Abstract
Objectives
Few studies have employed effective connectivity (EC) to examine the functional integrity of neural circuitry supporting abnormal emotion processing in bipolar disorder (BD), a key feature of the illness. We used Granger Causality Mapping (GCM) to map EC between the prefrontal cortex (PFC) and bilateral amygdala and a novel paradigm to assess emotion processing in adults with BD.
Methods
Thirty-one remitted adults with BD [(remitted BD), mean age = 32 years], 21 adults with BD in a depressed episode [(depressed BD), mean age = 33 years], and 25 healthy control participants [(HC), mean age = 31 years] performed a block-design emotion processing task requiring color-labeling of a color flash superimposed on a task-irrelevant face morphing from neutral to emotional (happy, sad, angry, or fearful). GCM measured EC preceding (top-down) and following (bottom-up) activity between the PFC and the left and right amygdalae.
Results
Our findings indicated patterns of abnormally elevated bilateral amygdala activity in response to emerging fearful, sad, and angry facial expressions in remitted-BD subjects versus HC, and abnormally elevated right amygdala activity to emerging fearful faces in depressed-BD subjects versus HC. We also showed distinguishable patterns of abnormal EC between the amygdala and dorsomedial and ventrolateral PFC, especially to emerging happy and sad facial expressions in remitted-BD and depressed-BD subjects.
Discussion
EC measures of neural system level functioning can further understanding of neural mechanisms associated with abnormal emotion processing and regulation in BD. Our findings suggest major differences in recruitment of amygdala–PFC circuitry, supporting implicit emotion processing between remitted-BD and depressed-BD subjects, which may underlie changes from remission to depression in BD.
Keywords: amygdala, bipolar disorder, connectivity, effective, emotion, functional magnetic resonance imaging, Granger Causality Mapping, prefrontal cortex
Bipolar disorder (BD) is one of the most debilitating illnesses (1), characterized by severe emotion dysregulation that may persist during remission (2). Understanding pathophysiological mechanisms of emotion dysregulation, and how they predispose to depressed or manic episodes, can help identify biological targets for novel treatments. The particular difficulty in finding effective treatments for bipolar depression (3) underscores the critical need for better understanding of biological processes underlying depression in BD to inform development of new treatments for this phase of illness.
Neuroimaging studies of emotion processing in BD have focused on examining functional abnormalities in the amygdala and ventral prefrontal cortex (PFC)/orbitofrontal cortex (OFC) and medial PFC (mPFC), highly interconnected regions that are well known from animal and human lesion (4, 5) and human neuroimaging (2, 6) studies to support emotion processing and regulation. These studies reported abnormal amygdala and different OFC and mPFC regional activity in BD subjects to emotional stimuli (7–9) during both depression and remission.
A limitation of these studies in BD is that they did not examine how the amygdala and OFC and mPFC are functionally integrated during emotion processing. Functional integration can be characterized by functional connectivity (FC), referring to correlations over time between activities in different neural regions (10). One study reported decreased amygdala–ventrolateral prefrontal cortical FC in manic-BD patients versus healthy control participants (HC) during fearful and angry face matching and labeling (11). We showed abnormally elevated right amygdala–OFC FC to sad faces in BD subjects during depression (depressed BD) and remission (remitted BD) versus HC, suggesting that this measure may represent trait vulnerability for BD, but abnormally elevated left amygdala–OFC FC to sad and abnormally reduced bilateral amygdala–OFC FC to intense happy faces only in depressed-BD subjects, suggesting that these latter measures may represent bipolar depression state markers (12). Using effective connectivity (EC), referring to the impact that activity in one region exerts over that in another, and estimating forward versus backward EC between regions, we showed abnormally reduced left OFC–left amygdala and right amygdala–right OFC EC during happy face emotion labeling in the depressed-BD group (13) but did not include remitted-BD subjects. Thus, little understanding exists of the extent to which differential patterns of abnormal amygdala–OFC and mPFC EC during emotion processing may differentiate remitted and depressed BD.
As a first step toward understanding how abnormal functional integration between the amygdala and different prefrontal cortical regions during emotion processing differentiates depression and remission in BD, we examined amygdala activity, and amygdala–prefrontal cortical EC, to positive and negative emotional faces in remitted-BD and depressed-BD individuals versus HC. We employed a novel, implicit emotion face processing task in which task-irrelevant faces morph from neutral to emotional. To examine between-group differences in EC, we employed Granger Causality Mapping (GCM) (14), in which whole brain EC to and from a specific region of interest (ROI) is examined.
Previous findings in remitted-BD individuals indicate abnormally elevated amygdala activity to fearful and happy facial expressions (8, 15), but also an absence of abnormal amygdala activity to happy facial expressions (16–18). In depressed-BD patients, studies indicate abnormally elevated amygdala activity to sad faces (17). Thus, we first hypothesized abnormally elevated amygdala activity to emerging fearful faces in remitted-BD subjects, and to emerging sad faces in depressed-BD subjects, versus HC. Second, our previous FC data in individuals with remitted and depressed BD (12) allowed us to hypothesize that remitted-BD and depressed-BD patients versus HC would both show abnormal right-sided amydgala–prefrontal cortical EC to emerging sad faces, while individuals with depressed BD, but not remitted BD, would also show abnormal left-sided amygdala–prefrontal cortical EC to these faces and abnormal bilateral EC to emerging happy faces. We also wished to directly compare amygdala–prefrontal cortical EC to emerging happy and sad faces in remitted BD and depressed BD, a comparison not yet performed in any previous study. Our novel paradigm also allowed us to examine between-group differences in amygdala–prefrontal cortical EC to other negative emotional faces (fearful or angry).
Materials and methods
Participants
Fifty-two adults with bipolar I disorder (BD-I) [mean age = 32.82, standard deviation (SD) = 8.20; male/female = 12/40] diagnosed according to criteria of the DSM-IV and the Structured Clinical Interview for DSM-IV, Research Version (SCID-P) (19) participated in the study. Of those, 31 (remitted BD: mean age = 32.63, SD = 8.21; male/female = 10/21) were in remission for at least two months at the time of scanning with a Hamilton Depression Rating Scale (HDRS-25) (20) score ≤ 7 and a Young Mania Rating Scale (YMRS) (21) score ≤ 10. All had experienced at least two episodes of illness in the last four years. An additional 21 participants (depressed BD: mean age = 33.09, SD = 8.38; male/female = 2/19) were in a current depressed episode based on SCID-P criteria (at least two weeks of depressed mood). Some individuals with BD had comorbid disorders and most were medicated (see Table 1). The remitted-BD and depressed-BD groups did not differ significantly in age, age of illness onset, illness duration, lifetime prevalence of other disorders, or medication use, but did differ from each other on the HDRS-25 [t(50) = 9.12, p < 0.001] and YMRS [t(50) = 2.22, p < 0.05] (see Table 1). Twenty-five HC (mean age = 31.75, SD = 6.46; male/female = 11/14) with no previous personal or family history of psychiatric illness in first- and second-degree relatives participated in the study. Gender ratio did not significantly differ between HC and remitted-BD patients, but differed between HC and depressed-BD patients [χ2 (1) = 6.69, p = 0.01] and marginally differed between remitted- BD and depressed-BD patients [χ2 (1) = 3.65, p = 0.06]. All participants were right-handed, native English speakers, and gave written informed consent prior to participation.
