Abstract
Background and Purpose
Remote ischemic postconditoning, a phenomenon in which brief ischemic stimuli of 1 organ protect another organ against an ischemic insult, has been demonstrated to protect the myocardium and adult brain in animal models. However, mediators of the protection and underlying mechanisms remain to be elucidated. In the present study, we tested the hypothesis that remote limb ischemic postconditioning applied immediately after hypoxia provides neuroprotection in a rat model of neonatal hypoxia–ischemia (HI) by mechanisms involving activation of the opioid receptor/phosphatidylinositol-3-kinase/Akt signaling pathway.
Methods
HI was induced in postnatal Day 10 rat pups by unilateral carotid ligation and 2 hours of hypoxia. Limb ischemic postconditioning was induced by 4 conditioning cycles of 10 minutes of ischemia and reperfusion on both hind limbs immediately after HI. The opioid antagonist naloxone, phosphatidylinositol-3-kinase inhibitor wortmannin, or opioid agonist morphine was administered to determine underlying mechanisms. Infarct volume, brain atrophy, and neurological outcomes after HI were evaluated. Expression of phosphorylated Akt, Bax, and phosphorylated ERK1/2 was determined by Western blotting.
Results
Limb ischemic postconditioning significantly reduced infarct volume at 48 hours and improved functional outcomes at 4 weeks after HI. Naloxone and wortmannin abrogated the postconditioning-mediated infarct-limiting effect. Morphine given immediately after hypoxia also decreased infarct volume. Furthermore, limb ischemic postconditioning recovered Akt activity and decreased Bax expression, whereas no differences in phosphorylated ERK1/2expression were observed.
Conclusions
Limb ischemic postconditioning protects against neonatal HI brain injury in rats by activating the opioid receptor/phosphatidylinositol-3-kinase/Akt signaling pathway.
Keywords: Akt, limb ischemic postconditioning, neonatal hypoxia–ischemia, opioid receptor
Ischemic preconditioning and ischemic postconditioning refers to the application of brief sublethal ischemia in 1 organ before (preconditioning) or after (postconditioning) a prolonged injurious ischemic insult generating tissueprotective mechanisms in the same organ.1 Ischemic preconditioning and postconditioning have been demonstrated to cause tissue salvage in the settings of ischemia/reperfusion injury in a similar degree in various organ systems, including myocardium, brain, etc.1–3 However, the applicability of ischemic preconditioning is limited by the unpredictable nature of ischemic events in clinical practice. Ischemic postconditioning can be conducted after the occurrence of an ischemic insult. However, inducing intermittent episodes of ischemia to the same vital organ, having suffered lethal ischemic damage by postconditoning, requires mechanical intervention and can only be translated to clinical practice in limited circumstances of ischemic events.
Recently, Ren et al reported that remote postconditioning reduces brain injury in an adult rat model of focal ischemia.4 The concept of remote postconditoning makes this treatment strategy against tissue ischemic damage clinically more feasible, because the ischemic conditioning stimulus can be performed at a remote site that is easily accessible and relatively resistant to ischemia. In the brain, remote preconditioning has been shown to provide neuroprotection in contexts of both focal and global ischemia.5,6 However, it is currently unknown whether remote ischemic postconditioning confers neuroprotection after neonatal hypoxia–ischemia (HI). The protective mechanisms underlying remote postconditioning have not been fully elucidated. Emerging evidence indicates that it may share common mechanistic signaling pathways with the conventional ischemic preconditioning, postconditioning, and remote preconditioning, including the intraorgan/interorgan transfer of protective factors (opioid, etc) and receptor stimulation,7 activation of prosurvival kinases (phosphatidylinositol-3-kinase [PI3K]/Akt, ERK),3 etc. The activation of opioid receptors has been shown to reduce brain injury and neurological deficits in focal ischemia.8 Opioid receptors are G-protein-coupled receptors and can activate PI3K/Akt on releasing the Gβγ subunit.9 Moreover, experimental studies have established that activation of the PI3K/Akt pathway contributes to postconditioning-mediated neuroprotection.3
Thus, the aim of the present study was to determine the effects of remote limb ischemic postconditioning in a rat neonatal HI model. We hypothesize that remote limb ischemic postconditioning exerts neuroprotection at a distance by activating the opioid receptor/PI3K/Akt signaling pathway.
Materials and Methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University. The neonatal HI model was performed in postnatal Day 10 Sprague-Dawley rat pups (Harlan Laboratories, Indianapolis, IN). Rat pups of both genders underwent right common carotid artery ligation under isoflurane anesthesia. Surgery time for each pup did not exceed 5 minutes. After recovery for 1 hour, pups were placed in a hypoxia chamber, which was submerged in a 37°C water bath, and subjected to 8%O2 in N2 for 2 hours. Sham-operated animals underwent anesthesia and neck incision only and did not receive vehicle (solvent for wortmannin) intracerebroventricularly. One hundred fifty-eight postnatal Day 10 rat pups were used in this study and randomly divided into the following groups: sham-operated (n=20); HI group (n=38); HI groups treated with limb ischemic postconditioning (PostC; n=37), naloxone+PostC (n=16), wortmannin+PostC (n=10), morphine (n=16), and wortmannin+morphine (n=8); HI group treated with naloxone alone (n=8); and HI group treated with wortmannin alone (n=5).
