Abstract
Endocrine-disrupting chemicals (EDCs), including bisphenol A (BPA), are environmental ubiquitous pollutants and associated with a growing health concern. Anecdotally, molar incisor hypomineralization (MIH) is increasing concurrently with EDC-related conditions, which has led us to investigate the effect of BPA on amelogenesis. Rats were exposed daily to BPA from conception until day 30 or 100. At day 30, BPA-affected enamel exhibited hypomineralization similar to human MIH. Scanning electron microscopy and elemental analysis revealed an abnormal accumulation of organic material in erupted enamel. BPA-affected enamel had an abnormal accumulation of exogenous albumin in the maturation stage. Quantitative real-timePCR, Western blotting, and luciferase reporter assays revealed increased expression of enamelin but decreased expression of kallikrein 4 (protease essential for removing enamel proteins) via transcriptional regulation. Data suggest that BPA exerts its effects on amelogenesis by disrupting normal protein removal from the enamel matrix. Interestingly, in 100-day-old rats, erupting incisor enamel was normal, suggesting amelogenesis is only sensitive to MIH-causing agents during a specific time window during development (as reported for human MIH). The present work documents the first experimental model that replicates MIH and presents BPA as a potential causative agent of MIH. Because human enamel defects are irreversible, MIH may provide an easily accessible marker for reporting early EDC exposure in humans.
CME Accreditation Statement: This activity (“ASIP 2013 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2013 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
The environment has become increasingly contaminated by various pollutants. This contamination has led to an increase in the incidence and gravity of known conditions and/or the emergence of new conditions. Recently, the appearance of a distinct enamel condition was identified and called molar incisor hypomineralization (MIH) in recognition that it is most likely to be found affecting permanent first molars with frequent involvement of the permanent incisors.1,2 MIH is diagnosed in children at approximately 6 to 8 years of age and presents as random white opacities on the enamel of affected teeth. MIH prevalence is highly variable, with 2.4% to 40.2% (mean of approximately 18%) of children affected.3 To date, the cause of MIH remains unclear. However, given that MIH affects those teeth that are undergoing mineralization around the time of birth, it is clear that the enamel-forming ameloblasts are only sensitive to the causative agent(s) responsible for MIH in a specific time window. MIH is indicative of some adverse event(s) occurring during early childhood that effect enamel development.1,2 Diverse environmental conditions, such as medication (amoxicillin), hypoxia, hypocalcaemia, dioxins, polychlorinated biphenyls, and prolonged breastfeeding, have been associated with MIH.4 The vast number of putative causative agents and the difficulty in retrospectively linking a specific exposure during the developmental window when enamel is susceptible to MIH make epidemiologic studies inconclusive.
An increasing prevalence of numerous adverse health effects, such as diabetes, obesity, infertility, cancers, and autism, has been linked to endocrine-disrupting chemicals (EDCs).5,6 Bisphenol A (BPA) is a typical EDC widely used in the production of polycarbonate plastics and epoxy resins. Its widespread use in food packaging and environment is controversial and hotly debated. Despite health agency concerns and safety policies, >95% of the population is contaminated by BPA.7 BPA affects different organs and physiologic key functions, such as reproduction8 and sex determinism, brain development, and behavior.6,9 It may also increase breast cancer risk10 and lead to obesity.6,11,12 Although molecular mechanisms of action are still being researched, specific BPA-target genes associated with specific disease states have been identified in differentiated cell types in epidemiologic surveys.8,13 The effects of BPA on dental cells are unknown. Interestingly, sensitivity to BPA in humans is highest during the perinatal period.8,14 This period corresponds to the temporal window when the enamel of the permanent incisors and first molars is being formed. Thus, our hypothesis is that EDCs such as BPA may be involved in MIH by having an adverse effect on amelogenesis.
Amelogenesis begins with a secretory stage during which a partially mineralized enamel matrix is elaborated. The enamel matrix is composed of amelogenins, enamelin, ameloblastin, and amelotin, which are subject to extracellular processing by matrix metalloprotease 20. The matrix proteins are essential for the correct enamel formation as evidenced by the fact that mutations in any of the proteins involved leads to amelogenesis imperfecta.15 Once the full thickness of the enamel has been deposited, amelogenesis enters the maturation phase during which the serine protease kallikrein 4 (KLK4) degrades the enamel matrix proteins. Abnormal retention of proteins or indeed any extraneous proteins, such as serum albumin, lead to the eruption of hypomineralized enamel.16 The rodent incisor provides a running record of how an effect on amelogenesis affects future development as the incisor continues to erupt.17,18 Once amelogenesis is complete, the ameloblasts and the overlying outer enamel epithelium cells degenerate and are ultimately lost through abrasion after tooth eruption. As a consequence, enamel defects are irreversible and provide a permanent record of any disturbances that occur during enamel development. This record of previous disturbances allows retrospective studies to be performed and provides a means of temporally fixing a pathologic event at some point during development.17
The objective of the present study was to assess the possible effect of BPA on enamel development and elucidate any underlying mechanism of action. Rodents were exposed daily in utero and after birth to a low dose of BPA to mimic human exposure occurring during the critical fetal and suckling periods when the teeth are developing. Bona fide human MIH enamel was also compared with enamel from BPA-treated rat to investigate whether any structural features of MIH were replicated in the BPA-treated rat teeth.
Materials and Methods
Animals and Biological Samples
Eight-week-old Wistar Han rats were purchased (Harlan France Sarl, Gannat, France). All animals were maintained in accordance with the French Ministry of Agriculture guidelines for care and use of laboratory animals (B2 231010EA).
Cages and bottles were made of polypropylene to avoid any contamination by BPA or phthalates, and drinking water was filtered through charcoal to eliminate pesticides. Animals were fed a purified phytoestrogen-free diet consisting of 18% casein, 40% corn starch, 20% maltodextrin, 6% sucrose, 5% corn oil, 5% cellulose, 5% mineral mixture, and 1% vitamin mixture (INRA, Jouy en Josas, France) and provided with water ad libitum.
At gestational day 1, determined by the presence of an intravaginal sperm plug, the dams were randomly divided into two groups. From gestational day 1 until weaning day 21, one group of pregnant females was orally administered 5 μg/kg of BPA daily (Sigma-Aldrich, St. Louis, MO) in 0.5 mL of corn oil, whereas the control group was administered corn oil alone. After weaning, young rats were exposed daily to BPA as described until sacrifice at day 30 or day 100.
At each stage, 16 male control rats and 16 male treated rats were used in the present study. Half (n = 8) of each group were randomly selected, anesthetized by isoflurane inhalation, and perfused with 4% paraformaldehyde (Sigma-Aldrich) in PBS (1×, pH 7.4). Perfused right hemimandibles were analyzed by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX), whereas perfused left hemimandibles were prepared for histologic analysis.
The remaining rats (n = 8) in each group were sacrificed, and their mandibles were immediately dissected. The surrounding soft tissues were removed and the mandibular bone encasing the lower incisors was carefully taken off under a stereomicroscope (Leica M125; Leica, Paris, France) to expose the entire labial surface. The incisors were then extracted, and the cervical loops were removed. For right hemimandibles, rat enamel organ, now easily accessible, was carefully harvested in its entirety and put in the Tri-Reagent (Euromedex, Paris, France) for RNA extraction. For left hemimandibles, epithelial cells from the secretion stage and the maturation stage were separately dissected using the molar reference line for isolation18,19 and placed in Tri-Reagent (Euromedex) for RNA extraction. Secretory-stage enamel was then harvested from all mandibles using a scalpel20 for the sequential extraction of matrix proteins.