Table 1.
Demographic and clinical variables and behavior performance
| Remitted BD (n = 31) | Depressed BD (n = 21) | HC (n = 25) | F/χ2 | |
|---|---|---|---|---|
| Age at scan, mean (SD) | 32.63 (8.21) | 33.09 (8.38) | 31.75 (6.46) | 0.18 |
| Gender, n (%) | ||||
| Male | 10 (32) | 2 (10) | 11 (44) | 6.62a |
| Female | 21 (68) | 19 (90) | 14 (56) | |
| Age of illness onset, mean (SD) | 18.03 (5.78) | 17.52 (6.01) | – | 0.09 |
| Illness duration, mean (SD) | 14.60 (7.37) | 15.56 (7.31) | – | 0.22 |
| HRSD-25 score, mean (SD) | 7.29 (5.53) | 24.62 (8.20) | – | 102.50a |
| YMRS score, mean (SD) | 2.39 (2.50) | 4.00 (2.72) | – | 14.64a |
| Lifetime presence of anxiety disorders, n (%) | 15 (48) | 14 (67) | – | 1.70 |
| Lifetime presence of somatoform disorders, n (%) | 0 (0) | 1 (5) | – | 1.51 |
| Lifetime presence of eating disorders, n (%) | 2 (6) | 3 (14) | – | 0.88 |
| Lifetime presence of alcohol/drug abuse or dependence, n (%) | 16 (52) | 9 (43) | – | 0.38 |
| Antidepressants, n (%) | 15 (48) | 8 (38) | – | 0.54 |
| Antipsychotics, n (%) | 16 (52) | 10 (48) | – | 0.08 |
| Mood stabilizers, n (%) | 23 (74) | 13 (62) | – | 0.89 |
| Benzodiazepines, n (%) | 5 (16) | 4 (19) | – | 0.08 |
| Percent emotional labeling accuracy for all faces, mean (SD) | 95 (0.08) | 93 (0.07) | 96 (0.03) | 0.86 |
| Reaction time during emotion labeling for all faces, msec, mean (SD) | 961.39 (139.27) | 989.68 (164.14) | 934.96 (102.66) | 0.92 |
Gender ratio did not significantly differ between the healthy control participants (HC) and the remitted-BD group, but differed between the HC and the depressed-BD group [χ2 (1) = 6.69, p = 0.01] and marginally differed between the remitted-BD group and the depressed-BD group [χ2 (1) = 3.65, p = 0.06]. Remitted BD = bipolar disorder patients in remission; depressed BD = bipolar disorder patients in a depressed episode; SD = standard deviation; HRSD-25 = 25-item Hamilton Rating Scale for Depression; YMRS = Young Mania Rating Scale.
p < 0.05.
Exclusion criteria for all participants included history of head injury (from medical records and participant report), systemic medical illness, cognitive impairment (score < 24 Mini-Mental State Examination; premorbid IQ estimate < 85 National Adult Reading Test), Axis-II borderline personality disorder, and general exclusion criteria for magnetic resonance imaging (MRI) (e.g., presence of metallic objects in the body). For HC, current or previous alcohol and illicit substance abuse (determined by SCID-I, and a saliva and urine screen) were further counted as exclusion criteria. Individuals with BD were excluded if they presented with rapid cycling BD-I (four or more episodes per year) or required immediate and intensive medical attention. BD participants also had to be free from alcohol or substance abuse or dependence for a minimum of two months. Of the 52 BD subjects in the study, 25 reported previous substance abuse or dependence. The mean number of months in remission was 99.72 (range 3–278).
Participants were recruited through local advertising. The participant population reflected the demographics of Pittsburgh, PA, USA and the surrounding area, and/or the patient population of the University of Pittsburgh Medical Center (UPMC). The study protocol was approved by the University of Pittsburgh Institutional Review Board.
Paradigm
Participants completed a 12.5-min emotional dynamic faces task during functional MRI (fMRI) scanning. Participants were asked to use one of three fingers to press a button indicating the color of a semi-transparent foreground color flash (orange, blue, or yellow) that appeared during the mid 200–650 msec of a one-sec presentation of a dynamically changing background face (neutral to emotional; see Fig. 1). These emotional faces were task-irrelevant and, thus, processed by the participants only implicitly. Faces from the Nim-Stim stimulus set (22) were morphed in 5% increments, from neutral (0% emotion) to 100% emotion for four emotions: happy, sad, angry, and fearful. Morphed faces were made into one-sec movies progressing from 0% to 100% emotion. In control trials, movies comprised a simple shape (dark oval) superimposed on a light gray oval, with similar structural characteristics to each face stimulus, which was subsequently morphed into a larger shape, approximating the movement shown by the morphed faces. There were three blocks for each of the above four types of emotion trial with 12 stimuli per block, and six control blocks with six stimuli per block. Emotional and control blocks were presented in a pseudorandomized order so that no two blocks of any condition were presented sequentially. Therefore, a total of 36 stimuli per condition was presented in the task. Our main dependent variables of interest were color labeling accuracy, reaction time, and activity and EC measures for all face emotion blocks.
Fig. 1.
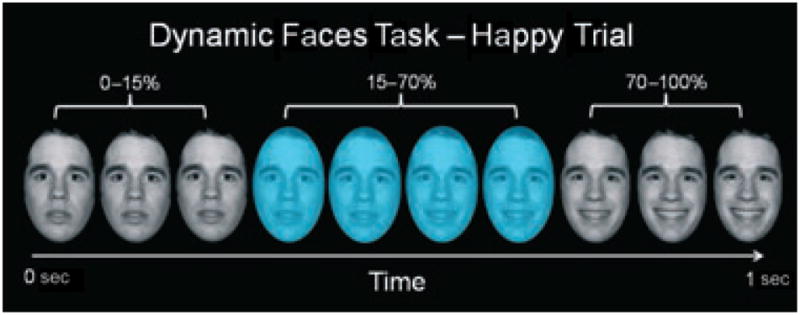
Graphic representation of a single happy trial of our emotional dynamic faces task. Over a one-sec duration, the face changed from neutral (0% emotion) to a happy, sad, angry, or fearful face (100% emotion). Participants were asked to identify the color flash presented in mid-dynamic change.