Limb Ischemic Postconditioning Treatment and Pharmacological Interventions
In the treatment group, limb ischemic postconditioning was induced immediately after hypoxia by 4 10-minute cycles of hind limb ischemia and reperfusion in awake rat pups with no restraint. The proximal hind limbs of each pup were encircled with rubber bands and their ends were threaded through a small rubber tube to form a reversible snare for hind limb ischemia. Reversible hind limb ischemia was induced by making the snare as tight as possible using a hemostatic forceps for 10 minutes followed by loosening the snare for 10 minutes. Four cycles of hind limb ischemia/reperfusion were performed.
For pharmacological interventions, the nonselective opioid antagonist naloxone (10 mg/kg in saline, intraperitoneally; naloxone+HI+PostC group)7 was given immediately before and after the hypoxia. The PI3K inhibitor wortmannin (86 ng/pup in dimethyl sulfoxide and phosphate-buffered saline, intracerebroventricularly; wortmannin [WM]+HI+PostC group and WM+HI+morphine group)10 was administered just before the HI surgery. The nonselective opioid receptor agonist morphine (2 mg/kg in saline, subcutaneously; HI+morphine group and WM+HI+morphine group)8 was given immediately after the hypoxia.
Infarct Volume and Brain Tissue Loss Quantification
Infarct volume was determined 48 hours after HI with 2,3,5-triphenyltetrazolium chloride monohydrate staining and was analyzed by Image J software (Version 1.40; National Institutes of Health, Bethesda, MD).10 Brain atrophy was evaluated at 1 week and 4 weeks after HI and was expressed as the mass ratio of the ipsilateral hemisphere compared with the contralateral hemisphere, as previously described.10
Neurobehavioral Tests
The following neurobehavioral tests were performed in a blinded setup at 4 weeks after HI.
In the modified grip-traction test, muscle strength of the rat was evaluated by hanging the rat to a horizontal rope by its forepaws.10 Time to falling (maximum 60 seconds) was recorded.
The forelimb placement test and the back pressure test were used to test the sensorimotor response, as previously described,11 and were scored as following: 0 for immediate and correct paw placing; 1 for delayed and/or incomplete placement; and 2 for no placement. Scores corresponded to raw value: 0 score=100; 1 score=50; 2 score=0.
Western Blotting
At 24 and 48 hours after HI, the brain samples were collected (n=5 to 6 in each group). Proteins of the ipsilateral hemisphere were extracted by homogenizing in RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA). Western blotting was performed as described previously10 using antiphospho-Akt (Ser473), anti-Bax, and antiphospho-p44/42 MAPK (pERK1/2) antibodies (Cell Signaling Technology, Danvers, MA).
Statistical Analysis
Data were expressed as the mean±SEM. Statistical differences among groups were analyzed by using 1-way analysis of variance followed by the Holm-Sidak method. P<0.05 was considered statistically significant.
Results
Limb ischemic postconditioning-induced reduction in infarct volume requires activation of opioid receptor/PI3K/Akt signaling pathway and morphine mimics a postconditioning effect.
