Abstract
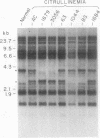
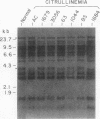
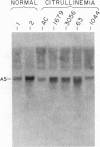
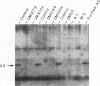
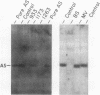
We have analyzed cultured skin fibroblasts derived from patients with argininosuccinate synthetase deficiency for alterations in gene structure, mRNA content, and protein structure. Genomic DNA was digested with the endonucleases EcoRI or HindIII, and the fragments were analyzed by Southern blotting and hybridization with a cDNA probe for argininosuccinate synthetase. The blot pattern is complex because there are at least 10 copies of argininosuccinate synthetase-like genes scattered over multiple human chromosomes. All nine patients studied showed patterns of DNA fragments that were indistinguishable from the normal control cell lines, and despite the possibility that the complexity could mask some changes, major deletions of the active gene(s) were not present. Blot hybridization of RNA indicated the presence of hybridizable mRNA of approximately normal size in seven of seven individuals examined with a suggestion of some heterogeneity. Analysis of enzyme antigen by protein transfer from NaDodSO4 containing polyacrylamide gels revealed considerable heterogeneity. This analysis revealed no cross-reacting material (CRM) in nine cell lines, CRM of normal molecular weight in one cell line, and CRM of reduced molecular weight in one cell line. These findings suggest that the genes for argininosuccinate synthetase in most citrullinemia patients are transcribed and produce stable mRNA. These mRNA either are not translated, or the translation product (enzyme) is rapidly degraded or is immunologically nonreactive. Defective gene expression in this disorder appears to involve abnormal mRNA, which may be altered by point mutations, frame shift mutations, deletions, insertions or particularly by abnormal RNA processing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batshaw M. L., Thomas G. H., Brusilow S. W. New approaches to the diagnosis and treatment of inborn errors or urea synthesis. Pediatrics. 1981 Aug;68(2):290–297. [PubMed] [Google Scholar]
- Beaudet A. L., Su T. S., O'Brien W. E., D'Eustachio P., Barker P. E., Ruddle F. H. Dispersion of argininosuccinate-synthetase-like human genes to multiple autosomes and the X chromosome. Cell. 1982 Aug;30(1):287–293. doi: 10.1016/0092-8674(82)90034-4. [DOI] [PubMed] [Google Scholar]
- Carritt B., Goldfarb P. S., Hooper M. L., Slack C. Chromosome assignment of a human gene for argininosuccinate synthetase expression in Chinese hamsterxhuman somatic cell hybrids. Exp Cell Res. 1977 Apr;106(1):71–78. doi: 10.1016/0014-4827(77)90242-7. [DOI] [PubMed] [Google Scholar]
- Christensen E., Brandt N. J., Philip J., Kennaway N. G. Citrullinaemia: the possibility of prenatal diagnosis. J Inherit Metab Dis. 1980;3(3):73–75. doi: 10.1007/BF02312528. [DOI] [PubMed] [Google Scholar]
- Daiger S. P., Wildin R. S., Su T. S. Sequences on the human Y chromosome homologous to the autosomal gene for argininosuccinate synthetase. Nature. 1982 Aug 12;298(5875):682–684. doi: 10.1038/298682a0. [DOI] [PubMed] [Google Scholar]
- FALLON H. J., SMITH L. H., GRAHAM J. B., BURNETT C. H. A GENETIC STUDY OF HEREDITARY OROTIC ACIDURIA. N Engl J Med. 1964 Apr 23;270:878–881. doi: 10.1056/NEJM196404232701705. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Orkin S. H., Hamer D. H. Abnormal RNA splicing causes one form of alpha thalassemia. Cell. 1982 Jul;29(3):895–902. doi: 10.1016/0092-8674(82)90451-2. [DOI] [PubMed] [Google Scholar]
- GAREN A., GAREN S. Complementation in vivo between structural mutants of alkaline phosphatase from E. coli. J Mol Biol. 1963 Jul;7:13–22. doi: 10.1016/s0022-2836(63)80015-7. [DOI] [PubMed] [Google Scholar]
- Gaull G., Sturman J. A., Schaffner F. Homocystinuria due to cystathionine synthase deficiency: enzymatic and ultrastructural studies. J Pediatr. 1974 Mar;84(3):381–390. doi: 10.1016/s0022-3476(74)80721-3. [DOI] [PubMed] [Google Scholar]
- Gillin F. D., Roufa D. J., Beaudet A. L., Caskey C. T. 8-Azaguanine resistance in mammalian cells. I. Hypoxanthine-guanine phosphoribosyltransferase. Genetics. 1972 Oct;72(2):239–252. doi: 10.1093/genetics/72.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. N., Levy R. I., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Adenine phosphoribosyltransferase deficiency: a previously undescribed genetic defect in man. J Clin Invest. 1968 Oct;47(10):2281–2289. doi: 10.1172/JCI105913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway N. G., Harwood P. J., Ramberg D. A., Koler R. D., Buist N. R. Citrullinemia: enzymatic evidence for genetic heterogeneity. Pediatr Res. 1975 Jun;9(6):554–558. doi: 10.1203/00006450-197506000-00008. [DOI] [PubMed] [Google Scholar]
- Ley T. J., Anagnou N. P., Pepe G., Nienhuis A. W. RNA processing errors in patients with beta-thalassemia. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4775–4779. doi: 10.1073/pnas.79.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda I., Anakura M., Arashima S., Saito Y., Oka Y. A variant form of citrullinemia. J Pediatr. 1976 May;88(5):824–826. doi: 10.1016/s0022-3476(76)81123-7. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E. Isolation and characterization of argininosuccinate synthetase from human liver. Biochemistry. 1979 Nov 27;18(24):5353–5356. doi: 10.1021/bi00591a015. [DOI] [PubMed] [Google Scholar]
- Saheki T., Ueda A., Hosoya M., Kusumi K., Takada S., Tsuda M., Katsunuma T. Qualitative and quantitative abnormalities of argininosuccinate synthetase in citrullinemia. Clin Chim Acta. 1981 Feb 5;109(3):325–335. doi: 10.1016/0009-8981(81)90318-1. [DOI] [PubMed] [Google Scholar]
- Su T. S., Beaudet A. L., O'Brien W. E. Increased translatable messenger ribonucleic acid for argininosuccinate synthetase in canavanine-resistant human cells. Biochemistry. 1981 May 12;20(10):2956–2960. doi: 10.1021/bi00513a037. [DOI] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., O'Brien W. E., Beaudet A. L. Cloning of cDNA for argininosuccinate synthetase mRNA and study of enzyme overproduction in a human cell line. J Biol Chem. 1981 Nov 25;256(22):11826–11831. [PubMed] [Google Scholar]
- Sundaram T. K., Fincham J. R. Nature of the complementation products formed by a complementing mutant of neurospora crassa. J Bacteriol. 1968 Mar;95(3):787–792. doi: 10.1128/jb.95.3.787-792.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco T. A., Mellman W. J. Argininosuccinate synthetase activity and citrulline metabolism in cells cultured from a citrullinemic subject. Proc Natl Acad Sci U S A. 1967 Mar;57(3):829–834. doi: 10.1073/pnas.57.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]