Data acquisition
Neuroimaging data were collected using a 3.0 Tesla Siemens Trio MRI scanner at the Magnetic Resonance Research Center (MRRC) at UPMC. Structural 3-D axial magnetized prepared rapid gradient echo (MPRAGE) images were acquired in the same session (TR/TE = 2200/3.29 msec, flip angle = 9°, FOV = 256 × 192 mm2, slice thickness = 1 mm, matrix = 256 × 256, 192 continuous slices). Blood oxygen level dependent (BOLD) images were then acquired with a gradient echo planar imaging (EPI) sequence during approximately 13 min (378 successive brain volumes) covering 39 axial slices (3.2 mm thick, TR/TE = 2000/28 msec/msec, FOV = 205 × 205 mm2, matrix = 64 × 64, flip angle = 90°).
Data analysis
Data were preprocessed and analyzed using Brain-Voyager QX2.2.1 (Brain Innovation, Maastricht, the Netherlands). Preprocessing included slice time correction (cubic spline interpolation), alignment of slices (cubic spline interpolation to the first non-discarded scan time), 3-D motion correction (trilinear interpolation), spatial smoothing (6-mm Gaussian kernel), linear trend removal, and temporal high-pass filtering (fast-Fourier transform based with a cutoff of three cycles/time course). The functional data sets were co-registered to the Talairach-transformed (23) T1-weighted anatomical image series to create a 4-D data representation. Participant movement was entered as a covariate of no interest at the individual subject level.
A multi-participant statistical analysis was performed by multiple linear regression of the time course of the BOLD response in each voxel in a priori ROIs and across the whole brain. The general linear model of the experiment was computed for each participant’s z-normalized volume time courses. Model predictors were defined by convolving an ideal boxcar response with a gamma-function model of the hemodynamic response (24). Boxcar values were equal to 1 during the emotion morph blocks and 0 during shape morph blocks.
ROI analyses to examine effects of individual emotion conditions between groups
First, we computed a 2 (condition: all faces, shape) × 3 (group: HC, remitted BD, depressed BD) ANOVA model to examine the main effects of condition and group and the group × condition interaction, as well as the main effect of condition in a specific a priori contrast of interest (all faces > shapes) in all participants. Then, to test our specific hypotheses, we entered all data into three separate random-effects 5 (condition: happy, sad, angry, fearful, and shapes) × 2 (group: HC versus remitted BD; or HC versus depressed BD; or remitted BD versus depressed BD) ANOVA models to examine specific pairwise between-group differences in activity to each of the five stimulus conditions. Analyses for all ANOVAs were focused on a single a priori ROI, by creating a structural mask encompassing the bilateral amygdala from the Analysis of Functional Neuroimages (National Institutes of Mental Health, Bethesda, MD, USA). Activation maps were visualized on a Talairach-transformed template brain, and displayed at a resolution of 1 mm3, and all p-values for all analyses were subjected to a multiplecomparison correction [FDR(q) < 0.05] (25). This procedure deals with the problem of multiple comparisons by automatically identifying a threshold for statistical significance that ensures that, on average, the proportion of false positives among the activated voxels will be less than q.
Effective connectivity analyses
We used GCM to determine the extent to which the BD groups displayed abnormal EC to and from the left and right structural amygdala seed regions (14). Using GCM, temporal information from the data is thus used to define the direction of influence, at the whole brain level, without establishing a model of assumed regional connectivity. Our GCM analysis was conducted at the individual level to generate a t-statistic image of the GCM map for each emotional condition. For each map, p-values were subjected to a multiple-comparison correction [FDR(q) < 0.01] at the whole brain level. Then these individual maps were compared across groups by computing a t-test for each emotional condition. This test was controlled at the p < 0.05 level across the whole brain and significant clusters of activation were limited to those >10 3 mm × 3 mm contiguous voxels. Note that there is some debate in the literature concerning the validity of GCM for measuring temporal precedence among functionally connected regions (26); that is, owing to interregional variation in timing of the hemodynamic response, GCM may determine temporal precedence between regions in which neuronal firing is instantaneously coupled. This limitation to the Granger method, however, is not relevant to the examination of differences in GCM maps between two or more experimental conditions employed in the current study.
Exploratory whole brain analyses
To examine specific pairwise, between-group differences in whole brain activity to each of the five stimulus conditions and to all emotional face conditions, we performed three separate random-effects 5 (condition: each emotion face condition and shape) × 2 (group: HC versus remitted BD; or HC versus depressed BD; or remitted BD versus depressed BD) ANOVA models.
Relationships between task performance and clinical variables and activity and EC
In all groups, relationships were examined between reaction time during color labeling in emotion blocks and extracted activity and EC values that differed between groups. The following demographic and clinical variables were selected for examination in remitted-BD and depressed-BD subjects: age, age of bipolar illness onset, bipolar illness duration, depression severity (HRSD-25), and mania severity (YMRS). Relationships between age and activity and EC values that differed between groups were examined in HC. We used Pearson correlational analyses. A problem for all neuroimaging studies of BD is the potential confounding effect of psychotropic medication, as it is difficult to recruit medication-free participants into such studies (27). Thus, activity and EC values that differed between groups, in those taking versus not taking each psychotropic medication class (mood stabilizer, antipsychotic, antidepressant, and anxiolytic medications), and for female subjects only in each group separately, were compared using independent t-tests. Due to the exploratory nature of these correlations we did not correct for multiple comparisons.
Results
Task performance
Color labeling accuracy and reaction times for emotion blocks were calculated based on individual subject task performance for an average of the four face conditions (faces) and each emotional face condition (fearful, angry, sad, and happy). Overall, task accuracy was high: 96%, 95%, and 93% face color labeling accuracy for HC, remitted BD, and depressed BD, respectively. There were no significant group differences in accuracy for faces, or any individual emotional face condition, nor were there any significant group differences in reaction time for faces or any individual emotional face condition (Table 1).
Activity
Our a priori contrast of interest (all faces>shapes) revealed significantly greater face-related activity in both right [peak: Talairach coordinates (TAL) x = 26, y = −8, z = −21; 2029 voxels] and left (peak: TAL x = −31, y = −9, z = −15; 852 voxels) amygdalae in all participants [t(304) ≥ 2.47, q < 0.05]. We then conducted amygdala ROI analyses using a 5 (condition) × 2 (group) ANOVA for two-group pairings across the three groups to test our specific hypotheses regarding group differences (HC versus remitted BD, HC versus depressed BD, and remitted BD versus depressed BD) first for amygdala activity to the a priori contrast of interest (all faces > shapes), and second for group differences in amygdala activity for each of the five different conditions (four different emotional face conditions and shapes). These analyses are described below.
