant functional impairment, and (4) the onset of the ADHD symptoms was prior to 12 years of age. This age threshold was used rather than age 7 because prior studies suggest it is more reliable and valid than the threshold specified in DSM-IV (e.g., Barkley & Biederman, 1997; Nigg et al., 2005).
Treatment history
Of the 23 individuals in the ADHD group, 22 had received a previous diagnosis of ADHD. Twenty individuals had been prescribed psychostimulant medication during their lifetime, and 14 individuals had a current prescription for mixed amphetamine salts (Adderall XR; N = 9), methylphenidate (Concerta, N = 3; Ritalin, N = 1), or dexmethylphenidate (Focalin, N = 1). Participants with a current prescription for stimulant medication agreed to refrain from taking the medication for 24 hours prior to their participation in the study. One participant with a current prescription for a nonstimulant medication (bupropion, Wellbutrin) was not asked to discontinue the medication.
Criteria for the comparison group
The comparison group for the fMRI study included 23 individuals who did not meet current or lifetime criteria for any DSM-IV ADHD subtype based on the rating scales and diagnostic interview. The control and ADHD samples were matched as a group on age, sex, and academic year.
Exclusion Criteria
Potential participants were excluded from both groups if they reported a previous diagnosis of a Learning Disability (LD) or met our study criteria for an LD on the measures of reading or math achievement described in the subsequent section. Individuals with bipolar disorder, severe major depressive disorder, obsessive-compulsive disorder, or substance-use disorder were also excluded, as were potential participants who had an estimated Full Scale IQ < 80, were pregnant, were left handed, had metal in their body that could not be removed (e.g., cardiac pacemaker), had a previous history of seizures or a head injury with loss of consciousness, or any other contraindication for the MR environment.
Measures
DSM-IV ADHD symptoms
Diagnostic interview
As part of the individual testing session at which the IQ, academic achievement, and neuropsychological measures were administered, each participant completed the Adult ADHD Interview described by Barkley and Murphy (1998). The interview assesses the 18 DSM-IV ADHD symptoms and the extent to which the symptoms lead to significant impairment in academic functioning, social functioning, job performance, operation of motor vehicles, and management of daily responsibilities.
Measures of functional impairment
All participants completed multiple measures to assess whether they met functional impairment across settings as specified in DSM-IV criteria C and D. Impairment was assessed by specific questions during the initial screening in the Current and Childhood Scales and interview regarding the impact of ADHD symptoms on the individual’s social, occupational, educational, and overall daily functioning (Barkley & Murphy, 1998). This data was supplemented by a more detailed impairment questionnaire developed for this study (Willcutt, Bidwell, Hitt-Laustsen, McHaffie, & Banich, in preparation) given during the initial screening. The impairment scale includes a broader range of questions regarding academic functioning (high school and college grade point average, completion of assignments, retention of academic material), interpersonal relationships (both friendships and romantic relationships), and specific aspects of adaptive functioning such as money management, driving performance, and occupational functioning. Finally, during the initial screening, participant and parent rating was obtained of the individual’s lowest overall functioning during the past year on a Global Assessment of Functioning Scale that corresponds directly to Axis V in DSM-IV.
The battery of impairment measures was used to derive composite measures of global, academic, social, and occupational functioning, management of daily responsibilities, and driving impairment. Significant impairment in each of these domains was defined by a score at or above the 93rd percentile of the total screening sample on the composite measure.
Intelligence and academic achievement
The Matrix Reasoning subtest from the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III; Wechsler, 1997) was administered to assess nonverbal abilities, and verbal abilities were measured by the WAIS-III Vocabulary subtest. The Woodcock-Johnson Tests of Achievement, Third Edition (WJ-III; Woodcock, McGrew, & Mather, 2001) was used to assess academic achievement in mathematics (Calculations and Math Fluency) and reading-related domains (Letter-Word Identification, Word Attack, and Spelling). Reading disability was defined by a standard score below 85 on the Letter-Word Identification subtest, and math disability was defined by a score below 85 on the Calculations subtest.
Stimuli and Experimental Design
A variant of the standard Color-Word Stroop task (Stroop, 1935) was used in which participants indicated via a key press the ink color (red, blue, green, or yellow) in which words were presented. There were three types of trials: congruent trials on which the word matched the ink color (e.g., “red” in red ink), incongruent trials on which the word conflicted with the ink color (e.g. “red” in green ink), and neutral trials in which the word did not name a color (e.g. “bond” in red ink).
A hybrid blocked/event-related fMRI design was utilized for examination of both blocked (sustained) and event-related (transient) effects. Three types of blocks were utilized: congruent, incongruent, and neutral. To allow event-related comparisons, half of the trials in each block were drawn from a set of 4 neutral words that were identical (i.e. same color-word pairings) across all three blocks (referred to as neutral frequent trials) and half were drawn from a set of 3 block-specific trials (incongruent, congruent, neutral infrequent).
Three fMRI runs were composed of 13 blocks each, with each run consisting of four fixation blocks (F) alternating with triads of non-fixation blocks, consisting of one incongruent block (I), one neutral block (N), and one congruent block (C) (e.g., FINCFNCIFICINF). Within each triad, no explicit signal was given to participants regarding when each of the blocks began and ended. The order of blocks within the triads was counterbalanced across the three runs (e.g., INC for the first run, NCI for the second run and CIN for the last run), and the order of triads across runs was counterbalanced across participants. Each block contained 12 trials. Seven blank trials from the beginning of each run and 1 blank trial from the end of the run were dropped to allow for stabilization of the magnetic field. Thus, in addition to 144 fixation trials, there were 324 Stroop trials, with 108 trials for each block type.