Teeth from MIH Patients
Patients were recruited in the Centre de Référence des Maladies Rares de la Face et de la Cavité Buccale MAFACE (Hôpital Rothschild, Paris, France). Inclusion and exclusion criteria were based on those established by the international consensus on MIH diagnosis.21 Positive diagnosis was based on the clinical singularity of enamel defects: random discolored patches, hypomineralized status assessed by probing, tooth-type selectivity, increased nociceptivity, and increased susceptibility to caries. Differential diagnosis with amelogenesis imperfecta, enamel fluorosis, and punctual inflammation–induced hypomineralization of permanent teeth was established by their distinct clinical features and familial and medical antecedent analysis. MIH and control teeth, obtained after elective extraction, were collected (n = 10) and immediately immersed in 4% paraformaldehyde in PBS buffer (pH 7.4) for 48 hours. Samples were then dehydrated using increasing concentrations of ethanol for 48 hours each and prepared for resin embedding as described previously.22
SEM-EDX Analysis
Human molars were sectioned parallel to the longitudinal axis of the tooth using cutting equipment (Exakt, Norderstedt, Germany). Sections were then polished with graded sandpaper to a thickness of 40 μm. Each section was bonded onto an aluminum pin stub using adhesive carbon disks. In the case of rat hemimandibles, the incisor cervical margin and the first molar furcation point were used as anatomical reference points so that specimens could be prepared consistently. Mandibles were cut transversely close to these anatomical reference points using a rotating diamond wheel and then ground back to the selected anatomical reference point with graded sandpaper. Samples were dehydrated in ethanol for 48 hours each at 4°C, stuck on a calibrated aluminum pin stub using clear polyester resin, and ultrasonicated for 15 minutes. Human and rat enamel surfaces were etched with 37% orthophosphoric acid for 30 and 15 seconds, respectively, to remove any smear layers. Each sample was coated with platinum 6 nm thick in a vacuum evaporator, and the enamel microstructure was observed with SEM (Carl Zeiss Supra 40; Carl Zeiss AG, Oberkochen, Germany) at 10 kV. After the initial observations, the samples were polished, etched, rinsed with 2.5% sodium hypochlorite for 2 minutes, and observed a second time after coating with platinum.
After SEM, the samples were analyzed by EDX using an X-ray detector system attached to an SEM (JSM-6100; Jeol, Tokyo, Japan) at 15 kV. For each specimen, 15 distinct points distributed within the enamel were analyzed to measure Ca and C content. Semiquantitative data were submitted to the ZAF correction method [atomic number effect correction (Z), absorption effect correction (A), and fluorescent excitation effect correction (F)].
IHC Assays
Left perfused hemimandibles were postfixed by immersion in 4% paraformaldehyde solution for 24 hours. After rinsing in PBS, hemimandibles were decalcified at 4°C in pH 7.4 PBS solution containing 4.13% EDTA (Sigma-Aldrich) and 0.2% paraformaldehyde for 2 months. The decalcification solution was changed twice weekly. After washing in PBS for 4 hours at 4°C, the samples were dehydrated in ethanol, rinsed in Safesolv (Labonord SASA, Templemars, France), and finally paraffin embedded (Paraplast Plus; Sigma-Aldrich). Serial frontal sections (8 μm thick) were cut using a microtome (RM 2145; Leica). Sections were deparaffinized and rehydrated in decreasing concentrations of ethanol. Endogenous peroxidases were blocked by incubation for 20 minutes in a freshly made solution of 3% H2O2 in PBS. Sections were then washed in PBS and blocked with 5% milk in PBS for 20 minutes at 4°C. Primary anti-amelogenin antibody (Kamya Biomedical Company, Seattle, WA) (1:300), anti-enamelin (1:300),23 anti-ameloblastin (M-300 sc-50534; Santa Cruz Biotechnology, Santa Cruz, CA) (1:300), or anti-albumin (M-140 sc-50536; Santa Cruz) (1:400) were applied for 1 hour at room temperature. Sections were incubated with Alexa Fluor 594 secondary antibody (A-11072; Life Technologies, Carlsbad, CA) (1:500) for 1 hour in the dark. After rinsing with PBS, section were immersed in DAPI (010M4003; Sigma-Aldrich) (1:100,000) for 1 minute. They were finally mounted with aquamount (13,800; Lerner Laboratories, Pittsburgh, PA). For albumin staining, sections were incubated for 30 minutes with Impress reagent (TM reagent ImmPRESS kit; Vector Laboratories, Burlingame, CA) containing secondary peroxidase-conjugated antibodies (1:5000) and immunocross-reactivity was visualized by adding peroxidase substrate (K3468; Dako, Carpinteria, CA). They were finally rinsed with water, dehydrated, and mounted with DePeX resin (BDH Laboratory, Poole, England).
Enamel Matrix Protein Extraction and Western Blot Analysis
Secretory-stage enamel matrix was sequentially extracted with 30 μL of 50 mmol/L Tris (pH 7.4) (to extract freely soluble proteins), then with 30 μL of 100 mmol/L PBS (pH 7.4) (to extract mineral bound proteins), and finally with 60 μL of 1% SDS (to extract remaining aggregated proteins). The use of phosphate buffer is akin to the elution buffer used in chromatography columns for protein adsorbed to hydroxyapatite. Phosphate buffer at 100 mmol/L is able to extract all mineral bound proteins from developing enamel.24,25
Twenty micrograms of the first (Tris) and third (SDS) fractions and 10 μg of the second fraction (PBS) extracted proteins were subjected to 12% SDS-PAGE. Gels were electrotransferred onto nitrocellulose membranes, which were subsequently blocked with 5% milk for 2 hours. Blocked membranes were incubated at 37°C for 90 minutes with anti-enamelin or anti-amelogenin polyclonal antibodies (1:500). The nitrocellulose membranes were washed and incubated for 45 minutes with a goat polyclonal anti–rabbit IgG antibody coupled to horseradish peroxidase (Sigma-Aldrich) diluted 1:2000. Immunoreactivity was visualized by chemiluminescence (ECL Western blotting detection system; Amersham Pharmacia Biotech, GE Healthcare Life Sciences, Velizy-Villacoublay, France) using a bioimager (ImageQuant LAS 4000; Uppsala, Sweden). Loading and electrotransfer efficiency were checked by staining membranes for total proteins with Ponceau red.
HAT-7 Rat Ameloblast-Like Cell Culture, Treatments, and Transfections
Rat HAT-7 ameloblastic cells26 were grown in Dulbecco’s modified Eagle’s medium/F-12 without phenol red supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and 50 U/mL of penicillin-streptomycin. HAT-7 cells were transfected with plasmids that contained the promoter regions of interest or the plasmids alone according to the manufacturer instructions (Qiagen, Courtaboeuf, France). Forty-eight hours after BPA treatments (Sigma-Aldrich), 5 × 106 cells were either collected for RNA extraction or lyzed for protein content determination and promoter activity assays. Briefly, protein content was determined using a bicinchoninic acid protein assay kit (Pierce, Paris, France) according to the manufacturer’s instructions, and luciferase activity was measured by mixing 25 μL of protein extract with 75 μL of luciferase assay substrate (Promega Corp., Madison, WI). Luciferase activity was measured with the B941 TriStar microplate reader (Berthold, Bruyères, France). The experiment was repeated four times independently.
Plasmid Constructions
Rat enamelin (−1468/+1 nt) and Klk4 (−1397/+1 nt) promoter regions were amplified using the Phusion high-fidelity Taq polymerase (ThermoScientific, Villebon-sur-Yvette, France) and the following primers: 5′-CCGGGTACCGGCTCACAGACTGAACCACC-3′ and 5′-TACACAGAACGAGGAACCGAGGAGCTCGCC-3′ for rat enamelin promoter and 5′-CCGGGTACCCTGAACTCCAGGGTCTCCCACTGG-3′ and 5′-CAGAAGTAAAGGTCCTCGGTTAGAGCTCGCC-3′ for rat Klk4 promoter. The purified fragments were cloned into PGL4.17 plasmid in front of luciferase reporter gene (Promega) by insertion in KpnI/XhoI (italicized) sites.