Remitted BD versus HC
Based on an a priori contrast of interest (all faces > shapes), remitted-BD subjects displayed significantly greater activity to all face conditions than HC in both the right (peak: TAL x = 17, y = −5, z = −24; 1428 voxels) and left (peak: TAL x = −28, y = −5, z = −18; 800 voxels) amygdalae [t(220) ≥ 2.09, q < 0.05]. Subsequently, we compared differences between groups in activity for each emotional condition and shapes within right and left amygdalae. Here, remitted-BD subjects relative to HC showed significantly greater bilateral amygdala activity for sad [t(220) ≥ 2.36, q < 0.05], angry [t(220) ≥ 2.42, q < 0.05], and fearful [t(220) ≥ 2.43, q < 0.05] faces, but not for happy faces or shapes (Table 2).
Table 2.
Differences in activity and effective connectivity in remitted BD, depressed BD, and healthy control participants (HC)a
| Amygdala activity
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Remitted BD > HC
|
Depressed BD > HC
|
Remitted BD versus depressed BD
|
||||||||||
| Hemisphere | BA | Voxelb | t | Hemisphere | BA | Voxelb | t | Hemisphere | BA | Voxelb | t | |
| Happy | – | – | – | – | – | – | – | – | – | – | – | – |
| Sad | Left/right | – | 1328/800 | 2.4 | – | – | – | – | – | – | – | – |
| Angry | Left/right | – | 930/425 | 2.4 | – | – | – | – | – | – | – | – |
| Fearful | Left/right | – | 986/218 | 2.4 | Right | – | 1385 | 2.59 | – | – | – | – |
|
| ||||||||||||
|
Prefrontal cortex connectivity
| ||||||||||||
| Remitted BD versus HC
|
Depressed BD versus HC
|
Remitted BD versus depressed BD
|
||||||||||
| Hemispherec | BA | Voxelb | t | Hemispherec | BA | Voxelb | t | Hemisphered | BA | Voxelb | t | |
|
| ||||||||||||
| Right amygdala | ||||||||||||
| Happy | Right (+) | 9 | 411 | 2.0 | Right (+) | 47 | 26855 | 2.0 | Bilateral (+) | 47/45 | 9844 | 2.0 |
| Sad | Midline (+) | 9 | 789 | 2.0 | – | – | – | – | Midline (−) | 9 | 726 | 2.0 |
| Angry | – | – | – | – | – | – | – | – | – | – | – | – |
| Fearful | Left (+) | 10 | 327 | 2.0 | Midline (+) | 10/11 | 575 | 2.0 | – | – | – | – |
| Left amygdala | ||||||||||||
| Happy | Right (+) | 8 | 1512 | 2.0 | Right (+) | 10 | 1952 | 2.0 | Midline (+) | 10 | 779 | 2.0 |
| Sad | Midline (−) | 32 | 693 | 2.0 | – | – | – | – | Midline (+) | 9 | 3113 | 2.0 |
| Angry | Right (+) | 10 | 1261 | 2.0 | – | – | – | – | – | – | – | – |
| Fearful | Midline (−) | 32 | 4881 | 2.0 | Midline (−) | 32 | 441 | 2.0 | – | – | – | – |
Remitted BD = bipolar disorder patients in remission; depressed BD = bipolar disorder patients in a depressed episode; BA = Brodmann Area; (+) = bottom-up connectivity; (−) = top-down connectivity.
All between-group differences at p < 0.05, corrected.
Voxel = number of 1 mm × 1 mm voxels in cluster. Voxel size is 1 mm3.
Direction HC.
Direction remitted BD.
Depressed BD versus HC
Depressed-BD individuals displayed significantly greater activity to all face conditions than HC in both right (peak: TAL x = 20, y = −1, z = −21; 1655 voxels) and left (peak: TAL x = −25, y = −1, z = −21; 1785 voxels) amygdalae [t(180) ≥ 2.06, q < 0.05]. Comparing differences in activity between groups for each emotional condition within bilateral amygdalae revealed that depressed-BD patients showed significantly greater right amygdala activity to emerging fearful faces [t(180) ≥ 2.59, q < 0.05] versus HC, but there were no between-group differences in activity to emerging happy, sad, or angry faces, or shapes (Table 2).
Depressed BD versus remitted BD
There were no significant between-group differences in amygdala activity to any of the face conditions or to shapes (Table 2).
EC analysis
We chose the right and left structural amygdalae as our seed regions, given our a priori hypotheses regarding EC to and from the amygdala. For our GCM, we identified PFC regions in which activity reliably preceded (top-down) or followed (bottom-up) right and left amygdala ROIs. We then conducted one-way ANOVAs for each emotion condition for two-group pairings across the three groups (HC versus remitted BD; HC versus depressed BD; remitted BD versus depressed BD) to test our specific hypotheses regarding between-group differences in amygdala–PFC EC to the different emotional conditions. These analyses are described below (Figs. 2 and 3, Table 2).
Fig. 2.
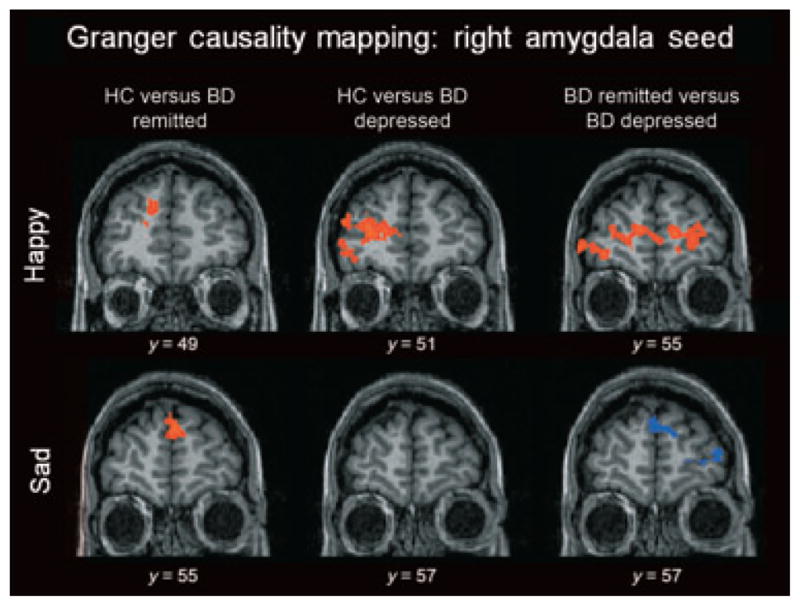
Areas of differential effective connectivity to and from the right amygdala seed for emerging happy and sad faces. Red indicates following connectivity to the right amygdala for group 1 and preceding connectivity for group 2. Blue indicates preceding connectivity to the right amygdala for group 1 and following connectivity for group 2. Here, healthy control participants (HC) displayed greater bottom-up connectivity from the amygdala to the prefrontal cortex for both bipolar disorder (BD) groups. BD patients in remission (remitted BD) displayed greater bottom-up connectivity than BD patients in a depressed episode (depressed BD) for the emerging happy condition but greater top-down connectivity for the emerging sad condition.