Data Acquisition
Functional imaging data were acquired with a GE Signal (3T) MRI scanner using a T2*-weighted gradient echo, echo-planar imaging (EPI) with ramp sampling. For each of the three runs, a total of 163 EPI volumes were collected (repetition time [TR] = 2000 ms, echo time [TE] = 32 ms, flip angle = 70°), each consisting of 29 slices (thickness = 4 mm, gap = 0 mm, field-of-view (FOV) = 220 mm, in-plane matrix = 64 × 64, in-plane resolution = 3.44 × 3.44mm2), parallel to the AC-PC line. T1 weighted 3D IR-SPGR anatomical images were also collected along the coronal plane (TR = 9 ms, TE = 2.0 ms, flip angle = 10•, • inversion time = 500 ms; 220 mm FOV, 256 × 256 matrix, 0.87 × 0.87 mm2 in-plane resolution, 124 slices, 1.7-mm slice thickness).
The scanner was equipped with a standard head coil fitted with a custom air pillow, consisting of polyurethane foam beads, inflated to mold to the participant’s head and neck to reduce motion. Stimuli were displayed with an LCD projector onto a Lucite screen, visible through a mirror mounted atop the head coil, and responses were collected with a four-button fiber optic response box, each button colored with one of four colors (red, green, blue, yellow). Data sets from 46 of our 50 participants met our criteria for high quality and scan stability with minimum motion correction (<2 mm displacement in any one direction) and were subsequently included in our fMRI analysis.
Image Analysis
Image processing and statistical analyses were conducted using FMRIB Easy Analysis Tool (FEAT; http://www.fmrib.ox.ac.uk/fsl/index.html). The first seven volumes from the time series of each run were discarded to allow the hemodynamic response to stabilize. Images were motion corrected using MCFLIRT (Jenkinson & Smith, 2001), and submitted to a brain extraction algorithm (BET) to remove all non-brain tissue from the images. Prior to statistical analysis, images were spatially smoothed using a Gaussian kernel (FWHM = 8mm) mean-based intensity normalized, and high-pass filtered to remove high-frequency noise (σ = 100 s).
Statistical Analyses
Statistical analyses were conducted using FMRIB improved linear model (FILM). Event-related changes in the time series were modeled by convolving binary regressors with a double-gamma hemodynamic response function. Error trials were excluded from event-related regressors, and were modeled as a separate regressor of no interest. FLAME was used to model the mixed-effects variance for each contrast of interest, taking into account both fixed effects (within-subjects variability) and random effects (between-subjects variability). Parameter estimates were subsequently registered to Montreal Neurological Institute standard stereotaxic space (MNI152) for comparisons across individuals.
For each contrast, we used an individual voxel threshold of Z = 2.58 (p = .01, two tailed) with a cluster-wise protection to guard against false positives, as determined by the AlphaSim software of AFNI. A whole brain mask was used for the analysis of blocked activity versus the fixation baseline as well as for activity in all contrasts that fell outside preselected ROIs, yielding an extent of 136 voxels for α <.10, 154 voxels for α <.05, and 171 for α <.025. A more restricted mask consisting of those regions in which we expected differences to occur (angular gyrus, supramarginal gyrus, superior parietal lobule, precuneus, superior temporal gyrus, occipital fusiform gyrus, middle frontal gyrus, superior frontal gyrus, inferior frontal gyrus, frontal operulculum, precentral gyrus, and the anterior cingulate cortex) was used for all other contrasts and yielded thresholds of 78 voxels for α <.10, 90 voxels for α <.05, and 103 voxels for α <.025.
We confirmed that our results obtained in the blocked contrasts were not contaminated by error trials by running an additional analysis that only included correct trials within a block of interest. For example, in this analysis, the incongruent vs. congruent contrast included all correct trials, both incongruent and neutral, within one EV to represent the incongruent block, and all correct trials within the congruent block, both congruent and neutral to represent the congruent block. All the effects listed below in our standard block analysis were significant in this additional analysis at a threshold of Z=2.24, p<.025 (two-tailed).
Results
Behavioral Results
Participant characteristics
Unpaired t-tests indicated that participants with ADHD exhibited significantly elevated symptoms of ADHD in childhood and as young adults, as would be expected (see Table 1). In contrast, the two groups did not differ significantly on measures of Full Scale, Verbal, or Performance IQ estimated from the Matrix Reasoning and Vocabulary subtests, and there were no group differences on measures of reading or mathematics achievement. Overall, the analysis of our samples indicates they are well matched with regards to overall intellectual ability, but the ADHD group shows clear evidence of attentional dysfunction and significant functional impairment.
Table 1.
Descriptive characteristics of the Control and ADHD samples
| GROUP | |||
|---|---|---|---|
| Control | ADHD | ||
| M (SD) | M (SD) | t | |
| Descriptive characteristics | |||
| Age | 19.0 (0.9) | 20.0 (1.7) | 2.61* |
| DSM-IV ADHD symptomsa | |||
| Childhood | |||
| Inattention | 0.8 (1.0) | 7.4 (2.0) | 14.07*** |
| Hyperactivity-impulsivity | 1.0 (1.3) | 6.8 (1.8) | 12.55*** |
| Current | |||
| Inattention | 0.9 (1.2) | 6.6 (2.3) | 11.81*** |
| Hyperactivity-impulsivity | 1.5 (1.2) | 5.0 (2.2) | 6.46*** |
| WAIS-III Estimated IQ scores | |||
| Performance | 113.5 (10.5) | 114.4 (8.7) | 0.31 |
| Verbal | 113.3 (10.5) | 118.9 (12.7) | 1.64 |
| Full Scale | 113.4 (8.3) | 116.6 (7.2) | 1.42 |
| WJ-III | |||
| Reading and Spelling | |||
| Letter Word ID | 105.1 (8.9) | 101.3 (9.8) | 1.40 |
| Word Attack | 100.4 (9.7) | 100.3 (9.4) | 0.05 |
| Spelling | 106.9 (7.9) | 103.6 (8.5) | 1.29 |
| Math | |||
| Calculations | 110.7 (13.5) | 106.2 (16.0) | 0.93 |
| Math Fluency | 102.3 (11.2) | 95.5 (11.6) | 1.93 |
| Percent Impaired | |||
| Control | ADHD | ||
| Domain of functioning | N (%) | N (%) | Χ2 |
| Global impairment (past 12 months) | 5 (22%) | 19 (83%) | 17.1*** |
| Management of responsibilities | 0 (0%) | 15 (65%) | 22.3*** |
| Academic functioning | 1 (4%) | 13 (57%) | 14.8*** |
| Social relationships | 0 (0%) | 16 (70%) | 24.5*** |
| Driving | 2 (9%) | 8 (35%) | 4.6* |
| Occupational functioning | 2 (9%) | 11 (48%) | 8.7** |
| Significant impairment in 1 or more domains | 5 (22%) | 23 (100.0%) | 29.6*** |
| Significant impairment in 2 or more domains | 0 (0%) | 19 (83%) | 32.4*** |
Parent and self-report ratings were combined to create the total symptom counts by coding each symptom as present if endorsed by either the parent or the participant (see Lahey et al., 1994).