RNA Extraction and qPCR Analysis
Total RNA extraction was performed using Tri-Reagent (Euromedex) according to the manufacturer procedure. RNA concentration and purity were determined by a spectrophotometry at 260 nm (NanoDrop 1000; ThermoScientific). Reverse transcription was performed on 1 μg of total RNA for 45 minutes at 42°C, using a primer mix oligodT and random primers according to the manufacturer instructions (Superscript II; Invitrogen). Quantitative real-time PCR (qPCR) was performed using Opticon Monitor device (Bio-Rad Laboratories, Hercules, CA). Each PCR was repeated in triplicate independently, and the results were normalized against Gapdh and Rs15. Details of the primers and the corresponding amplicon sizes are presented in Table 1. Results were calculated by the method of standard curves. Similar data were obtained when ΔΔCt method was applied.
Table 1.
Primer Sequences Used for Real-Time PCR Analysis
| cDNA | Amplicon size (pb) | Primer sequences |
|---|---|---|
| Rs15 | 315 | 5′-GGCTTGTAGGTGATGGAGAA-3′ |
| 5′-CTTCCGCAAGTTCACCTACC-3′ | ||
| Gapdh | 260 | 5′-GACCCCTTCATTGACCTCAACTAC-3′ |
| 5′-AAGTTGTCATGGATGACCTTGGCC-3′ | ||
| Amelogenin | 271 | 5′-ACACCCTTCAGCCTCATCAC-3′ |
| 5′-GAGAACAGTGGAGGCAGAGG-3′ | ||
| Enamelin | 439 | 5′-CATGTGGCCTCCGCCAGTCC-3′ |
| 5′-GTCATCTGGGGGCGGGTCCT-3′ | ||
| Ameloblastin | 258 | 5′-TGCAGCCTCACCAGCCAGGA-3′ |
| 5′-CCCGAGACAGCGAATGGGCG-3′ | ||
| Tuftelin | 202 | 5′-CTCCCCTGTCCGCAGCAAGC-3′ |
| 5′-GGCGTCCATGTGCTGCTGGT-3′ | ||
| Amelotin | 379 | 5′-GCAACAAAACCGACTCCAG-3′ |
| 5′-CTCCATTCTGCACATCTGG-3′ | ||
| Mmp20 | 320 | 5′-CTGGGCCTGGGCCATTCCAC-3′ |
| 5′-CTGGTGATGGTGCTGGGCCG-3′ | ||
| Klk4 | 320 | 5′-GCATCCGCAGTGGGTGCTGT-3′ |
| 5′-CACACTGCAGGAGGCTGGGC-3′ |
Statistical Analysis
Data resulted from at least three independent experiments are presented as means ± SEM and were analyzed on GraphPad Prism software version 4.0 using the two-tailed nonparametric U-test. Values were considered significantly different at P < 0.05.
Results
BPA-Affected Rat Enamel and Human MIH Enamel Appear to be Hypomineralized
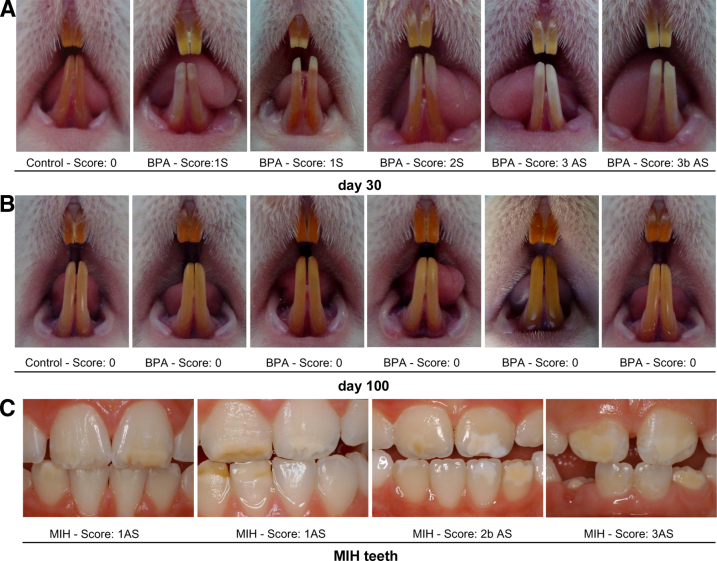
Extracted human MIH teeth were compared with BPA-treated rat incisors (Figure 1), and both presented asymmetrical white spots that affected the enamel. At day 30, the lower incisors of 12 of 16 rats (75%) were affected after administration of BPA, whereas 100% of control rats were unaffected. Control mandibular incisor enamel was homogeneously yellow to orange, whereas incisors from BPA-treated rats had enamel white spots either symmetrically or asymmetrically affecting the incisors to varying degrees (Figure 1 and Table 2). The severity of the phenotype was scored using criteria based on various indices previously reported for scoring human hypomineralization.21,27 Interestingly, the day 30 phenotype was completely consistent with the human MIH phenotype (Supplemental Figure S1) observed in the present and previous studies.21 In complete contrast, incisors from BPA-treated rats at day 100 were no longer affected and were indistinguishable from controls (Figure 1B), indicating that rat amelogenesis has a window of susceptibility that does not extend to the period when the incisor enamel in day 100 rats was being laid down. This finding provides a clear parallel between the cause associated with the effect of BPA on rat amelogenesis and the cause associated with human MIH (Supplemental Figure S1).
Figure 1.
Phenotypic comparison between BPA-treated rat incisors and human teeth affected by MIH. A: Both incisors in all control rats were free of defects (scored 0). Mandibular incisors from BPA treated-rats exhibited symmetrical (S) or asymmetrical (AS) white opacities and were scored as described in the Table 2. In the case of AS-affected teeth, the score for the most affected incisor was recorded. B: On day 100, mandibular incisors from BPA-treated rats were similar to those of control rats and scored 0. C: Human incisors affected by MIH and scored using the same criteria as for rat incisors.
Table 2.
Scoring of Enamel Defects Observed on Rat Mandibular Incisors on Day 30 after BPA Exposure
| Score | Proportion of tooth surface presenting white opacities | No. of affected animals | No. of animals with symmetric affection | No. of animals with asymmetric affection |
|---|---|---|---|---|
| 0 | Enamel defect free | 4 | ||
| 1 | One-third of tooth surface or less affected | 4 | 3 | 1 |
| 2 | Two-thirds of tooth surface affected | 2 | 2 | |
| 3 | Total tooth surface affected | 6 | 6 | |
| b | With enamel breakdown | 3 | 1 | 2 |
b, breakdown.