Fig. 3.
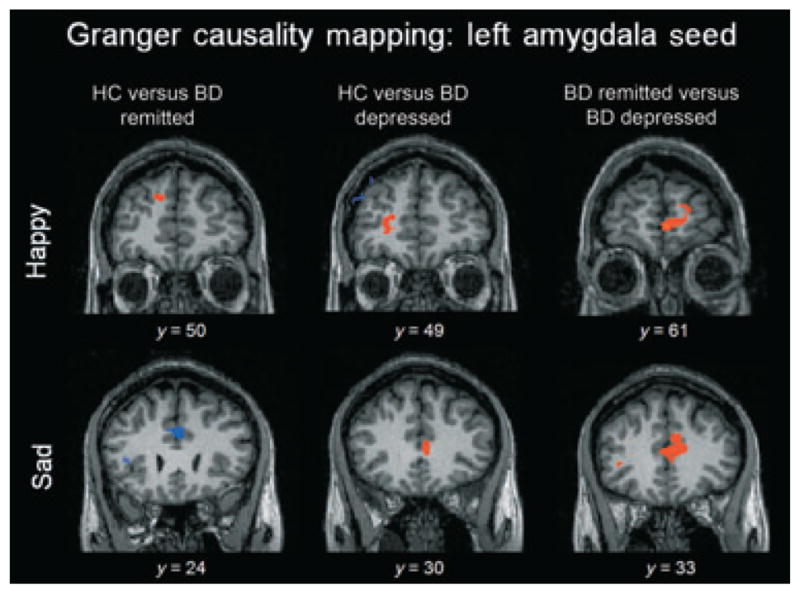
Areas of differential effective connectivity to and from the left amygdala seed for emerging happy and sad faces. Red indicates following connectivity to the right amygdala for group 1 and preceding connectivity for group 2. Blue indicates preceding connectivity to the right amygdala for group 1 and following connectivity for group 2. Here, bipolar disorder (BD) patients in a depressed episode (depressed BD) displayed greater top-down connectivity from the amygdala to the prefrontal cortex in comparison to both healthy control participants (HC) and BD patients in remission (remitted BD). The remitted-BD group displayed greater top-down connectivity than HC for the emerging happy condition but greater bottom-up connectivity for the emerging sad condition.
Remitted BD versus HC
Right amygdala seed
In the emerging happy condition, there was one area [peak: TAL x = 11, y = 49, z = 30; 411 voxels; Brodmann Area (BA) 9] in which EC to the right amygdala significantly differed between groups [t(54) = 2.01, p < 0.05]. For the emerging sad and fearful conditions, there was a single cluster in each analysis [(sad: peak: TAL x = −7, y = 58, z = 27; 789 voxels; BA 9) and (fearful: peak: TAL x = −28, y = 67, z = 6; 327 voxels; BA 10)] in which EC significantly differed between groups. For the emerging happy and sad conditions, activity in PFC regions followed activity in the right amygdala for HC but preceded activity in the right amygdala for remitted-BD patients. For the emerging fearful condition, activity in the PFC region preceded activity in the right amygdala for HC, but followed activity in the right amygdala for remitted-BD patients. There were no significant between-group differences in the PFC for the emerging angry condition.
Left amygdala seed
In the emerging happy (peak: TAL x = 32, y = 28, z = 36; 1512 voxels; BA 8) and emerging angry (peak: TAL x = 8, y = 61, z = 27; 1261 voxels; BA 10) conditions, there were PFC areas in which EC followed activity in the left amygdala for HC, but preceded activity in the left amygdala for remitted-BD individuals [t(54) = 2.01, p < 0.05]. In the emerging sad (peak: TAL x = −1, y = 25, z = 33; 693 voxels; BA 32) and emerging fearful (peak: TAL x = 5, y = 40, z = 9; 4,881 voxels; BA 32) conditions, there were PFC areas in which EC preceded activity in the left amygdala for HC, but followed activity in the left amygdala for remitted-BD individuals [t(54) = 2.01, p < 0.05].
Depressed BD versus HC
Right amygdala seed
In the emerging happy condition, one large area (peak: TAL x = 47, y = 4, z = −15; 26855 voxels; BA 47) within the PFC significantly differed between groups in EC with the right amygdala [t(41)=2.02, p < 0.05]. In this area, activity in the PFC followed activity in the amygdala for HC, but preceded it for the depressed-BD group. Two significantly differing regions of EC were found for the emerging fearful condition. In the first (peak: TAL x = −1, y = 61, z = 24; 344 voxels; BA 10), activity in the PFC preceded activity in the right amygdala for HC, but followed it for the depressed-BD group. In the second (peak: TAL x = −1, y = 28, z = −12; 575 voxels; BA 11), activity in the PFC followed activity in the right amygdala for HC, but preceded it for depressed-BD individuals. There were no differing areas of EC between groups within the PFC for the emerging sad or angry conditions.
Left amygdala seed
In the emerging happy (peak: TAL x = 20, y = 46, z = 15; 1952 voxels; BA 10) condition, there was one area within the PFC that significantly differed between groups in EC with the left amygdala [t(41) = 2.02, p < 0.05]. In this area, activity in the PFC followed activity in the left amygdala for HC, but preceded it for depressed-BD subjects. For the emerging fearful condition, there was an area within the PFC in which EC with the amygdala significantly differed between groups (peak: TAL x = 8, y = 43, z = 9; 441 voxels; BA 32). In this area, activity in the PFC preceded activity in the left amygdala for HC, but followed it for the depressed-BD group. There were no differing areas of EC within the PFC for the emerging sad or angry conditions.