Stroop Task
Reaction time
An ANOVA on mean reaction time with a between-subjects factor of GROUP (ADHD, Control), BLOCK (congruent, neutral, incongruent) and TRIAL TYPE (block specific, neutral frequent) yielded a significant three-way GROUP by BLOCK by TRIAL TYPE interaction (F(2,88)=5.96, p<.005), which was driven entirely by block specific trials (TRIAL by GROUP F(2,88)=7.87, p< .001) and not by the neutral frequent trials (TRIAL by GROUP F(2,88)=.393, p=.69) (see Figure 1a). Interference and facilitation were calculated as a percentage of RT on neutral trials. For across-block analyses, mean RT for all trials within a block were used (e.g., interference: mean RT for the incongruent block – mean RT for the neutral block/mean RT for the neutral block), while for within-block analysis, RT for the block-specific trials were compared to RT for the neutral frequent trials within that block (e.g., mean RT for the neutral frequent trials in the congruent block – RT for the congruent trials in the congruent block/RT for the neutral frequent trials in the congruent block). Thus, interference represents the percentage increase to which RT is slowed on incongruent trials relative to neutral trials. Likewise, facilitation represents the percentage increase to which RT is speeded on congruent trials relative to neutral trials. Both interference and facilitation index the degree to which individuals have difficulty complying with task demands and pay attention to the word rather than the ink color.
Figure 1.
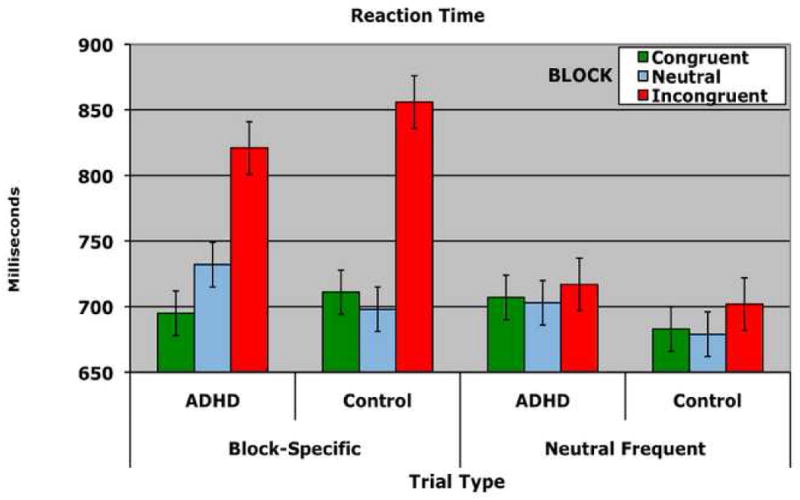
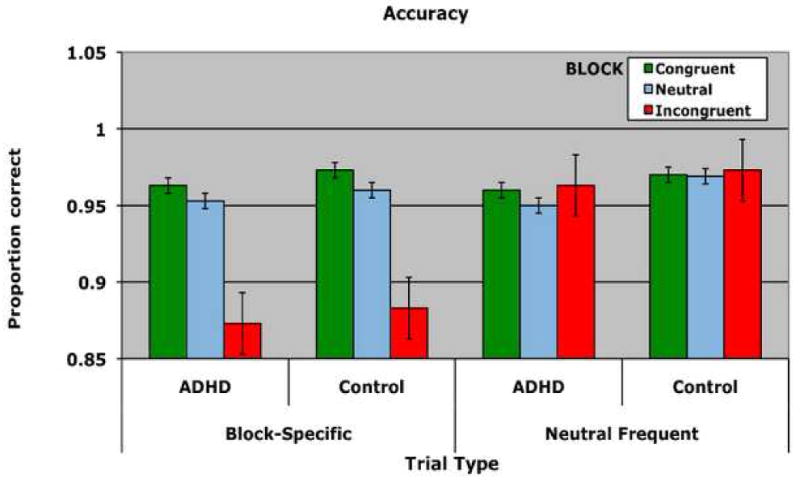
Behavioral performance on the Stroop task for individuals with ADHD and controls. A) Reaction Time B) Accuracy. Performance is shown for each group separately for each of the three blocks. Within each block, performance on block-specific trials (left) as well as performance on neutral frequent trials (right) is shown. Errors bars represent plus and minus on standard deviation.
Because meta-analyses suggest that poor performance on the Stroop task is often observed in individuals with ADHD (van Mourik, Oosterlaan & Sergaent, 2005; Willcutt, Doyle, Nigg, Faraone & Pennington, 2005), the results were surprising as they revealed that interference was significantly greater for the control group than for the ADHD group both across blocks (t=2.93, df=44, p<.005) (ADHD: 9.8% Controls 15.5%) and within block (t=−2.40, df=44, p<.025) (ADHD: 17.9% Controls: 26.5%). In consideration of the fact that the ADHD group showed less interference, this measure was used as a covariate in specific fMRI analyses noted below to determine whether group differences in activation still existed even when behavioral performance was taken into account.