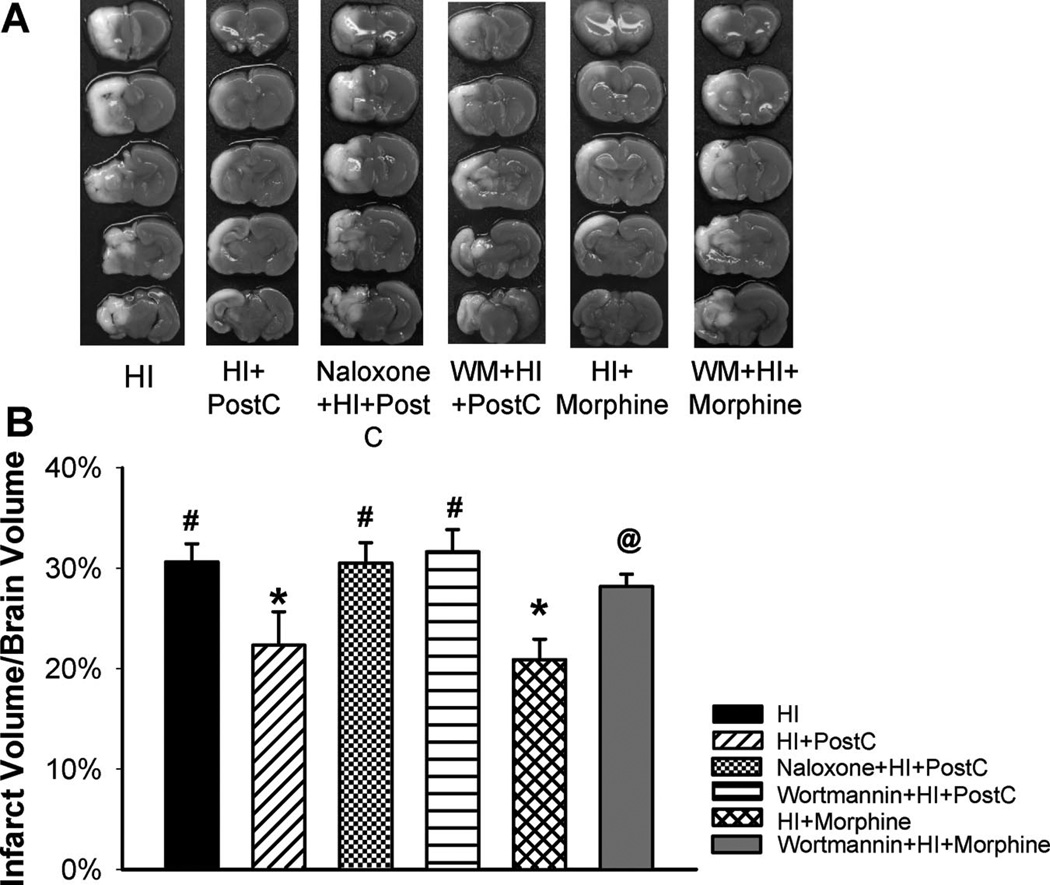
Figure 1 demonstrated that limb ischemic postconditioning significantly reduced infarct volume compared with the HI group (22.3%±3.3% and 30.6%±1.8%, respectively; Figure 1) at 48 hours after neonatal HI brain injury. To test if the stimulation of opioid receptor/PI3K/Akt signaling pathway is crucial to limb ischemic postconditioning-mediated reduction in infarct volume, we used pharmacological interventions and pretreated pups with the nonselective opioid antagonist naloxone or the PI3K inhibitor WM. Administration of naloxone or WM abolished the protective effect by limb ischemic postconditioning (30.5%±2.0% and 31.6%±2.2%, respectively; Figure 1). Naloxone or WM did not affect the infarct volume when administered alone to the HI pups (data not shown).
Figure 1.
Representative pictures of 2,3,5-triphenyltetrazolium chloride monohydrate-stained coronal brain slices from each group (A) and quantitative analysis of infarct volume (B) in the HI group and groups treated with limb ischemic postconditoning (HI+PostC), naloxone+postconditioning (naloxone+HI+PostC), wortmannin+postconditioning (WM+HI+PostC), morphine (HI+morphine), and wortmannin+morphine (WM+HI+morphine) at 48 hours after HI; n=8 to 10 in each group. Limb ischemic postconditioning and morphine treatment resulted in significant reduction in infarct volume, respectively. Pretreatment with naloxone or wortmannin abolished the reduction in infarct volume induced by postconditioning. Wortmannin also blocked morphineinduced infarct sparing effect. Values are the mean±SEM; *P<0.05 versus HI group; #P<0.05 versus postconditioning-treated group; @P<0.05 versus morphine-treated group.
As a pharmacological mimic for limb ischemic postconditioning-mediated opioid receptor activation, we administrated morphine, a nonselective opioid agonist, immediately after HI. Morphine treatment resulted in a significant reduction in infarct volume compared with the HI group (Figure 1). WM also abrogated this protection. Taken together, these findings indicate that the limb ischemic postconditioning-induced activation of the opioid receptor/PI3K/Akt signaling pathway mediates the neuroprotective effect after HI.