BPA Increases Organic Content of Enamel
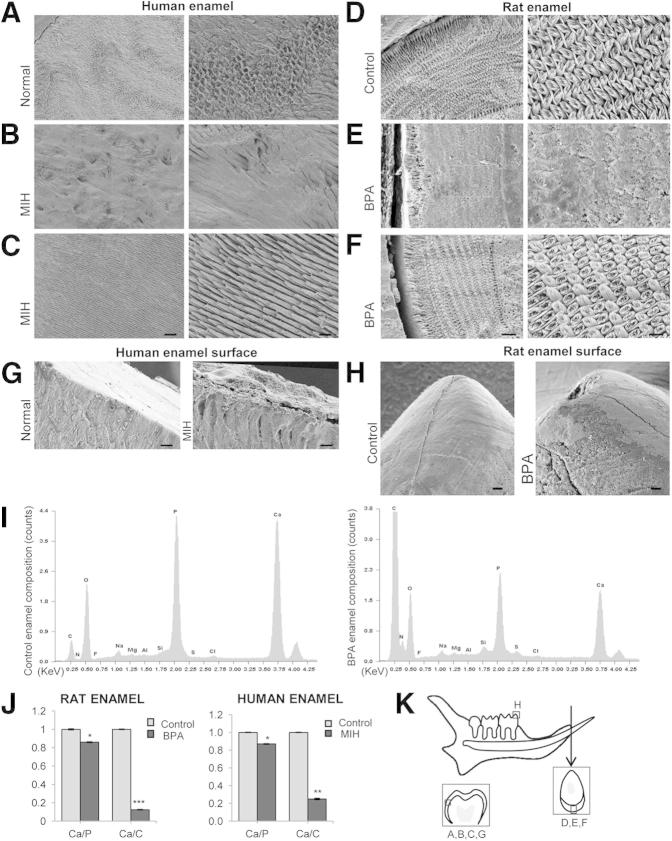
The underlying prismatic structure in human MIH enamel was obscured by a covering layer as reported previously.28 This layer was eliminated by hypochlorite treatment (Figure 2, B and C) to reveal the underlying enamel structure. Enamel sections from BPA-treated rats also exhibited a similar layer obscuring the underlying prismatic structure (Figure 2E and Supplemental Figure S2). This layer was also eradicated by hypochlorite, and its removal revealed the classic decussating prismatic structure of rodent enamel (Figure 2F). In addition, human MIH and BPA-affected rat teeth had broken enamel in areas where the teeth occlude: molar cusps and incisor tips (Figure 2, G and H, and Supplemental Figure S2). These secondary defects induced by mastication emphasize the fragility of hypomineralized enamel. Such defects were present in neither normal human enamel nor control rats.
Figure 2.
SEM and EDX analysis of rat and human enamel. A: Sound human enamel was characterized by well-organized prismatic and interprismatic structure clearly visualized at higher magnification. B: Enamel affected by MIH was hidden by a covering layer. C: After an NaOCl rinse, MIH enamel exhibited prismatic and interprismatic structure. Scale bars: 20 μm (left); 10 μm (right). D: Enamel of control rats on day 30. E: BPA-treated rat enamel exhibited a covering layer obscuring the underlying prismatic structure. F: After an NaOCl rinse, enamel from BPA-treated rats exhibited the classic architecture seen in controls. Scale bars: 15 μm (left); 5 μm (right). G: Human control enamel exhibited a smooth surface, whereas MIH enamel presented a rough surface. Scale bars: 10 μm. H: Rat control molars showed an unbroken smooth surface, whereas the molars from BPA-treated rats had a rough surface with enamel breakdown. Scale bars: 30μm. I: Typical EDX spectra. J: Ca/P and Ca/C ratios of BPA (n = 8) and MIH (n = 10) enamel. K: Diagram showing the location of SEM images of human molars and rat incisors. Arrow shows the plane of sectioning. All data are means ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, U-test.
Enamel elemental composition was determined using EDX (Figure 2I). There was a noticeable difference between the elemental composition of incisors from BPA-treated and control rats (Figure 2J). The Ca/C and Ca/P ratios were lower in BPA-treated rats than in controls, indicating a significant increase in the concentration of organic material relative to the mineral phase and a calcium deficiency compared with controls. These compositional differences were also mirrored in human MIH (Figure 2J).
BPA Increases the Albumin and Enamelin Content of Enamel
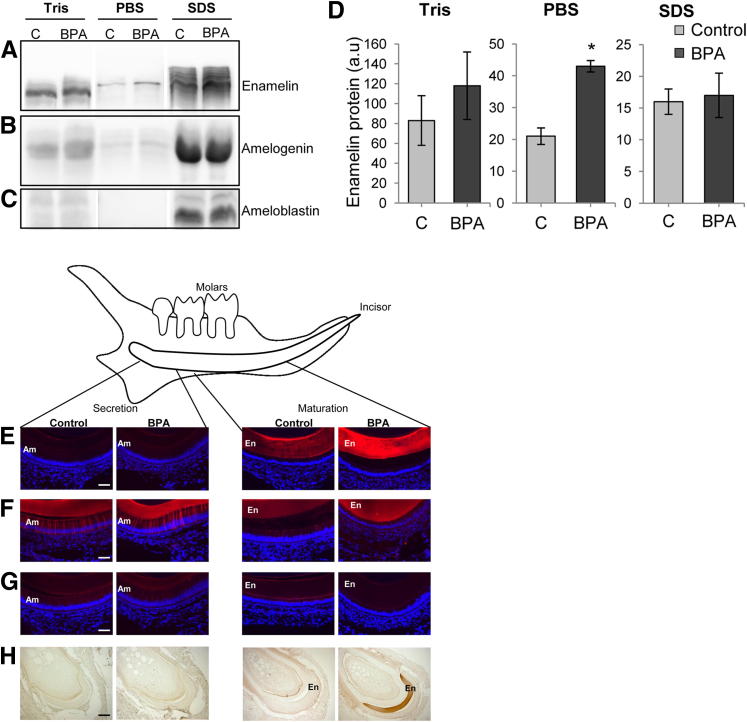
Enamel matrix proteins were sequentially extracted from BPA-effected and control incisors. Western blotting experiments revealed a clear and specific signal for enamelin at 66 kDa in all fractions (Figure 3A). Enamelin content was higher in all BPA-treated fractions, with a significant difference when comparing the fractions that contained mineral bound enamelin (PBS-extracted fractions) (Figure 3, A and D). BPA has no significant effect on amelogenin and ameloblastin expression as determined by Western blotting (Figure 3, B and C).
Figure 3.
Enamel matrix proteins in rat mandibular incisors on day 30. Enamel matrix proteins were sequentially extracted from secretory-stage enamel on day 30 and analyzed by Western blotting with specific antibodies for enamelin (A), amelogenin (B), and ameloblastin (C). D: Densitometric quantification revealed a significant increase (2.12-fold ± 0.02) of enamelin adsorbed to the enamel crystals (PBS fractions) in BPA-treated rats (P = 0.02). All data are means ± SEM (n = 3). ∗P < 0.05. IHC analysis of enamelin (E), amelogenin, (F), ameloblastin (G), and serum albumin (H). Scale bars: 70 μm (G); 200 μm (H). En, enamel; Am, ameloblasts.
Similarly, immunohistochemistry (IHC) revealed that the enamelin content was increased during the maturation stage of BPA-treated rats compared with controls (Figure 3E). Consistent with the Western data, IHC also indicated that BPA had no effect on the amelogenin and ameloblastin content (Figure 3, F and G). In addition, IHC revealed that the early maturation stage of BPA-affected enamel was clearly positive for serum albumin, whereas the corresponding control enamel was negative (Figure 3H).