Compared to controls, the ADHD group exhibited marginally increased facilitation across blocks (ADHD: 1.7% Controls: −0.6%) (t=1.90, df=44, p=.064 two-tailed) and significant greater facilitation within blocks (ADHD: .5%, Controls: -.4.3%) (t=2.76, df=44, p<.01). MacLeod and MacDonald have argued that facilitation (i.e., faster responses on congruent than neutral trials) indexes the degree to which individuals do not comply with task demands on congruent trials. Rather than identifying the ink color on these trials, individuals “cheat” on a certain proportion of congruent trials and read the word. Because reading is faster than ink color identification, their responses are speeded is comparison to neutral trials, on which no such cheating is possible. Hence, individuals with ADHD appear do not appear to be complying with task demands. In contrast, the control group is actually slowed by congruent trials compared to neutral trials, which is not without precedence (Nealis, 1973; Schulz, 1979). Finally, the degree to which the ink color interfered with processing, as defined by the sum of interference and facilitation, was shown not to differ between the two groups via an independent sample t-test (t =−1.72, df=44, p>,05).
Accuracy
An ANOVA on mean accuracy with the between-subject factor of GROUP (ADHD, Control) and the within-subject factors of BLOCK (congruent, neutral, incongruent) and TRIAL TYPE (block specific, neutral frequent) yielded no effects or interactions with the factor of GROUP (See Figure 1b).
Imaging Results
GROUP DIFFERENCES IN ACTIVATION
Blocked analyses
Conditions vs. Fixation
The contrast of each block (i.e. congruent, incongruent, neutral) vs. fixation calculated separately was used to examine brain mechanisms engaged when ink color identification must be selected over word reading. Similar group differences emerged across all three types of blocks (see Table 2). Individuals with ADHD showed significantly less activity in left posterior DLPFC (BA 8/6) and left middle DLPFC (BA 9/46) than controls. On the other hand, individuals with ADHD showed more activity than controls across all three contrasts in a number of regions including the right insula, left and right superior temporal gyri, and posterior cingulate cortex. Inspection of the maps individually for each group revealed that individuals with ADHD did not deactivate these regions relative to fixation baseline as much as controls. Deactivation of the insula and superior temporal gyri may occur as a means to preclude linguistic processing of the word, while deactivation of posterior cingulate, a region considered part of the default network, may aid in meeting attentional demand.
Table 2.
Clusters that yielded significant differences between groups for blocked activity vs. fixation baseline
| Region | BA | Max Z | No. of voxels | x | y | z | Z value for each group individually | |
|---|---|---|---|---|---|---|---|---|
| Neutral vs. Fixation Baseline | ||||||||
| Control > ADHD | ||||||||
| *Middle Frontal Gyrus (L) | 46 | 3.69 | 140 | −42 | 30 | 24 | CTRL ADHD |
5.03 2.42 |
| *Precentral Gyrus (L) | 6 | 3.40 | 77 | −50 | 4 | 36 | CTRL ADHD |
6.32 3.52 |
| ADHD > Control | ||||||||
| *Posterior Cingulate (R) | 23 | 4.59 | 1140 | 8 | −46 | 20 | CTRL ADHD |
−2.74 2.59 |
| *Insula (R) | 13 | 3.54 | 802 | 34 | −26 | 12 | CTRL n.s. ADHD |
−2.10 2.78 |
| *Superior Temporal Gyrus (R) | 22 | 3.61 | 408 | 60 | −10 | 2 | CTRL ADHD n.s. |
−3.22 1.06 |
| 22 | 3.09 | 103 | 42 | −58 | 12 | CTRL ADHD n.s. |
−3.93 −0.07 |
|
| *Superior Temporal Gyrus (L) | 41 | 3.14 | 277 | −46 | −26 | 4 | CTRL n.s. ADHD n.s. |
−2.41 2.37 |
| *Superior Frontal Gyrus (L) | 8 | 3.94 | 338 | −14 | 46 | 46 | CTRL ADHD n.s. |
−4.27 1.59 |
| *Lingual Gyrus (L) | 17 | 3.68 | 266 | −2 | −94 | −4 | CTRL ADHD |
2.90 4.97 |
| *Precuneus (L) | 19 | 3.20 | 123 | −34 | −82 | 38 | CTRL ADHD n.s. |
−4.34 0.34 |
| Medial Frontal Gyrus (R) | 10 | 3.10 | 113 | 6 | 54 | 14 | CTRL ADHD n.s. |
−3.48 0.46 |
| *Inferior Frontal Gyrus (R) | 47 | 3.40 | 112 | 26 | 16 | −28 | CTRL ADHD n.s. |
−4.07 −0.75 |
| Congruent vs. Fixation Baseline | ||||||||
| Control > ADHD | ||||||||
| *Middle Frontal Gyrus (L) | 46 | 3.32 | 154 | −46 | 32 | 28 | CTRL ADHD |
4.65 2.70 |
| *Middle Frontal Gyrus (L) | 8 | 3.62 | 152 | −48 | 6 | 40 | CTRL ADHD |
5.33 2.77 |
| ADHD > Controls | ||||||||
| *Superior Temporal Gyrus (R) | 22 | 3.39 | 269 | 60 | −4 | −2 | CTRL ADHD n.s. |
−4.15 −0.64. |
| *Superior Temporal Gyrus (L) | 41 | 3.36 | 215 | −40 | −34 | 8 | CTRL ADHD n.s. |
−4.32 −0.61 |
| *Cingulate Gyrus (R) | 31 | 3.11 | 96 | 4 | −48 | 30 | CTRL ADHD n.s. |
−4.87 −2.17 |
| Incongruent vs. Fixation Baseline | ||||||||
| Control >ADHD | . | |||||||
| *Middle Frontal Gyrus (L) | 9 | 3.29 | 54 | −46 | 32 | 30 | CTRL ADHD n.s. |
4.80 2.72 |
| ADHD > Controls | ||||||||
| * Insula (R) | 13 | 3.37 | 571 | 44 | −14 | 6 | CTRL ADHD n.s. |
−4.31 −0.22 |
| *Insula (L) | 13 | 3.21 | 224 | −40 | −22 | 6 | CTRL ADHD |
−5.63 −3.94 |
| *Posterior Cingulate (R) | 29 | 3.39 | 469 | 6 | −48 | 6 | CTRL ADHD n.s. |
−4.66 −2.10 |
| *Superior Temporal Gyrus (R) | 22 | 3.26 | 254 | 42 | −60 | 12 | CTRL ADHD n.s. |
−3.90 0.65 |
| Superior Temporal Gyrus (L) | 38 | 3.18 | 178 | −34 | 0 | −28 | CTRL ADHD n.s. |
−4.89 −2.36 |
| *Lingual (L) | 18 | 3.48 | 153 | 0 | −92 | −2 | CTRL n.s. ADHD |
2.55 5.34 |
| Inferior Occipital Gyrus (R) | 17 | 3.11 | 110 | 16 | −90 | −6 | CTRL ADHD |
3.68 4.80 |
Clusters sizes marked with *are also significant at p<.05 (Z=1.96) when the behavioral covariate of mean RT for the relevant block (e.g., RT across all trials in the incongruent block) is included as a covariate.