Limb Ischemic Postconditioning Improved Neurological Outcomes After HI
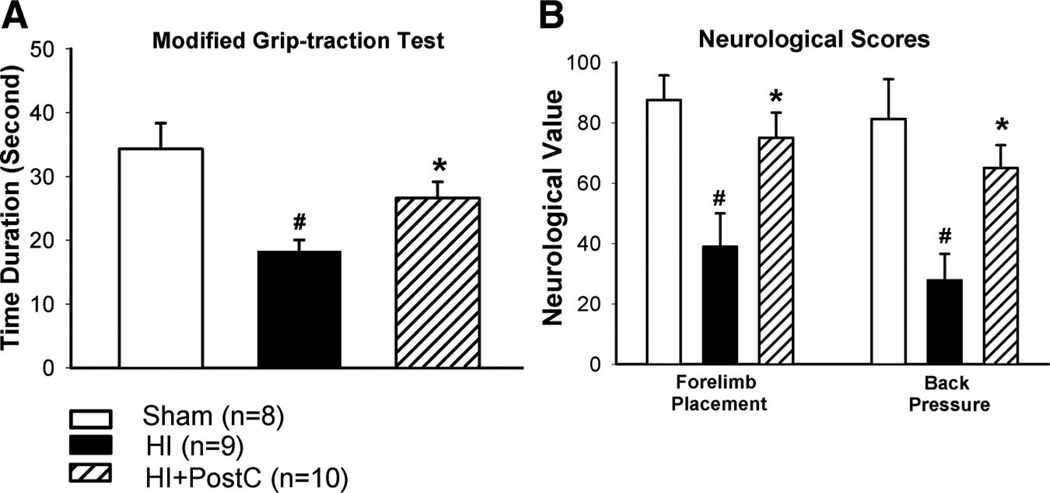
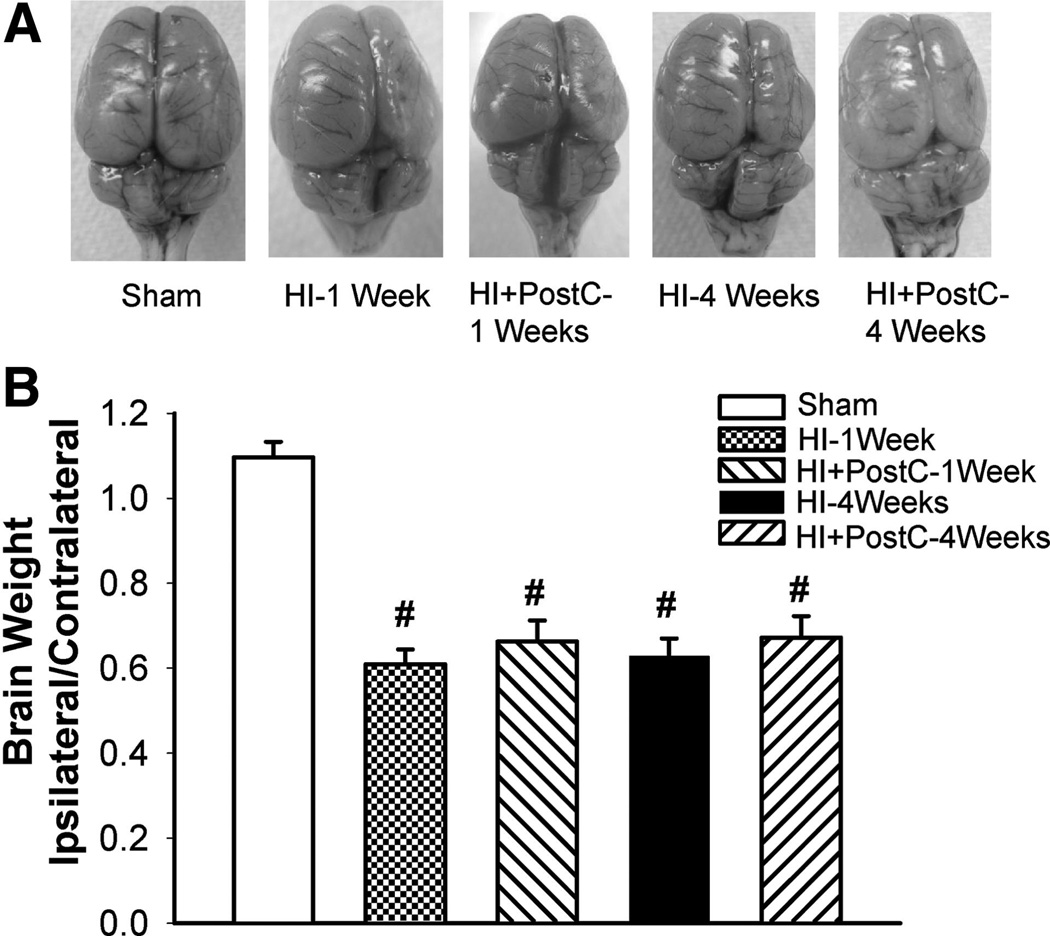
Limb ischemic postconditioning reduced infarct volume after neonatal HI brain injury; we next sought to determine whether limb ischemic postconditioning also improved neurological outcomes. Four weeks after HI, rats in shamoperated, HI, and postconditioning groups were evaluated in the modified grip-traction test, forelimb placement test, and back pressure test. In the modified grip-traction test, rats in the postconditioning group hung significantly longer to the rope than those in the HI group (Figure 2A). In the forelimb placement and back pressure tests, rats in the HI group demonstrated a significantly worse performance, whereas limb ischemic postconditioning significantly improved the sensorimotor response in these tests (Figure 2B). At 1 week and 4 weeks after HI, no significant differences of brain tissue loss were observed between the postconditioning group and HI group (Figure 3).
Figure 2.