Remitted BD versus depressed BD
Right amygdala seed
In the emerging happy (peak: TAL x = 26, y = 37, z = 6; 9844 voxels; BA 47/45) condition, there was one large area within the mPFC that significantly differed between groups in EC with the right amygdala [t(50) = 2.01, p < 0.05]. In this area, activity in the PFC followed activity in the right amygdala for remitted-BD subjects, but preceded it for depressed-BD subjects. In the emerging sad condition, there was also an area within the PFC in which EC with the amygdala differed significantly between groups (peak: TAL x = −1, y = 55, z = 24; 726 voxels; BA 9). In this area, however, activity in the PFC preceded activity in the right amygdala for remitted-BD subjects, but followed it for depressed-BD subjects. There were no areas of EC within the PFC that differed between groups for the emerging fearful or angry conditions.
Left amygdala seed
In both the emerging happy (peak: TAL x = −4, y = 64, z = 0; 779 voxels; BA 10) and emerging sad (peak: TAL x = −13, y = 37, z = 18; 3113 voxels; BA 9) conditions, there were areas within the PFC that significantly differed between groups in EC with the left amygdala [t(50) = 2.01, p < 0.05]. In both of these areas, activity in the PFC followed activity in the left amygdala for remitted-BD individuals, but preceded it for depressed-BD individuals. There were no areas of EC within the PFC that differed between groups for the emerging fearful or angry conditions.
Exploratory whole brain analyses
Using three separate 5 (condition: all emotional conditions and shapes) × 2 (group) GCMs, we compared whole brain activity in HC versus remitted BD, HC versus depressed BD, and remitted BD versus depressed BD to examine between-group differences to each of the five stimulus conditions and to all faces. For remitted BD versus HC for all faces, across the whole brain, only two significant clusters of activation were revealed, located in the right (peak: TAL x = 17, y = −2, z = −21; 407 voxels) and left (peak: TAL x = −28, y = −5, z = −18; 419 voxels) amygdalae [t(220 ≥ 4.01, q < 0.05]. Here, remitted BD showed significantly greater bilateral amygdala activity to all faces versus HC. No significant clusters of activation were found within the entire brain for the same interaction contrast for HC versus depressed BD or for remitted BD versus depressed BD.
Exploratory relationships between EC, task performance, age, sex, illness history variables, and on/off different medications
For the remitted-BD group, age was positively correlated with reaction time for emerging fearful [r(29) = 0.40, p = 0.03] and sad [r(29) = 0.41, p = 0.02] faces. Reaction time for emerging fearful faces was also positively correlated with illness duration [r(29)=0.40, p =0.03]. The use of antipsychotics was related to slower reaction times for emerging angry faces [t(29) = −2.10, p = 0.05]. The use of mood stabilizers was marginally significantly related to greater left amygdala activity for emerging sad faces [t(29) = −2.82, p = 0.06] and angry faces [t(29) = −2.99, p = 0.06], and greater right amygdala activity for emerging angry faces in comparison with HC [t(29) = −2.69, p = 0.06]. Age [r(29) = −0.36, p < 0.05] and illness duration [r(29) = −0.40, p < 0.05] were both negatively correlated with preceding EC from the PFC to the amygdalae to emerging happy faces in comparison to depressed BD. For female remitted-BD patients only, the use of mood stabilizers was related to lower preceding EC from the PFC to the left amygdala to sad faces [t(19) = −2.35, p = 0.03], where all remitted-BD patients had shown greater EC relative to HC, i.e., a normalizing effect of mood stabilizers in female remitted-BD patients upon the abnormally elevated left PFC–amygdala EC shown by remitted-BD patients as a group. (See Supplementary Tables 1 and 2 for more information.)
For the depressed-BD group, age was correlated with right amygdala activity for emerging angry faces in comparison to HC [r(19) = −0.57, p < 0.01]. The use of benzodiazepines was related to slower reactions times for all conditions [t(19)= −2.97–2.29, p < 0.05]. The use of antidepressants was related to greater preceding EC from the PFC to the left amygdala to emerging happy faces [t(19) = 2.50, p = 0.02], but only in comparison with remitted BD. For female depressed-BD patients only, the use of antidepressants was related to lower preceding EC from PFC (BA 11 region) to the right amygdala for emerging fearful faces [t(17) = 0.98, p = 0.02], where all depressed-BD patients had shown greater EC relative to HC, and lower preceding EC from PFC to the left amygdala for emerging happy [t(17) = 21.20, p < 0.001] and sad [t(17) = 7.96, p = 0.01] faces, where the depressed-BD group had shown greater EC relative to the remitted-BD group, i.e., a normalizing or attenuating effect, respectively, upon the pattern of elevated preceding EC from the PFC to the amygdala shown by depressed BD as a group relative to HC and remitted BD.
There were no relationships between any demographic or behavioral variables and activity or EC for HC.
Discussion
The goal of the present study was to examine functional integration between the bilateral amygdala and PFC during emotion processing in order to differentiate patterns of abnormal amygdala–PFC EC which may distinguish remitted BD from depressed BD. Our findings indicate patterns of abnormally elevated bilateral amygdala activity to emerging fearful, sad, and angry faces in remitted- BD subjects versus HC; and abnormally elevated right amygdala activity to emerging fearful faces in the depressed-BD group versus HC. We also show distinguishable patterns of abnormal EC between amygdala and dorsomedial and ventrolateral PFC, especially to emerging happy and sad faces in remitted-BD and depressed-BD individuals. In employing GCM, we build upon previous studies of amygdala–PFC EC in BD (11, 28–30) by not only investigating a directional relationship between interconnected neural regions, but by directly comparing amygdala–PFC EC in remitted BD and depressed BD.
In partial support of our first hypothesis, remitted- BD individuals displayed significantly greater bilateral amygdala activity than HC to all negative emotional conditions, which was paralleled by our finding in whole brain analyses of significantly greater bilateral amygdala activity in remitted-BD subjects relative to HC to all face conditions, while depressed-BD subjects displayed significantly greater right amygdala activity than HC only to the fearful condition. The presentation of emerging, rather than static, emotional faces in the present study may have contributed to the differences between the present findings and those of previous studies using static facial expressions (7–9, 15, 17, 31). Our findings do, however, add to the growing body of literature demonstrating significantly greater amygdala activity to predominantly negative emotional stimuli in BD relative to HC during depression (17, 32) and remission (8, 15, 33).