In sum, these findings suggest that compared to controls, young adults with ADHD show less engagement of brain regions that support top-down attentional control to task-relevant processes, show less disengagement of regions that process task-irrelevant material, and less disengagement of the default network.
Contrasts across conditions
The control group showed more activity in posterior regions related to attentional control for the contrast of congruent>neutral blocks (left precuneus) and incongruent>neutral blocks (left inferior parietal lobule) (see Table 3). ADHD individuals exhibited more activity in right middle frontal gyrus for the contrast of incongruent>neutral and incongruent>congruent trials. This region often seems to become activated to meet increased attentional demand, (e.g., Milham, Banich & Barad, 2003), and as such this finding may indicate that ADHD individuals need to recruit more brain regions than controls to meet similar attentional demands. The alternative possibility that engagement of this region by ADHD individuals undergirds their reduced behavioral interference is unlikely because when the degree of behavioral interference in RT is used as a covariate, these group differences remain.
Table 3.
Clusters that yielded significant differences between individuals with ADHD and controls in comparisons across blocks
| Region | BA | Max Z | No. of voxels | x | y | z | Z value for each group separately | |
|---|---|---|---|---|---|---|---|---|
| CONGRUENT > NEUTRAL | ||||||||
| Controls > ADHD | ||||||||
| Precunues (L) | 7 | 3.38 | 116 | −6 | −60 | 32 | CTRL n.s. ADHD n.s. |
2.44 −1.72 |
| INCONGRUENT> NEUTRAL | ||||||||
| Controls >ADHD | ||||||||
| *Supramarginal Gyrus (L) | 40 | 3.31 | 81 | −58 | −50 | 34 | CTRL ADHD n.s. |
4.32 1.53 |
| ADHD >Control | ||||||||
| *Cuneus(R) | 7 | 3.22 | 140 | 2 | −76 | 30 | CTRL n.s. ADHD |
0.50 4.75 |
| *Middle Frontal Gyrus (R) | 46 | 3.47 | 83 | 48 | 40 | 20 | CTRL n.s. ADHD |
1.03 4.77 |
| INCONGRUENT > CONGRUENT | ||||||||
| ADHD >Control | ||||||||
| ^ Middle Frontal Gyrus (R) | 46 | 3.18 | 72 | 54 | 34 | 18 | CTRL n.s. ADHD |
−1.32 3.09 |
Clusters sizes marked with *are also significant at p<.05 (Z=1.96) when interference (incongruent RT mean RT to all trials in the incongruent block - neutral RT mean RT to all trials in the neutral block /mean RT neutral RT to all trials in the neutral block) is considered as a covariate.
Also significant at p<.05 (Z=1.96) when the difference between interference and facilitation (Mean RT to all trials in the neutral block – Mean RT to all trials in the congruent block/Mean RT to all trials in the neutral block) is included as a covariate
Single-Trial Analyses
Within block analyses
The purpose of the single-trial within-block analysis was to examine the response to transient attentional demands that cannot be controlled via a static top-down attentional set across a block of trials. Because the single-trial contrasts compare trial types within a block, any attentional set for the block will be equivalent across the two trial types and hence will not contribute to any observed differences. The results from these analyses are presented in Table 4.
Table 4.
Clusters that yielded significant differences between individuals with ADHD and controls in the single-trial comparisons within block
| Region | BA | Max Z | No | x | y | z | Z value for each group separately | |
|---|---|---|---|---|---|---|---|---|
| INCONGRUENT>NEUTRAL (inc) | ||||||||
| Control > ADHD | ||||||||
| * Right Thalamus | 3.44 | 748 | 6 | −22 | 0 | CTRL ADHD n.s. |
4.51 .702 |
|
| * Cingulate Gyrus (R) | 24 | 3.16 | 138+ | 6 | 14 | 28 | CTRL ADHD n.s. |
4.39 1.25 |
| CONGRUENT > NEUTRAL (con) | ||||||||
| Control > ADHD | ||||||||
| ^ Inferior Parietal Lobule (L) | 40 | 3.8 | 546 | −38 | −58 | 46 | CTRL ADHD n.s. |
4.05 −.22 |
| NEUTRAL BLOCK SPECIFIC > NEUTRAL FREQUENT NEUTRAL | ||||||||
| Control > ADHD | ||||||||
| Parahippocampal Gyrus (R) | 30 | 3.33 | 459 | 24 | −50 | 8 | CTRL ADHD |
2.456 −2.22 |
| Lingual Gyrus (R) | 18 | 3.25 | 115+ | 28 | −76 | −10 | CTRL n.s. ADHD |
1.57 −2.96 |
Clusters sizes marked with + are corrected for multiple comparisons within an a priori Stroop mask; other regions are corrected for multiple comparisons within a whole-brain search;
Remains significant at p<.05, Z=1.96 when covariate of interference (RT incongruent trials within the incongruent block – RT neutral trials within the incongruent block/ RT neutral trials within the incongruent block) is used as a covariate;
Remains significant at p<.05, Z=1.96 when covariate of facilitation (RT neutral trials within the congruent block – congruent trials within the congruent block/ RT neutral trials within the congruent block) is used as a covariate.