Evaluation of neurological outcomes in the modified grip-traction test (A), forelimb placement test and back pressure test (B) in groups of sham operation (sham), HI or treated with limb ischemic postconditoning (HI+PostC) at 4 weeks after HI; n=8 to 10 in each group. Animals in the HI group exhibited severe neurological deficits. Limb ischemic postconditioning significantly improved neurological outcomes. Values are the mean±SEM; *P<0.05 versus HI group; #P<0.05 versus sham-operated group.
Figure 3.
Representative pictures (A) and quantification of brain tissue loss (B) in groups of sham operation (sham), HI or treated with limb ischemic postconditoning (HI+PostC) at 1 week and 4 weeks after HI; n=7 to 10 in each group. Limb ischemic post-treatment did not reduce brain tissue loss. Values are the mean±SEM; #P<0.05 versus sham-operated group.
Limb Ischemic Postconditioning Induces Akt Activation by Stimulating Opioid Receptors
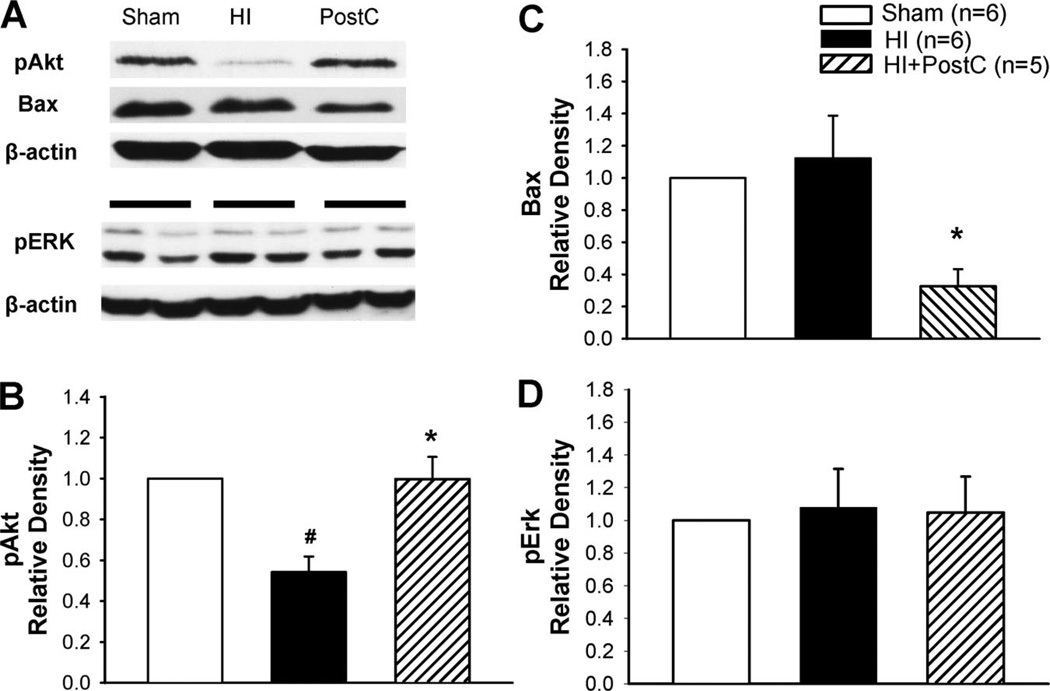
To examine whether Akt/Bax and ERK underlie limb ischemic postconditioning-mediated neuroprotection in neonatal HI, we measured phosphorylated Akt, Bax, and pERK in the ipsilateral hemisphere at 24 hours and 48 hours after HI (Figures 4 and 5). Although phosphorylation of Akt significantly decreased in the HI group at both 24 and 48 hours, the Akt activity was restored in the postconditioning group (Figures 4A–B and 5). On the other hand, limb ischemic postconditioning significantly reduced the Bax expression at 24 hours compared with the HI group (Figures 4A and 4C). No significant differences of pERK expression were detected among the sham-operated, HI, and limb ischemic postconditioning groups at 24 hours after HI (Figures 4A and 4D).
Figure 4.
Representative Western blots (A) and quantitative analysis of pAkt (B), Bax (C), and pERK (D) expression in groups of sham operation (sham), HI or treated with limb ischemic postconditoning (HI+PostC); n=5 to 6 in each group at 24 hours after HI. The HI brain injury induced decrease in pAkt levels. Limb ischemic postconditioning resulted in a recovery of pAkt expression and reduction in Bax expression (A–C). No significant differences of pERK expression were observed among these groups (D). Values are the mean±SEM; *P<0.05 versus HI group; #P<0.05 versus sham-operated group.