BPA Modulates Enamelin and Klk4 mRNA Expression
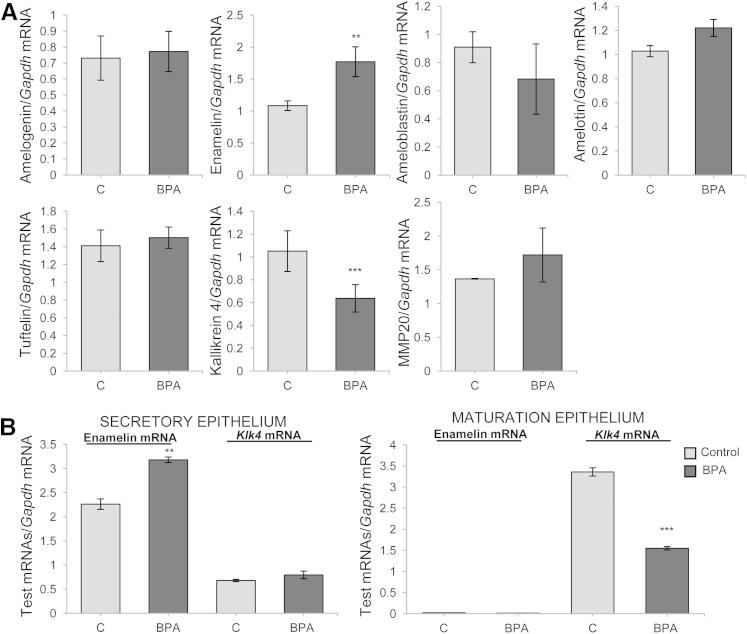
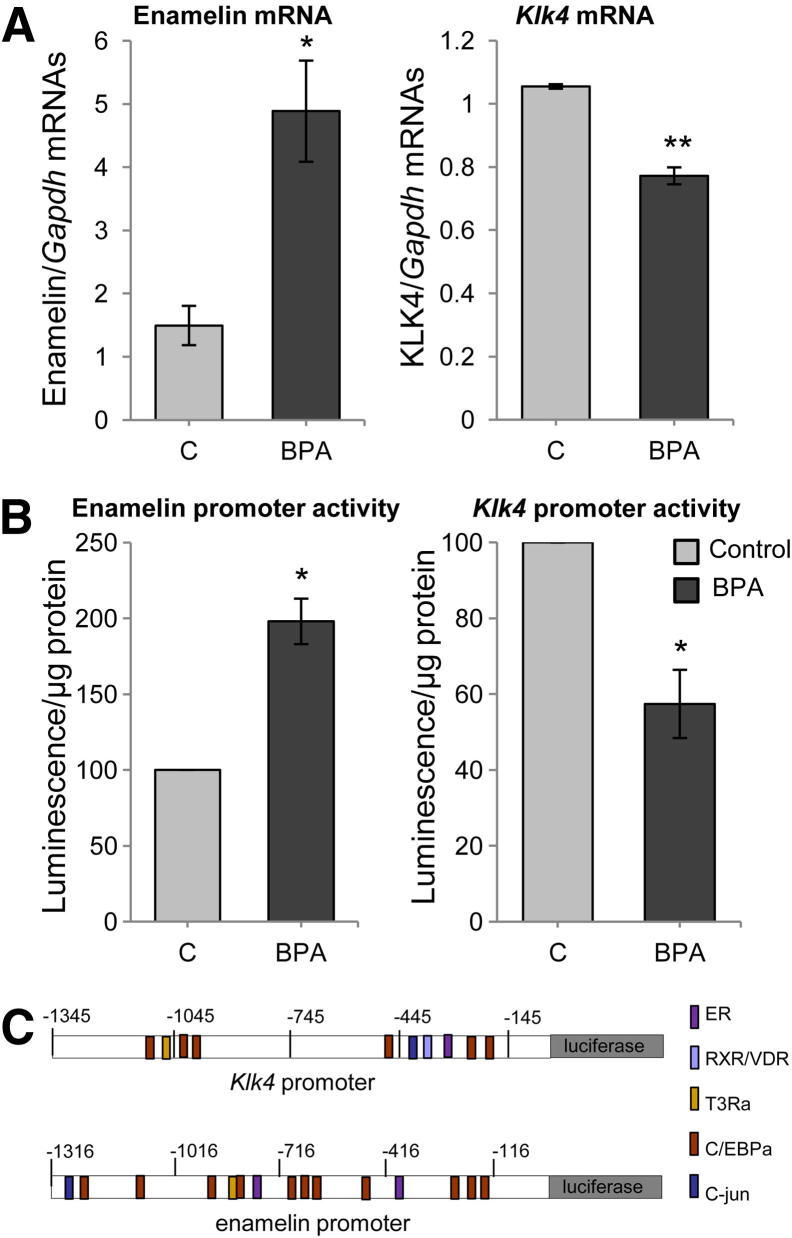
Levels of amelogenin, ameloblastin, amelotin, tuftelin, and matrix metalloprotease 20 mRNAs in whole dental enamel epithelia were unaffected by BPA. In contrast, enamelin mRNA levels were significantly increased, whereas the Klk4 mRNA level were significantly decreased (Figure 4A). Microdissection was used to isolate the secretion and maturation-stage enamel epithelia. In both BPA-treated and control rats, enamelin mRNA was essentially localized to the secretory-stage tissue (as previously described by Lacruz et al29). Consistent with the results from whole dental enamel epithelia, secretory-stage enamelin expression was increased in BPA-treated rats. Klk4 mRNA expression was detected in secretory- and maturation-stage epithelia from both BPA-treated and control rats. Expression levels were greater in maturation-stage enamel epithelia, and consistent with the results from whole dental enamel epithelia, levels of maturation stage Klk4 expression were markedly reduced in BPA-treated rats (Figure 4B). These modulations were not observed in rats exposed to BPA at the late postnatal stage of day 100 (Supplemental Figure S3).
Figure 4.
Enamel gene expression analyzed by qPCR. A: Analysis of lower incisor dental epithelium mRNAs on day 30 demonstrated the presence of all enamel matrix mRNAs and of the 2 main protease mRNAs (n = 4). B: qPCR analysis of mRNAs extracted from microdissected dental epithelium (n = 4). All data are means ± SEM. Each sample was analyzed in 3 independent experiments. ∗∗P < 0.01, ∗∗∗P < 0.001.
The effect of BPA on enamelin and Klk4 expression was examined in ameloblastic HAT-7 cells. Both genes were expressed by HAT-7 cells and were modulated by 10−9 mol/L BPA. Enamelin mRNA levels were significantly increased and Klk4 levels were significantly decreased by BPA (Figure 5A). Transcriptional regulation by BPA was investigated by measuring its effect on enamelin and Klk4 promoter activity. Cells transfected with these promoters controlling the luciferase reporter gene were treated with 10−9 mol/L BPA. Luminescence was significantly increased when luciferase expression was driven by the enamelin promoter and decreased when driven by the Klk4 promoter (Figure 5B). Similar modulations were observed using 10−6 mol/L to 10−12 mol/L BPA (Supplemental Figure S4).
Figure 5.
Modulation of enamelin and Klk4 expression by BPA. Enamelin and Klk4 expression was measured in rat epithelial cell line HAT-7 after 48 hours of treatment with 10−9 mol/L BPA. A: Enamelin and Klk4 mRNAs were assayed by qPCR (n = 4). B: Enamelin and Klk4 promoter activities were measured in transfected cells (n = 4). C: BIOBASE biological databases were used for promoter sequence analysis. All data are means ± SEM (n = 4). ∗P < 0.05, ∗∗P < 0.01, U-test.
Discussion
MIH is a recently described pathology1,2 that is frequently diagnosed in many populations throughout the world, although precise etiologic factors remain unclear.4,30 Comparing the features of human MIH previously reported, and those reported here, to the features of BPA-affected rat enamel, there are apparent similarities between BPA-induced enamel defects in the rat model and human MIH lesions.
A key characteristic of human MIH is that it preferentially affects permanent incisors and first molars. The causative agents underpinning human MIH thus appear to exert their effects during a specific developmental time window. Teeth that develop outside this critical window are not affected. Likewise, the present study indicates that the continually growing rat incisor is only susceptible to BPA during a specific developmental time window because enamel erupting in rats on day 100 is unaffected (in contrast to affected enamel erupting on day 30).