The results indicate that brain activation also differs between ADHD individuals and controls in the face of transient attentional demands. For the contrast of incongruent > neutral trials within the incongruent blocks, controls exhibited more activity than individuals with ADHD in two regions, the thalamus and anterior cingulate cortex (BA 24) directly above the callosum (See Figure 2b). As the thalamus serves to activate cortical regions, we speculate that contributions of this region to phasic increases to attentional demand are smaller in ADHD individuals. The lack of anterior cingulate cortex activity in the ADHD group is consistent with reports by others of atypical activation in adults with ADHD in this portion of the cingulate (Bush et al., 1999). An analysis including the degree of behavioral interference as a covariate indicated that these group differences remained, indicating that the smaller degree of behavioral interference in the ADHD was unlikely to be generating these group differences.
Figure 2.
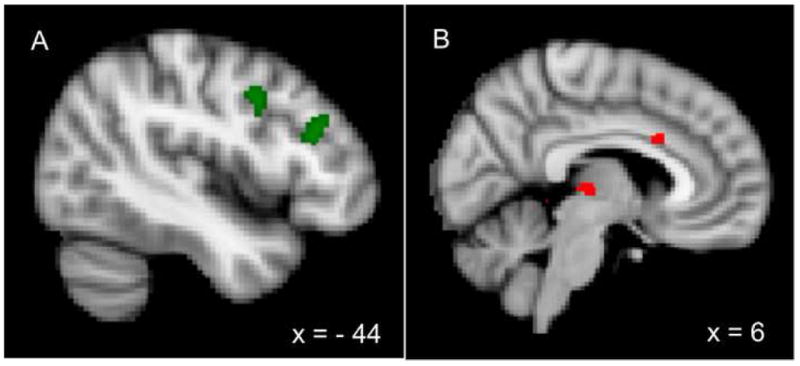
Regions of significantly greater activity for controls than individuals with ADHD. A) Blocked activity: Regions of mid- and posterior dorsolateral prefrontal cortex in the contrast of congruent blocks vs. fixation blocks. B) Single-trial activity: Regions of the anterior cingulate cortex and thalamus in the contrast of incongruent trials vs. neutral trials within the incongruent block.
The contrast of congruent>neutral trials within congruent block revealed that controls but not individuals with ADHD activated a large region of the inferior parietal lobe (BA 40), spanning the intraparietal sulcus, which has been heavily implicated in attentional control. We speculate that for controls, top-down control is relatively low on congruent blocks, so intraparietal regions are recruited on congruent trials to help deal with the increased attentional demand (relative to neutral trials within the block). In contrast, based on the blocked analysis and their increased behavioral facilitation, individuals with ADHD are not complying with task demands, and may be reading the word on a certain proportion of congruent trials. Hence, congruent trials are not likely to engage these attentional control regions.
The contrast of neutral infrequent vs. neutral frequent trials within the neutral block, yielding marginally more activation in controls for a set of posterior regions including the right parahippocampal, right lingual and bilateral fusiform gyri, and right cuneus. These finding suggests more sensitivity on the part of the controls to stimulus-specific perceptual characteristics of the lower frequency of occurrence of the neutral infrequent items.
In sum, these data suggest that certain portions of the brains of individuals with ADHD are not as sensitive as controls to the transient increase in attentional demands that occur on a trial-by-trial basis, including regions of posterior cortex sensitive to the perceptual characteristics of an item, thalamic regions most likely involved in cortical arousal, and portions of the dorsal anterior cingulate cortex, which we have previously argued are involved in late-stage selection (Milham & Banich, 2005).
Analyses of trial types in different blocks
In the within-block single-trial analyses discussed above, the neutral frequent trials serve as our baseline. To examine whether this baseline was similar across blocks, we performed an across-block comparison of activation to the neutral frequent trials using the orthogonalization procedures within FSL. This procedure allowed us to calculate the event-related activity for neutral frequent trials independent of activity common to all trials within the block from which they were drawn (e.g., incongruent trial). What remains then, is the transient response to these item types. These analyses suggested that there were minimal differences in activation between the groups for the neutral frequent trials across blocks (see Table 5).
Table 5.
Clusters that yielded significant differences between individuals with ADHD and controls in the single-trial comparisons across block.
| Region | BA | Max Z | No. of voxels | x | y | z | Z value for each group separately | |
|---|---|---|---|---|---|---|---|---|
| Neutral Frequent (inc) > Neutral Frequent (neut) | ||||||||
| Control > ADHD | ||||||||
| Hippocampus (L) | 3.37 | 173 | −34 | −18 | −16 | ADHD n.s. Control |
2.11 2.62 |
|
| Substantia Nigra (R) | 3.18 | 171 | 14 | −14 | −12 | ADHD n.s. Control n.s. |
−2.37 2.13 |
|
| Neutral Frequent (Inc) > Neutral Frequent (cong) | ||||||||
| Control > ADHD | ||||||||
| Substantia Nigra (R) | 3.65 | 789 | 10 | −28 | −16 | ADHD n.s. Control |
−2.04 3.02 |
|
| Incongruent – Congruent | ||||||||
| Control > ADHD | ||||||||
| Inferior Frontal Gyrus (R) | 47 | 3.06 | 112+ | 48 | 16 | −6 | ADHD n.s. Control |
.27 4.00 |
Clusters sizes marked with + are corrected for multiple comparisons within an a priori Stroop mask; other regions are corrected for multiple comparisons within a whole-brain search.