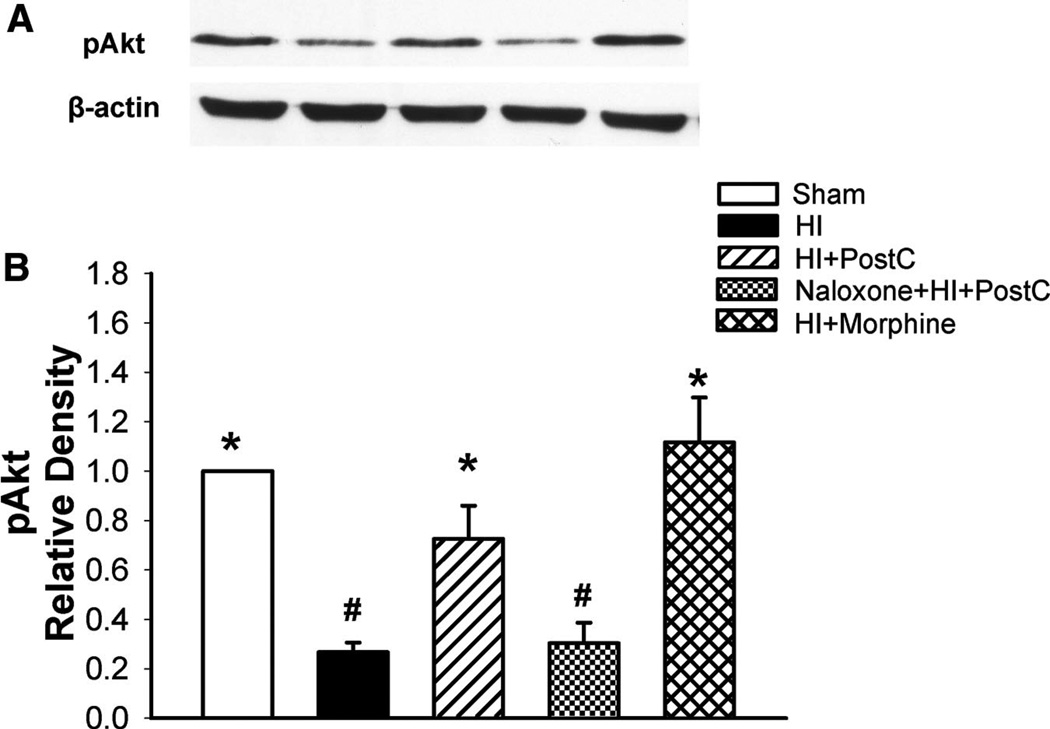
Figure 5.
Representative Western blots (A) and quantitative analysis of pAkt (B) expression in groups of sham operation (sham), HI or treated with limb ischemic postconditoning (HI+PostC), naloxone+postconditioning (naloxone+HI+PostC), and morphine (HI+morphine); n=6 in each group at 48 hours after HI. The pAkt expression remained decreased in the HI group at 48 hours after HI. Limb ischemic postconditioning and morphine treatment restored the Akt activity. Naloxone blocked this postconditioning-mediated effect. Values are the mean±SEM; *P<0.05 versus HI group; #P<0.05 versus sham-operated group.
To investigate whether the stimulatory effect of limb ischemic postconditioning on Akt is an opioid receptor-mediated event, we pretreated pups with the nonselective opioid receptor antagonist naloxone. Figure 5 shows that naloxone completely blocked the stimulatory effects of remote postconditioning on Akt phosphorylation. Furthermore, morphine, a nonselective opioid receptor agonist, used as a pharmacological mimic for the limb ischemic postconditioning, also increased the expression levels of phosphorylated Akt (Figure 5), accompanying its neuroprotective effect in the same neonatal HI model (Figure 1). Taken together, these results suggest that limb ischemic postconditioning-mediated Akt activation depends on the opioid receptor stimulation.
Discussion
The present study demonstrated that remote limb ischemic postconditioning reduced infarct volume in the short term and improved neurological outcomes in the long term in a rat model of neonatal HI. Blockage of opioid receptor with naloxone or inhibiting PI3K by WM abolished the infarct reduction provided by limb ischemic postconditioning. Given as a pharmacological mimic for limb ischemic postconditioning, morphine, the nonselective opioid receptor agonist, also provided the infarct sparing effect after HI. Furthermore, the remote postconditioning-induced neuroprotection has been shown to be associated with activation of Akt and deactivation of Bax.