To explain the existence of this time window of susceptibility to BPA in the rat, it is useful to consider the biochemical elimination of xenoestrogens, such as BPA. In adult rats, BPA is eliminated in the bile after extensive glucuronidation (to yield BPA-GA) by an isoform of hepatic uridine 5′-diphospho-glucuronosyltransferase (UGT2B1).31 BPA-GA conjugates are excreted into the bile via a pathway that involves the hepatic protein Mrp2, although in the gut BPA-GA is deconjugated and BPA is readsorbed. This enterohepatic circulation of BPA prolongs clearance times in rats.32 During pregnancy, maternal rat hepatic Mrp2 expression is reduced by 50%,33 and bilious excretion of BPA-GA is decreased in favor of a reciprocal release of BPA-GA into the systemic circulation.34 BPA-GA in the maternal circulation can cross the rat placenta and be deconjugated back to BPA in the fetus.31 The fact that this, together with the fact that UGT2B1 glucuronidase activity against xenoestrogens appears undetectable in rat fetus and activity in the neonate, only reaches adult levels by day 21 after a linear increase from birth35 indicates that the risk of BPA exposure is greatest in the fetus and neonate, a time corresponding exactly to when the affected enamel described in this study would have been under formation. We assume that once young rats have reached day 21 and UGT2B1 glucuronidase activity has reached adult levels, the BPA load previously affecting amelogenesis is relieved and formation of enamel forming thereafter is unaffected (as seen rats on day 100).
In the case of humans, the teeth mostly affected by MIH (ie, permanent first molars and permanent incisors) begin to mineralize between birth and 5 months of age, which identifies the window of susceptibility for human MIH. It has been argued using a physiologically based pharmacokinetic modeling approach that human newborns exhibit plasma BPA concentration 11 times greater than that found in adults, whereas at 3 months of age the ratio has decreased to 2.36 This finding agrees well with the hypothesis that exposure to BPA during the first months of life affect the development of the teeth that are developing during that window time, thus giving rise to MIH.
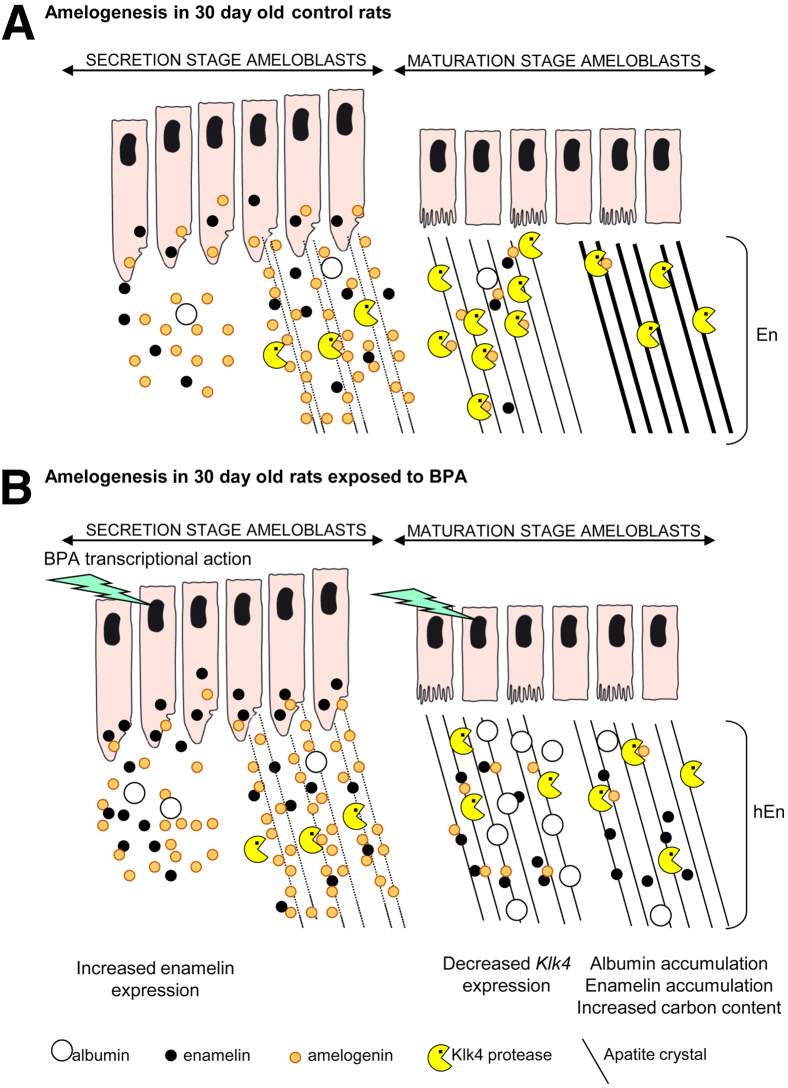
Our results also provide information of the possible molecular mechanisms by which BPA affects enamel formation in the rat model. Gene expression data obtained from BPA-treated rats on day 30 indicated that enamelin expression was significantly increased, whereas Klk4 gene expression was significantly decreased. In addition, early maturation stage enamel of BPA-treated rats contained significantly increased levels of serum albumin. It is unclear whether increased albumin levels are a consequence of increased ingress or reduced proteolytic degradation due to reduced Klk4 expression, but the presence of mineral bound albumin and enamel matrix proteins in the early maturation stage could inhibit enamel crystal growth, leading to hypomineralization37,38 (Figure 6). Inhibition of crystal growth during the maturation stage by the organic material revealed by SEM and EDX would not affect the underlying prism structure because the actual prismatic architecture of the enamel is laid out during the secretory stage. Retention of protein in the later maturation stage would inhibit the growth of the crystals already laid out in the classic decussating prism pattern, but the prism architecture would still be present, albeit in a hypomineralized state. The presence of the organic layer obscuring the underlying prism structure in both rat and human teeth studied here is in accordance with previously published data on MIH.28,39,40 The nature of this organic material is unclear at present, but its sensitivity to hydrolysis by hypochlorite suggests it may be proteinacious.
Figure 6.
Scheme of the effects of BPA on amelogenesis. A: Amelogenesis in control rats. B: Disturbed amelogenesis in BPA-treated rats. Decreased Klk4 expression and protein accumulation led to enamel hypomineralization. En, enamel; hEn, hypomineralized enamel.
The mechanism by which BPA affects ameloblast gene expression is unclear. However, BPA has been reported to interact with steroid, retinoid, and thyroid nuclear receptor pathways.41 BPA has been reported to bind multiple receptors, including estrogen receptors,6,41,42 GPR30,43 retinoid receptor-γ,44 and estrogen-related receptor-γ.45 It modulates androgen, thyroid, and glucocorticoid receptor activities and expression levels of key transcription factors, such as CREB, CEBP, STAT3, THR, PPAR-γ, or GATA-4. BPA may have a direct influence on ameloblasts by binding to a BPA sensitive receptor. The presence of estrogen receptor-α, vitamin D, and thyroid receptors in ameloblasts17,46 at the developmental stages where both enamelin and Klk4 expression is occurring maximally invites the suggestion that BPA affects the expression of these genes through nuclear hormone pathway. This is evidenced by our luciferase data that suggest BPA influences enamelin and Klk4 expression by transcriptional regulations.
In conclusion, the present data indicate that ameloblasts are susceptible to BPA and that BPA may be a causative agent in human MIH etiology. The rat model has enabled us to propose a hypothetical scheme for MIH pathophysiology at the molecular level. MIH may thus represent a permanent record of exposure to BPA (or EDCs sharing similar molecular effects) and could be easily used as a biomarker for retrospective analysis of infant exposure to EDCs and the effect of such exposure on health in later life.
Acknowledgments
We thank Prof. Chantal Naulin-Ifi for MIH image and experience, and Prof. Pascal Ferré (UPMC, Paris, France) for his pertinent comments on the manuscript, Laurence Decoq, Bruno Pasquis, and Elise Lalarme (UMR 1324 INRA, Dijon, France) for animal breeding, Hélène Lecoq, David Montero, Dominique LeDenmat, and Frédéric Herbst (Université Paris Diderot, Paris, France) for their help in SEM and EDX analysis; and Christophe Klein for his assistance in confocal microscopy.