We similarly examined event-related activity for incongruent>congruent trials, orthogonal of the effect of block. Controls activated a region of right inferior frontal gyrus, implicated in inhibitory control (Arons, Robbins, & Poldrack, 2004) significantly more for incongruent than congruent trials, a difference that was absent for individuals with ADHD. This region did not yield a group difference in the blocked contrast, suggesting this region becomes involved in transient rather than sustained aspects of attentional control.
Discussion
The Neural Substrates of ADHD in Young Adults
Our results provide new insights into a number of issues regarding the neural systems underlying attentional control in individuals with ADHD. First, they suggest that atypical activation in neural networks engaged to exert attentional control can be observed in adults with ADHD, even high functioning ones. Second, the results are noteworthy because ADHD is likely to be causing the observed effects. Our population was selected via rigorous methods to ensure that a) individuals exhibited both childhood and current symptoms of ADHD, b) showed significant impairment on neuropsychological tests and in everyday activities, and c) were not comorbid for psychiatric or learning disorders. Moreover, because neither overall IQ nor accuracy of performance differed between the groups, we can have a high degree of confidence that the differences we observe between the two groups are indeed driven by the presence or absence of attention deficit-hyperactivity disorder. Finally, a separate voxel-based morphometric analysis of the groups examined in this study (Depue et al., in preparation) did not yield any significant differences in grey matter volume, indicating that the differences in activation we observed between the groups are unlikely to be an artifact of anatomical variation.
Our results do not suggest a simple locus of neural dysfunction in ADHD but rather suggest that a variety of regions are involved. For example, we did not find differences limited to or predominantly in cingulate regions as has been reported by other groups (e.g., Bush et al., 1999) or prefrontal regions (e.g., Valera et al., 2005). Rather, our results suggest that a large number of regions show differential activity between ADHD adults and controls. Some of these regions are conceptualized as being sources of attentional control: DLPFC, anterior cingulate, posterior parietal cortex, and right inferior frontal cortex. Supporting the idea that these regions exerted control less effectively in individuals with ADHD than controls, ADHD individuals had more activation in brain regions, such as posterior language areas, that are involved in processing the task-irrelevant word.
Transient vs. Sustained Aspects of Attentional Control
The results also suggest that both sustained and transient aspects of attentional control are affected in young adults with ADHD. Regardless of the type of block (incongruent, neutral, congruent), reduced DLPFC activity was observed in ADHD individuals compared to a fixation baseline, suggesting dysregulation of mechanisms involved in top-down attentional control. Supporting this interpretation, the reduction was largest for the congruent condition, which provides the least support or reinforcement of the task-relevant (i.e., ink identification) process (e.g., Kane & Engle, 2003). In addition, individuals with ADHD exhibited greater behavioral facilitation than controls, also consistent with the idea that they were not effectively exerting control. Finally, individuals with ADHD exhibited a greater degree of activation across all blocks in regions related to linguistic processing, such as the left temporal gyrus, which suggests increased processing of task-irrelevant information.
Group differences were also noted in transient responses within block to attentional demand. In particular, the control group exhibited increased thalamic activation as well as increased activation of the cingulate and inferior parietal cortex, effects not observed for individuals with ADHD. And a direct single-trial comparing only incongruent and congruent trials (with the effect of blocked partialled out) revealed greater activation in right inferior frontal regions involved in response inhibition (Aron, Robbins, & Poldrack, 2004). As our previous research (e.g., Milham et al., 2001) suggests that anterior cingulate and right inferior frontal mechanisms are involved in late-stage, usually response-related, aspects of attentional control, the results suggest that such mechanisms may also be disrupted in ADHD.
Limitations
Our results are somewhat limited by the fact that the group we selected was rather high functioning, as they all were enrolled in a four-year college program Hence, whether the results we obtained would generalize to individuals more severely affected with ADHD remain to be seen. The high degree of functioning of our sample may explain why we did not find group differences in subcortical regions that have previously yielded differences in activation between children with and without ADHD, such as the basal ganglia and the cerebellum (for a review, see Seiman, Valera & Bush, 2004). It is impossible to tell from the current study whether this null result occurs because of the level of functioning in our sample or because these brain regions have overcome the hypothesized maturational lag in brain development in individuals with ADHD by young adulthood (Rubia et al., 2000; Shaw et al. 2007). Nonetheless, the current results suggest that by college age, not all aspects of brain function are normalized in ADHD.
Another example of the high degree of functioning of our sample is that they did not show increased behavioral interference compared to controls, and, on the contrary, showed less. However, the fact that the group difference in activation results remained significant when behavioral data were entered as covariates suggests that they were not driving the group differences. Furthermore, the consistent pattern of reduced prefrontal activation both in blocks in which the performance of the ADHD group was better than controls (i.e. incongruent blocks) and in which performance was worse than controls (i.e. congruent blocks) also reduced the likelihood that performance is the basis of the group difference.
Another limitation of our study is that the hybrid blocked/event-related design used cannot totally distinguish transient and sustained aspects of attentional control. The activity that is assessed via the blocked regressor includes not only activity that is consistent across block, but also includes some contribution of the transient activity that is engendered by each individual trial within the block. Although state-item designs (Donaldson et al. 2001) may separate these factors more cleanly, they require fixation trials interspersed within a block, and it remains a question as to whether task-set is maintained during fixation trials as would have to be assumed by such designs. The within-block comparison, in contrast, is a relatively clean measure of transient activity. Given the trade-offs for various fMRI designs, the hybrid blocked/event-related design provides at least some ability to distinguish sustained versus transient aspects of attentional control. Consistent with the idea that such an approach was effective, the regions that varied between the groups differed for blocked versus single-trial analyses, and their function, as identified by previous research, was consistent with being involved in either more sustained or more transient aspects of attentional control.