Ischemic postconditioning has been proven to confer neuroprotection in experimental cerebral ischemia.3,12,13 Wang et al reported that ischemic postconditioning reduced neuronal death and neurobehavioral deficit after global ischemia.12 Experimental data from Pignataro et al showed that postconditioning reduced infarct volume after focal ischemia and attenuated neuronal death in cortical neuronal cultures, and Akt appeared to mediate this postconditioning-induced neuroprotection.13 We expand further on this concept by applying postconditioning at a distance (hind limbs) in a model of neonatal HI and showed that the remote ischemic postconditioning significantly reduced the infarct volume after HI. These results are consistent with those of Ren et al, reporting that remote postconditioning diminished brain injury after focal ischemia.4 For the underlying molecular mechanisms, both humoral and neurogenic pathways have been suggested to contribute to the transfer of remote protection from 1 distant organ to the other.4,7 Ren et al showed that remote postconditioning-mediated neuroprotection depends on neurogenic pathway and modulation of protein synthesis.4 Research in the setting of myocardial infarction implicates that release of mediators such as opioids, etc, by remote preconditioning and postconditioning may account for their cardioprotective effects.7 In addition to the brain, endogenous opioids have been shown to be expressed in other organ systems such as skeletal muscle, myocardium, intestine, etc.14 Moreover, the skeletal muscle has been shown to be capable of releasing opioids.14 On the other hand, the brain contains opioid receptors that can induce neuroprotection against ischemic brain injury on its stimulation.8 Presently, we showed that naloxone, a nonselective opioid receptor antagonist, blocked the postconditioning-mediated infarct reduction. In the Western blot assay, we found (Figure 5) that the prosurvival phosphorylated Akt (pAkt) expression decreased at 48 hours after HI alone. Although limb ischemic postconditioning and morphine treatment (as a remote postconditioning mimic) restored pAkt level, naloxone blocked this effect, which confirms our data gained by stroke volume evaluation. We proposed that limb ischemic postconditioning confers neuroprotection through releasing endogenous opioids from the skeletal muscle (hind limbs), which reach the brain through the circulation and subsequently activate opioid receptor leading to remote protection. Furthermore, we showed that morphine significantly reduced infarct volume after HI, accompanying the restoration of pAkt. Taken together, our findings suggest that endogenous opioids are the protective mediators of remote postconditioning, which are likely released through the ischemic conditioning stimuli in skeletal muscle (hind limbs).
In the literature, both pERK and pAkt have been implicated to be involved in postconditioning-induced protection.3 Cao et al demonstrated that opioids mediated cardioprotection by activating the PI3K/Akt and ERK pathway.15 In our study, we showed that neonatal HI resulted in a significant decrease in pAkt levels; however, no change in pERK was observed at 24 hours after HI, which is consistent with the findings of van den Tweel et al.16 In their study, the authors found no changes in pERK levels at 24 and 48 hours after neonatal HI. Thus, we did not examine pERK at 48 hours and believe that at 48 hours after HI, there is no difference in pERK expression between the sham-operated group and HI group. However, at 48 hours, we examined the pAkt expression under pharmacological interventions to further verify role of PI3K/Akt in the postconditioning mechanism. Consequently, we showed that WM, a PI3K inhibitor, blocked both remote postconditioning-mediated and morphine-induced infarct sparing effects, indicating indicating that the remote postconditioning-mediated neuroprotection requires activation of the opioid receptor/PI3K/Akt signaling pathway. Intracellular downstream targets of PI3K/Akt include Bax, Bad, caspase 9, GSK-3β, etc.17 Presently, we focused on Bax. Akt phosphorylates Bax at Ser-184 and prevents its insertion into mitochondrial membranes and reduces its half-life, thus blocking the Bax-mediated proapoptotic pathway.17 We found that remote postconditioning restored pAkt expression and concurrently reduced Bax levels at 24 hours after HI indicating the involvement of the prosurvival PI3K/Akt/Bax cascade in the remote postconditoning-induced protection.
Of note, vehicle-only treatments were not included in the present experimental design. It is however unlikely that used solvents had significant effect on the investigated parameters. First, when WM or naloxone alone was used with their solvents, no effect on the infarction size was detected. Second, to our knowledge, no effect of single injection dimethyl sulfoxide or phosphate-buffered saline on opioid receptors or PI3K pathway has been ever reported. In fact, studies have shown that dimethyl sulfoxide fails to affect PI3K.18 Moreover, dimethyl sulfoxide has no analgesic activity by itself and is commonly used as a solvent for pharmacological agents binding to opioid receptors. Therefore, we believe that naloxone and WM abrogated the postconditioning-mediated infarct reduction unaffected by solvent effects.
Our results showed that remote postcondtioning reduced the infarct volume at 48 hours but failed to restore the brain weight in long-term study, suggesting that the neonatal HI brain injury is a lasting process, which continues to cause cell death after 48 hours. However, postconditioning treatment significantly improved the neurological functional outcomes, consistent with previous data of animal studies suggesting that the recovery of neurological functions is not totally dependent on restoring the majority of brain tissue.19 Iwai et al reported that delayed erythropoietin treatment failed to reduce brain tissue loss but was capable of improving functional outcomes by promoting oligodendrogenesis and neuronal migration in neonatal HI.19 Thus, we speculated that limb ischemic postconditioning stimulates the protective opioid receptor/PI3K/Akt signaling pathway in the subacute phase of neonatal HI, which leads to subtle cellular effects or remodeling of survival brain tissue in the long term, thus resulting in improved neurological outcomes. In this regard, further studies are needed.