Both groups of individuals with BD relative to HC showed significantly greater bilateral PFC–amygdala preceding EC during the happy condition and significantly greater bilateral amygdala–PFC following EC during the fearful condition. Contrary to our second hypothesis, however, these findings suggest significantly greater top-down EC from the PFC to the amygdala during processing of emerging happy faces, but significantly greater bottom-up EC from the amygdala to the PFC during processing of fearful faces in remitted-BD and depressed-BD subjects. These findings may reflect greater regulation of amygdala to emerging positive emotional stimuli, but greater feedforward processing from the amygdala to the PFC during appraisal of emerging threat relative to HC in both the remitted and depressed phases of BD. Only the remitted-BD individuals showed significantly greater right PFC–amygdala preceding EC and significantly greater left amygdala–PFC following EC relative to HC during the sad condition, and significantly greater left PFC–amygdala preceding EC relative to HC during the angry condition. Only the depressed-BD individuals showed significantly greater right PFC–amygdala preceding EC relative to HC during the fearful condition. In the remitted-BD group, these findings indicate additional left- and right-sided abnormalities in EC between the amygdala and PFC during the angry and sad conditions, in turn suggesting abnormal top-down regulation of the amygdala to sad conditions in the right hemisphere, and abnormal top-down regulation of the amygdala to emerging angry and abnormal bottom-up processing of emerging sad stimuli in the left hemisphere. In depressed-BD subjects, these findings indicate abnormal top-down regulation of the amygdala to emerging fearful conditions, but no abnormal EC between the amygdala and PFC to emotional conditions other than emerging happy and fearful stimuli in the right hemisphere.
The direct contrast of EC between the amygdala and PFC between remitted BD and depressed BD revealed that patterns of abnormal EC between the amygdala and PFC during the happy and sad conditions best discriminated the two BD groups. Here, depressed-BD patients showed significantly greater bilateral PFC–amygdala preceding EC than remitted-BD patients during the happy condition, suggesting greater top-down regulation of the amygdala to emerging happy stimuli in depressed BD relative to remitted BD in both hemispheres. The remitted-BD group showed significantly greater right-sided PFC–amygdala preceding EC and significantly greater left-sided amygdala–PFC following EC than the depressed- BD group during the sad condition, suggesting significantly greater regulation of the amygdala to emerging sad faces in the right hemisphere and significantly greater feedforward processing and appraisal of emerging sad faces in the left hemisphere in remitted BD than depressed BD.
Our present findings of significantly greater abnormal bilateral PFC–amygdala top-down preceding EC in both BD groups (which were evident to a greater extent in depressed BD relative to remitted BD) during the happy condition are in contrast with our previous findings of abnormally reduced OFC–amygdala EC and FC to happy faces in depressed BD relative to HC (12, 29). Similarly, our present findings of abnormal patterns of bilateral EC between the amygdala and PFC during the sad condition only in remitted-BD, and not in depressed-BD subjects also contrast with our previous findings of abnormal right-sided EC between the amygdala and OFC to sad faces in both remitted-BD and depressed-BD subjects (12), but abnormal left-sided EC between the amygdala and OFC to sad faces only in the depressed-BD group (12). In the present study, the initial presentation of neutral faces that morphed into emotional faces, which is quite distracting, together with the employment of an implicit emotion processing task (color labeling) during a short, one-sec time period, may have made the present emotion processing task more difficult than our previous task, involving explicit emotion labeling of static facial expressions (12, 29). This switch to an emotional distracter in the face of a challenging motor/cognitive task, combined with an overall increase in task difficulty level, in turn may have been associated with greater recruitment of top-down prefrontal cortical–amygdala circuitry in BD relative to HC in order to direct attention away from emerging emotion and toward color labeling, as was the case during the happy condition. While the remitted-BD group also showed abnormal right PFC–amygdala top-down preceding EC during the sad condition, it is interesting that the depressed-BD group showed no abnormal EC between the amygdala and PFC in either hemisphere during this condition. This may reflect the absence of additional recruitment (relative to HC) of top-down PFC–amygdala circuitry to direct attention away from emerging sad emotion in depressed BD. The abnormal amygdala–PFC bottom-up following EC during the sad condition in remitted BD suggests abnormal feedforward processing of emerging sad faces in this group.
The majority of PFC regions observed to be differentially connected with the amygdala across groups were in ventromedial PFC (BA 11) and dorsomedial prefrontal cortical regions (BA 9, BA 10, and BA 32). These regions have been implicated in reward and in different components of self-related processing, respectively. BA 10 and BA 32, specifically, have been implicated in reflection on one’s own and others’ emotions (34). In the depressed-BD group only, however, the PFC region showing abnormal PFC–amygdala preceding top-down EC during the happy condition in the right hemisphere was in ventrolateral PFC (BA 47). Furthermore, the PFC region with which EC with the amygdala differed between the BD groups during the happy condition was also in the ventrolateral PFC (BA 45/47). These regions are implicated in reversal learning (35, 36) and in voluntary emotion regulation strategies, including reappraisal and redirection of attention away from emotional material (2). Our findings, therefore, suggest that the patterns of abnormal EC between the amygdala and medial prefrontal cortical regions observed in remitted BD in both hemispheres, in addition to reflecting abnormal regulation or feedforward processing and appraisal of emotion, may also reflect abnormal reward and self-referential processing in the context of implicit emotion processing. For the depressed-BD group, our findings also suggest abnormal reward and self-referential processing in the context of implicit emotion processing during fearful conditions in both hemispheres and during the happy condition in the left hemisphere. During the happy condition in the right hemisphere, however, our findings suggest that the depressed-BD group adopted an abnormal reappraisal strategy to regulate to emerging happy stimuli rather than showing abnormal self-referential processing of these stimuli.
Taken together, our findings point to distinct pathological changes in PFC–amygdala circuitry in both hemispheres—and in the right hemisphere in particular—during implicit processing of emotional faces in depressed BD relative to remitted BD. Consistent with this possibility are our previous findings of right-sided prefrontal white matter abnormalities in depressed BD (12, 37). The significance of the right hemisphere to understanding pathophysiological processes in bipolar depression is unclear, but given the putative role of the right hemisphere in withdrawal-related negative emotion processing (38, 39), it is possible that pathological changes in right prefrontal cortical regions supporting a combination of self-referential and negative emotion processing may underlie the switch to depression in bipolar illness. There have, however, been no studies to date to our knowledge that have examined amygdala–PFC EC in longitudinal studies of BD as mood state changes from remission to depression or vice versa. This should be a focus of future neuroimaging studies of BD.