Conclusion
Our study is the first to demonstrate extensive neural dysfunction in young adults with ADHD during performance of an attentionally-demanding task. Because of the sample selection procedures, the results are most likely due to ADHD and not other factors such as comorbidity of other psychiatric disorders, nor group differences in intelligence. The results suggest that attentional dysregulation involves a large number of brain regions including regions related to overall arousal and attention (brainstem, thalamus), those involved in top-down biasing of attention (DLPFC) and those involved in late-stage selection and inhibition (anterior cingulate cortex and right inferior frontal regions). Furthermore, this dysregulation occurs for both relatively sustained as well as transient aspects of attentional control. These results suggest that in young adults with ADHD, the functioning of a wide network of brain regions functions atypically in the face of attentional demand.
Acknowledgments
This study was supported by NIMH grant R01 070037 (M.Banich, P.I.) as well as providing support for the first, second, third author, fourth and seventh authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cognitive Science. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z, Wright A, Shenker J, Magin R. fMRI Studies of Stroop Tasks Reveal Unique Roles of Anterior and Posterior Brain Systems in Attentional Selection. Journal of Cognitive Neuroscience. 2000a;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang Z, Barad V, Gullett D, Shah C, Brown C. Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMRI. Cognitive Brain Research. 2000b;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Biederman J. Toward a broader definition of the age-of-onset criterion for attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1204–1210. doi: 10.1097/00004583-199709000-00012. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy K. Attention-deficit hyperactivity disorder: A clinical workbook. 2. New York: Guilford Press; 1998. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biological Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen BR, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends in Cognitive Science. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-Precuneus Interactions: A New Locus of Dysfunction in Adult Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue BE, Burgess GC, Ames BJ, Ruzic L, Bidwell LC, Hitt-Lausten S, Wilcutt EG, Banich MT. Brain morphology in young adults with ADHD: Relations to symptomatology in preparation. [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. Neuroimage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- DuPaul JG, Anastopoulos AD, Power TJ, Reid R, Ikeda MJ, McGoey KE. Parent Ratings of Attention-Deficit/Hyperactivity Disorder Symptoms: Factor Structure and Normative Data. Journal of Psychopathology and Behavioral Assessment. 1998;20:83–102. [Google Scholar]
- Hale TS, Bookheimer S, McGough JJ, Phillips JM, McCracken JT. Atypical brain activation during simple & complex levels of processing in adult ADHD: an fMRI study. Journal of Attentional Disorders. 2007;11:125–40. doi: 10.1177/1087054706294101. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimization method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. NeuroImage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Functional dissociation of attentional selection within PFC: response and non-response related aspects of attentional selection as ascertained by fMRI. Cerebral Cortex. 2006;16:827–834. doi: 10.1093/cercor/bhj026. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends in Cognitive Science. 2000;4:383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, Caviness VS, Faraone SV, Seidman LJ. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cerebral Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: An fMRI analysis of conflict specificity and functional differentiation. Human Brain Mapping. 2005;25:328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: an event-related fMRI study of the stroop task. Cognitive Brain Research. 2003;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. NeuroImage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional Control in the aging brain: Insights from an fMRI study of the Stroop task. Brain and Cognition. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cognitive Brain Research. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Nealis PM. The Stroop phenomenon: Some critical tests of the response competition hypothesis. Perceptual and Motor Skills. 1973;37:147–153. doi: 10.2466/pms.1973.37.1.147. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, Henderson JM. Executive functions and ADHD in adults: Evidence for selective effects on ADHD symptom dimensions. Journal of Abnormal Psychology. 2005;114:706–717. doi: 10.1037/0021-843X.114.3.706. [DOI] [PubMed] [Google Scholar]
- Nolan EE, Volpe RJ, Gadow KD, Sprafkin J. Developmental, gender, and comorbidity differences in clinically referred children with ADHD. Journal of Emotional and Behavioral Disorders. 1999;7:11–20. [Google Scholar]
- Nolan EE, Gadow KD, Sprafkin J. Teacher reports of DSM-IV ADHD, ODD, and CD symptoms in school children. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:241–249. doi: 10.1097/00004583-200102000-00020. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ, Parasuraman R. Executive attention: Conflict, target detection, and cognitive control. In: Parasuraman Raja., editor. The attentive brain. Cambridge, MA: The MIT Press; 1998. pp. 401–423. [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Schulz T. Components of the reaction time Stroop-task. Psychological Research. 1979;40:377–395. [Google Scholar]
- Seidman LJ, Valera E, Bush G. Brain function and structure in adults with attention-deficit/hyperactivity disorder. Psychiatric Clinics of North America. 2004:323–347. doi: 10.1016/j.psc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L, Kelly A, Biswal B, Margulies D, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler L, Casetellanos F. Network homogeneity reveals decreased integrity of default-mode network in ADHD. Journal of Neuroscience Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Valera EM, Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-Analysis of Structural Imaging Findings in Attention Deficit/Hyperactivity Disorder. Biological Psychiatry. 2007:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- van Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): the role of error processing. Psychiatry Research. 2007:211–20. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- van Mourik R, Oosterlaan J, Sergeant JA. The Stroop revisited: a meta-analysis of interference control in AD/HD. Journal of child psychology and psychiatry, and allied disciplines. 2005;46:150–165. doi: 10.1111/j.1469-7610.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Visscher KM, Miezin FM, Kelly JE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Willcutt EG, Bidwell LC, Hitt-Laustsen S, Banich MT. Internal and external validity of ADHD in young adults. Manuscript submitted for publication. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wolf R, Plichta M, Smbataro F, Fallgatter A, Jacob C, Lesch K, Herrmann M, Schönfeldt-Lecuona C, Connemann B, Grön G, Vasic N. Regional brain activation changes and abnormal functional connectivity of the ventrolateral prefrontal cortex during working memory processing in adults with attention-deficit hyperactivity disorder. Human Brain Mapping. 2009 doi: 10.1002/hbm.20665. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock R, McGrew K, Mather N. Woodcock-Johnson III. Itasca, IL: Riverside Publishing Company; 2001. [Google Scholar]
- Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, Hamburger S, Cohen RM. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. The New England Journal of Medicine. 1990;323:1361–1366. doi: 10.1056/NEJM199011153232001. [DOI] [PubMed] [Google Scholar]