In the present study, we suggested that endogenous opioids was involved in remote postconditioning-induced neuroprotection. However, we did not test whether other humoral factors or neurogenic pathway play a role in transfer of remote protection. In regard to these issues, further studies are needed.
We conclude that limb ischemic postconditioning reduced infarct volume in the short term and promoted long-term functional recovery after neonatal HI brain injury. Activation of the prosurvival opioid receptor/PI3K/Akt signaling pathway plays a vital role in this remote ischemic postconditioning-mediated neuroprotection. Because remote limb ischemic postconditioning could be performed in clinical practice as a noninvasive simple maneuver, it may serve as an alternative effective treatment strategy combating neonatal HI brain injury.
Acknowledgments
Sources of Funding
This study is partially supported by National Institutes of Health (NIH) NSHD43120 and NIHNS54685 to J.H.Z. and NIH NS60936 to J.T.
Footnotes
Disclosure
None
References
- 1.Yellon DM, Hausenloy DJ. Realizing the clinical potential of ischemic preconditioning and postconditioning. Nat Clin Pract Cardiovasc Med. 2005;2:568–575. doi: 10.1038/ncpcardio0346. [DOI] [PubMed] [Google Scholar]
- 2.Gidday JM. Cerebral preconditioning and ischemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 3.Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29:873–885. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren C, Yan Z, Wei D, Gao X, Chen X, Zhao H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009;1288:88–94. doi: 10.1016/j.brainres.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151:1099–1103. doi: 10.1016/j.neuroscience.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin RL, Li WB, Li QJ, Zhang M, Xian XH, Sun XC, Zhao HG, Qi J. The role of extracellular signal-regulated kinases in the neuroprotection of limb ischemic preconditioning. Neurosci Res. 2006;55:65–73. doi: 10.1016/j.neures.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Patel HH, Moore J, Hsu AK, Gross GJ. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol. 2002;34:1317–1323. doi: 10.1006/jmcc.2002.2072. [DOI] [PubMed] [Google Scholar]
- 8.Zhao P, Huang Y, Zuo Z. Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J Neuropathol Exp Neurol. 2006;65:945–952. doi: 10.1097/01.jnen.0000235123.05677.4b. [DOI] [PubMed] [Google Scholar]
- 9.Tegeder I, Geisslinger G. Opioids as modulators of cell death and survival-unraveling mechanisms and revealing new indications. Phamacol Rev. 2004;56:351–369. doi: 10.1124/pr.56.3.2. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, Zhang JH. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by sphingosine 1-phosphate/phosphatidylinositol 3-kinase/Akt pathway. Stroke. 2010;41:1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Fathali N, Lekic T, Tang J, Zhang JH. Glibenclamide improves neurological function in neonatal hypoxia-ischemia in rats. Brain Res. 2009;1270:131–139. doi: 10.1016/j.brainres.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, Liang HW, Bruce IC, Luo BY, Xia Q. Ischemic postconditioning protects against global cerebral ischemia/reperfusion induced injury in rats. Stroke. 2008;39:983–990. doi: 10.1161/STROKEAHA.107.499079. [DOI] [PubMed] [Google Scholar]
- 13.Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Gala R, Simon RP. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008;28:232–241. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- 14.Denning GM, Ackermann LW, Barna TJ, Armstong JG, Stoll LL, Weintraub NL, Dickson EW. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissue. Peptides. 2008;29:83–92. doi: 10.1016/j.peptides.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Cao Z, Liu L, Van Winkle DM. Met5-enkephalin-induced cardioprotection occurs via transactivation of EGFR and activation of PI3K. Am J Physiol Heart Circ Physiol. 2005;288:H1955–H1964. doi: 10.1152/ajpheart.00256.2004. [DOI] [PubMed] [Google Scholar]
- 16.Van den Tweel ER, Kavelaars A, Lombardi MS, Nijboer CH, Groenendaal F, van Bel F, Heijnen CJ. Bilateral molecular changes in a neonatal rat model of unilateral hypoxic–ischemic brain damage. Pediatr Res. 2006;59:434–439. doi: 10.1203/01.pdr.0000200799.64038.19. [DOI] [PubMed] [Google Scholar]
- 17.Matheny RW, Jr, Adamo ML. Current perspectives on Akt Akt-ivation and Akt-ions. Exp Biol Med (Maywood) 2009;234:1264–1270. doi: 10.3181/0904-MR-138. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Su T, Li F, Li L, Qin X, Pan W, Feng F, Chen F, Liao D, Chen L. PI3K/Akt signaling transduction pathway is involved in rat vascular smooth muscle cell proliferation induced by apelin 13. Acta Biochim Biophys Sin (Shanghai) 2010;42:396–402. doi: 10.1093/abbs/gmq035. [DOI] [PubMed] [Google Scholar]
- 19.Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, Chen J, Cao G. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxia/ischemic brain injury. Stroke. 2010;41:1032–1037. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]