There were limitations to the present study. The study was cross-sectional and therefore inferences about pathophysiological changes underlying the change from remission to depression in BD are provisional. Age and illness duration were related to longer reaction times in individuals with BD; however, for the fearful condition this was only true in the remitted-BD group and not in the depressed-BD group. Age and illness duration were also negatively correlated with preceding EC from the PFC to the amygdala in remitted-BD individuals, but in the left hemisphere this correlated only to the happy condition. All of the individuals with BD were taking psychotropic medication, as is typical of the majority of BD-I patients in neuroimaging studies because of the difficulty in coping without medication. There were, however, no consistent relationships between psychotropic medication and reaction time in remitted-BD or depressed-BD patients; benzodiazepine, antipsychotic, and mood-stabilizer medications were associated with slower reaction times, but only for some conditions and not consistently across both BD groups. Numerous previous neuroimaging studies have demonstrated that psychotropic medication is more likely to have a normalizing rather than confounding effect upon neuroimaging measures (27). The present study’s findings also indicate no consistent pattern of findings regarding relationships between psychotropic medication and activity and EC measures differing between groups. Specifically, our findings indicate that while mood stabilizers may have marginally contributed to the pattern of greater amygdala activity to negative emotional faces in the remitted-BD subjects relative to the HC, this was not shown consistently across both hemispheres and did not fully reach the conventional p < 0.05 level of significance. The pattern of abnormally elevated preceding EC from the PFC to the left amygdala was attenuated in female remitted-BD patients taking mood stabilizers. Additionally, while antidepressants may have contributed to the observed pattern of greater preceding EC from the PFC to the amygdala during the happy condition in depressed-BD versus remitted-BD patients, this was only in the left hemisphere. Furthermore, in the female depressed-BD group, antidepressants diminished the pattern of greater preceding EC to the right amygdala from the PFC relative to female HC for the fearful condition, and also diminished this pattern to the left amygdala for the happy and sad conditions in the female depressed-BD group relative to the female remitted-BD group. Antidepressants therefore attenuated patterns of EC shown by all depressed-BD subjects relative to all HC for the fearful condition, and by all depressed-BD subjects relative to all remitted-BD subjects for the happy condition. While both remitted-BD and depressed-BD subjects had comorbid anxiety and substance use, there were no significant relationships between these measures and activity and EC measures.
This study is among the first to examine amygdala to PFC EC in emotion processing neural systems during an implicit emotion processing paradigm in remitted-BD and depressed-BD patients, as well as HC. Our findings suggest that these activity and EC measures are useful indices of neural system level functioning that can help further understanding of neural mechanisms differentiating bipolar remission and depression. Future studies should aim to examine activity and EC in emotion processing neural circuitry in larger samples of BD subjects, including those who are unmedicated whenever possible. Furthermore, longitudinal studies would be ideal to better understand neural mechanisms of emotion processing that may provide insights into pathophysiological processes associated with the switch from bipolar remission to depression and help provide biological targets for future treatments for bipolar depression.
Supplementary Material
Acknowledgments
All work was carried out within the Department of Psychiatry, University of Pittsburgh, Pittsburgh, PA, USA and neuroimaging data was collected at the Magnetic Resonance Research Center (MRRC), University of Pittsburgh. We thank Dr. Fernando Boada and his staff for their help acquiring neuroimaging data and Dr. Jay Fournier for his statistical advice.
Research support was received from an R01 MH076971 grant to MLP; and K01 MH094467 and T32 MH18951 grants to SBP. AV received support from a NARSAD Young Investigator Award.
Footnotes
Disclosures
The authors of this paper report no biomedical financial interests or potential conflicts of interest in connection with this manuscript.
Additional supporting information may be found in the online version of this article:
Table S1. Exploratory relationships between amygdala activity and age, illness history, and medication.
Table S2. Exploratory relationships between effective connectivity and age, illness history, and medication.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (Other than missing material) should be directed to the corresponding author for the article.
References
- 1.Murray CJL, Lopez AD. Evidence-based health policy - Lessons from the global burden of disease study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 2.Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833– 857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieta E. Long-term treatment of bipolar depression and other issues. J Clin Psychiatry. 2010;71:e07. doi: 10.4088/JCP.8125tx4c. [DOI] [PubMed] [Google Scholar]
- 4.Bechara A, Tranel D, Damasio H, et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dannlowski U, Ohrmann P, Konrad C, et al. Reduced amygdala-prefrontal connectivity is associated with symptom severity in major depression. Pharmacopsychiatry. 2007;40:206. [Google Scholar]
- 7.Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacol. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumberg HP, Donegan NH, Sanislow C, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacol. 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 9.Altshuler L, Bookheimer S, Townsend J, et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RSJ. Functional topography: multidimensional scaling and functional connectivity in the brain. Cereb Cortex. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- 11.Foland LC, Altshuler LL, Bookheimer S, et al. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Versace A, Thompson WK, Zhou D, et al. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almeida JRC, Mechelli A, Hassel S, et al. Increased functional connectivity in the paralimbic-ventral prefrontal cortical system during emotion processing in bipolar disorder: a dynamic causal modelling approach. Biol Psychiatry. 2008;63:148S. [Google Scholar]
- 14.Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25:230–242. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Hassel S, Almeida JRC, Kerr N, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biol Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida JR, Versace A, Mechelli A, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M, Willians JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID, version 2.0) New York: Biometric Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 22.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talairach JTP. Co-planar stereotaxic atlas of the human brain: 3 dimensional proportional system: An approach to cerebral imaging. New York: Thieme Medical; 1988. [Google Scholar]
- 24.Friston KJ, Holmes AP, Worsley K, et al. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- 25.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 26.David O, Guillemain I, Saillet S, et al. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 2008;6:2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Kalmar JH, He Y, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida JR, Mechelli A, Hassel S, et al. Abnormally increased effective connectivity between parahippocampal gyrus and ventromedial prefrontal regions during emotion labeling in bipolar disorder. Psychiatry Res. 2009;174:195– 201. doi: 10.1016/j.pscychresns.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Versace A, Almeida JR, Hassel S, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Archiv Gen Psychiatry. 2008;65:1041– 1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord. 2007;103:29–42. doi: 10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Malhi GS, Lagopoulos J, Sachdev P, et al. Cognitive generation of affect in hypomania: an fMRI study. Bipolar Disord. 2004;6:271–285. doi: 10.1111/j.1399-5618.2004.00123.x. [DOI] [PubMed] [Google Scholar]
- 33.Malhi GS, Lagopoulos J, Owen AM, et al. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. J Affect Disord. 2007;97:109– 122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 35.Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12:142– 162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- 36.Budhani S, Marsh AA, Pine DS, Blair RJ. Neural correlates of response reversal: considering acquisition. Neuroimage. 2007;34:1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 37.Zanetti MV, Jackowski MP, Versace A, et al. State-dependent microstructural white matter changes in bipolar I depression. Eur Arch Psychiatry Clin Neurosci. 2009;259:316–328. doi: 10.1007/s00406-009-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JD. Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport. 1998;9:3233–3239. doi: 10.1097/00001756-199810050-00019. [DOI] [PubMed] [Google Scholar]
- 39.Sutton SK, Davidson RJ. Prefrontal brain electrical asymmetry predicts the evaluation of affective stimuli. Neuropsychologia. 2000;38:1723–1733. doi: 10.1016/s0028-3932(00)00076-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