Footnotes
Supported by the University Paris-Diderot, the French National Institute of Health and Medical Research (INSERM), the National Research Program on Endocrine Disruptors (CIME), WELMEC (a Wellcome-EPSRC Centre of Excellence in Medical Engineering; S.J.B. and J.K.), the NIHR Leeds Musculoskeletal Biomedical Research Unit (S.J.B. and J.K.) and Wellcome Trust grant 093113 (S.J.B. and J.K.).
Supplemental Data
Tooth mineralization chronology. A: Teeth affected by MIH (the first permanent molars and incisors) (red). Nonaffected teeth are formed later (green). The panoramic radiograph of an 8-year-old MIH patient: possibly affected teeth (red) and nonaffected teeth (green) that mineralized later (after approximately 2 years) before eruption. B: Rat incisors that developed before day 30 are affected (red). The nonaffected incisors are formed later at the adult stage (green).
SEMs of rat enamel. A: on day 30, mandibular molar enamel of control rats exhibited a normal structure characterized by decussating prism architecture. BPA-treated rat molar enamel exhibited a magmatic-like layer obscuring the underlying prismatic structure. B: Mandibular incisor tips of control rats were unbroken, whereas the BPA-affected incisors had enamel breakdown.C: At higher magnification mandibular incisor enamel of control rats exhibited a smooth surface, whereas BPA-treated rats presented a rough surface. D: A schematic showing the location of rat molar and incisor SEM images. Arrow shows the plane of sectioning. Scale bars: 2 μm (A); 200 μm (B); 10 μm (C).
Enamelin and Klk4 mRNA levels analyzed by qPCR. Analysis of lower incisor dental epithelium mRNAs on day 100 revealed that enamelin and Klk4 mRNA levels were not significantly modulated by BPA. All data are means ± SEM (n = 4).
BPA dose-effect response. Transcriptional modulation of enamelin and Klk4 expression after exposure to different concentrations of BPA. A: Enamelin promoter activity measured in transfected cells was significantly induced by all three concentrations of BPA tested 48 hours after treatment. B:Klk4 promoter activity was inhibited by the different BPA concentrations used. Four independent experiments were performed, and data are means ± SEM. *P < 0.05, U-test.
References
- 1.Jälevik B., Klingberg G., Barregård L., Norén J.G. The prevalence of demarcated opacities in permanent first molars in a group of Swedish children. Acta Odontol Scand. 2001;59:255–260. doi: 10.1080/000163501750541093. [DOI] [PubMed] [Google Scholar]
- 2.Weerheijm K.L., Merjare I. Molar incisor hypomineralisation: a questionnaire inventory on its occurrence in member countries of the European Academy of Paediatric Dentistry (EAPD) Int J Paediatr Dent. 2003;13:411–416. doi: 10.1046/j.1365-263x.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- 3.Jälevik B. Prevalence and diagnosis of molar-incisor-hypomineralisation (MIH): a systematic review. Eur Arch Paediatr Dent. 2010;11:59–64. doi: 10.1007/BF03262714. [DOI] [PubMed] [Google Scholar]
- 4.Alaluusua S. Aetiology of Molar-Incisor Hypomineralisation: a systematic review. Eur Arch Paediatr Dent. 2010;11:53–58. doi: 10.1007/BF03262713. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Magdalena P., Quesada I., Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;7:346–353. doi: 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- 6.Vom Saal F.S., Nagel S.C., Coe B.L., Angle B.M., Taylor J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol. 2012;354:74–84. doi: 10.1016/j.mce.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirard C., Sagot C., Deville M., Dubois N., Charlier C. Urinary levels of bisphenol A, triclosan and 4-nonylphenol in a general Belgian population. Environ Int. 2012;48:78–83. doi: 10.1016/j.envint.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Hunt P.A., Lawson C., Gieske M., Murdoch B., Smith H., Marre A., Hassold T., Vandevoort C.A. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012;109:17525–17530. doi: 10.1073/pnas.1207854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poimenova A., Markaki E., Rahiotis C., Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167:741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 10.Tharp A.P., Maffini M.V., Hunt P.A., VandeVoort C.A., Sonnenschein C., Soto A.M. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci U S A. 2012;109:8190–8195. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadal A. Obesity: fat from plastics? linking bisphenol A exposure and obesity Nat Rev Endocrinol. 2012;9:9–10. doi: 10.1038/nrendo.2012.205. [DOI] [PubMed] [Google Scholar]
- 12.Rubin B.S., Soto A.M. Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol. 2009;304:55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano S., Alonso-Magdalena P., García-Arévalo M., Novials A., Muhammed S.J., Salehi A., Gustafsson J.A., Quesada I., Nadal A. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS One. 2012;7:e31109. doi: 10.1371/journal.pone.0031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varayoud J., Ramos J.G., Bosquiazzo V.L., Lower M., Muñoz-de-Toro M., Luque E.H. Neonatal exposure to bisphenol A alters rat uterine implantation-associated gene expression and reduces the number of implantation sites. Endocrinology. 2011;152:1101–1111. doi: 10.1210/en.2009-1037. [DOI] [PubMed] [Google Scholar]
- 15.Wright J.T. The molecular etiologies and associated phenotypes of amelogenesis imperfecta. Am J Med Genet A. 2006;140:2547–2555. doi: 10.1002/ajmg.a.31358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson C., Kirkham J., Brookes S.J., Bonass W.A. The chemistry of enamel development. Int J Dev Biol. 1995;39:145–152. [PubMed] [Google Scholar]
- 17.Berdal A., Hotton D., Pike J.W., Mathieu H., Dupret J.M. Cell- and stage-specific expression of vitamin D receptor and calbindin genes in rat incisor: regulation by 1,25-dihydroxyvitamin D3. Dev Biol. 1993;155:172–179. doi: 10.1006/dbio.1993.1016. [DOI] [PubMed] [Google Scholar]
- 18.Lacruz R.S., Smith C.E., Chen Y.B., Hubbard M.J., Hacia J.G., Paine M.L. Gene-expression analysis of early- and late-maturation-stage rat enamel organ. Eur J Oral Sci. 2011;119:149–157. doi: 10.1111/j.1600-0722.2011.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith C.E., Nanci A. A method for sampling stages of amelogenesis on mandibular rat incisors using the molars as a reference for dissection. Anat Rec. 1989;225:257–266. doi: 10.1002/ar.1092250312. [DOI] [PubMed] [Google Scholar]
- 20.Hiller C.R., Robinson C., Weatherell J.A. Variations in the composition of developing rat incisor enamel. Calcif Tissue Res. 1975;18:1–12. doi: 10.1007/BF02546222. [DOI] [PubMed] [Google Scholar]
- 21.Weerheijm K.L., Duggal M., Mejàre I., Papagiannoulis L., Koch G., Martens L.C., Hallonsten A.L. Judgement criteria for molar incisor hypomineralisation (MIH) in epidemiologic studies: a summary of the European meeting on MIH held in Athens. Eur J Paediatr Dent. 2003;4:110–113. [PubMed] [Google Scholar]
- 22.Molla M., Descroix V., Aïoub M., Simon S., Castañeda B., Hotton D. Enamel protein regulation and dental and periodontal physiopathology in MSX2 mutant mice. Am J Pathol. 2010;177:2516–2526. doi: 10.2353/ajpath.2010.091224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brookes S.J., Kingswell N.J., Barron M.J., Dixon M.J., Kirkham J. Is the 32-kDa fragment the functional enamelin unit in all species? Eur J Oral Sci. 2011;119(Suppl 1):345–350. doi: 10.1111/j.1600-0722.2011.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brookes S.J., Lyngstadaas S.P., Robinson C., Shore R.C., Wood S.R., Kirkham J. Enamelin compartmentalization in developing porcine enamel. Connect Tissue Res. 2002;43:477–481. doi: 10.1080/03008200290000862. [DOI] [PubMed] [Google Scholar]
- 25.Brookes S.J., Kirkham J., Shore R.C., Wood S.R., Slaby I., Robinson C. Amelin extracellular processing and aggregation during rat incisor amelogenesis. Arch Oral Biol. 2001;46:201–208. doi: 10.1016/s0003-9969(00)00121-7. [DOI] [PubMed] [Google Scholar]
- 26.Kawano S., Morotomi T., Toyono T., Nakamura N., Uchida T., Ohishi M. Establishment of dental epithelial cell line (HAT-7) and the cell differentiation dependent on Notch signaling pathway. Connect Tissue Res. 2002;43:409–412. doi: 10.1080/03008200290000637. [DOI] [PubMed] [Google Scholar]
- 27.Chawla N., Messer L.B., Silva M. Clinical studies on molar-incisor-hypomineralisation part 2: development of a severity index. Eur Arch Paediatr Dent. 2008;9:191–199. doi: 10.1007/BF03262635. [DOI] [PubMed] [Google Scholar]
- 28.Jälevik B., Dietz W., Norén J.G. Scanning electron micrograph analysis of hypomineralised enamel in permanent first molars. Int J Paediatr Dent. 2005;15:233–240. doi: 10.1111/j.1365-263X.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 29.Lacruz R.S., Smith C.E., Bringas P., Jr., Chen Y.B., Smith S.M., Snead M.L. Identification of novel candidate genes involved in mineralization of dental enamel by genome-wide transcript profiling. J Cell Physiol. 2012;227:2264–2275. doi: 10.1002/jcp.22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crombie F., Manton D., Kilpatrick N. Aetiology of molar-incisor hypomineralization: a critical review. Int J Paediatr Dent. 2009;19:73–83. doi: 10.1111/j.1365-263X.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 31.Nishikawa M., Iwano H., Yanagisawa R., Koike N., Inoue H., Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ Health Perspect. 2010;118:1196–1203. doi: 10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pottenger L.H., Domoradzki J.Y., Markham D.A., Hansen S.C., Cagen S.Z., Waechter The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol Sci. 2000;54:3–18. doi: 10.1093/toxsci/54.1.3. [DOI] [PubMed] [Google Scholar]
- 33.Cao J., Stieger B., Meier P.J., Vore M. Expression of rat hepatic multidrug resistance associated proteins and organic anion transporters in pregnancy. Am J Physiol Gastrointest Liver Physiol. 2002;283:757–766. doi: 10.1152/ajpgi.00126.2002. [DOI] [PubMed] [Google Scholar]
- 34.Inoue H., Tsuruta A., Kudo S., Ishii T., Fukushima Y., Iwano H., Yokota H., Kato S. Bisphenol a glucuronidation and excretion in liver of pregnant and non-pregnant female rats. Drug Metab Dispos. 2005;33:55–59. doi: 10.1124/dmd.104.001537. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto J., Yokota H., Yuasa A. Developmental increases in rat hepatic microsomal UDP-glucuronosyltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect. 2002;110:193–196. doi: 10.1289/ehp.02110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edginton A.N., Ritter L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ Health Perspect. 2009;117:645–652. doi: 10.1289/ehp.0800073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmer J.P., Hu Y., Lertlam R., Yamakoshi Y., Hu J.C. Hypomaturation enamel defects in Klk4 knockout/LacZ knockin mice. J Biol Chem. 2009;284:19110–19121. doi: 10.1074/jbc.M109.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson C., Kirkham J., Brookes S.J., Shore R.C. The role of albumin in developing rodent dental enamel: a possible explanation for white spot hypoplasia. J Dent Res. 1992;71:1270–1274. doi: 10.1177/00220345920710060101. [DOI] [PubMed] [Google Scholar]
- 39.Mangum J.E., Crombie F.A., Kilpatrick N., Manton D.J., Hubbard M.J. Surface integrity governs the proteome of hypomineralized enamel. J Dent Res. 2010;89:1160–1165. doi: 10.1177/0022034510375824. [DOI] [PubMed] [Google Scholar]
- 40.Farah R.A., Monk B.C., Swain M.V., Drummond B.K. Protein content of molar incisor hypomineralisation enamel. J Dent. 2010;38:591–596. doi: 10.1016/j.jdent.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Paris F., Balaguer P., Térouanne B., Servant N., Lacoste C., Cravedi J.P., Nicolas J.C., Sultan C. Phenylphenols, biphenols, bisphenol-A and 4-tert-octylphenol exhibit alpha and beta estrogen activities and antiandrogen activity in reporter cell lines. Mol Cell Endocrinol. 2002;193:43–49. doi: 10.1016/s0303-7207(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 42.Delfosse V., Grimaldi M., Pons J.L., Boulahtouf A., le Maire A., Cavailles V., Labesse G., Bourquet W., Balaguer P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc Natl Acad Sci U S A. 2012;109:14930–14935. doi: 10.1073/pnas.1203574109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alonso-Magdalena P., Ropero A.B., Soriano S., García-Arévalo M., Ripoll C., Fuentes E., Quesada I., Nadal Á. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol Cell Endocrinol. 2012;355:201–207. doi: 10.1016/j.mce.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Nishigori M., Nose T., Shimohigashi Y. Highly potent binding and inverse agonist activity of bisphenol A derivatives for retinoid-related orphan nuclear receptor RORγ. Toxicol Lett. 2012;212:205–211. doi: 10.1016/j.toxlet.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Liu X., Matsushima A., Nakamura M., Costa T., Nose T., Shimohigashi Y. Fine spatial assembly for construction of the phenol pocket to capture bisphenol A in the human nuclear receptor estrogen related receptor γ. J Biochem. 2012;151:403–415. doi: 10.1093/jb/mvs008. [DOI] [PubMed] [Google Scholar]
- 46.Ferrer V.L., Maeda T., Kawano Y. Characteristic distribution of immunoreactions for estrogen receptor alpha in rat ameloblasts. Anat Rec. 2005;284:529–536. doi: 10.1002/ar.a.20190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tooth mineralization chronology. A: Teeth affected by MIH (the first permanent molars and incisors) (red). Nonaffected teeth are formed later (green). The panoramic radiograph of an 8-year-old MIH patient: possibly affected teeth (red) and nonaffected teeth (green) that mineralized later (after approximately 2 years) before eruption. B: Rat incisors that developed before day 30 are affected (red). The nonaffected incisors are formed later at the adult stage (green).
SEMs of rat enamel. A: on day 30, mandibular molar enamel of control rats exhibited a normal structure characterized by decussating prism architecture. BPA-treated rat molar enamel exhibited a magmatic-like layer obscuring the underlying prismatic structure. B: Mandibular incisor tips of control rats were unbroken, whereas the BPA-affected incisors had enamel breakdown.C: At higher magnification mandibular incisor enamel of control rats exhibited a smooth surface, whereas BPA-treated rats presented a rough surface. D: A schematic showing the location of rat molar and incisor SEM images. Arrow shows the plane of sectioning. Scale bars: 2 μm (A); 200 μm (B); 10 μm (C).
Enamelin and Klk4 mRNA levels analyzed by qPCR. Analysis of lower incisor dental epithelium mRNAs on day 100 revealed that enamelin and Klk4 mRNA levels were not significantly modulated by BPA. All data are means ± SEM (n = 4).
BPA dose-effect response. Transcriptional modulation of enamelin and Klk4 expression after exposure to different concentrations of BPA. A: Enamelin promoter activity measured in transfected cells was significantly induced by all three concentrations of BPA tested 48 hours after treatment. B:Klk4 promoter activity was inhibited by the different BPA concentrations used. Four independent experiments were performed, and data are means ± SEM. *P < 0.05, U-test.