Abstract
Objective
To evaluate the anti-bacterial and anti-oxidant activity of andrographolide (AND) and echiodinin (ECH) of Andrographis paniculata.
Methods
In this study, an attempt has been made to demonstrate the anti-microbial and anti-oxidant activity of isolated AND and ECH by broth micro-dilution method and 2,2-diphenyl-2-picryl-hydrazyl (DPPH) assay, respectively. Structure elucidation was determined by electro-spray ionization-MSD, NMR (1H and 13C) and IR spectra.
Results
AND was effective against most of the strains tested including Mycobacterium smegmatis, showing broad spectrum of growth inhibition activity with Minimum inhibitory concentration values against Staphylococcus aureus (100 µg/mL), Streptococcus thermophilus (350 µg/mL) Bacillus subtilis (100 µg/mL), Escherichia coli (50 µg/mL), Mycobacterium smegmatis (200 µg/mL), Klebsiella pneumonia (100 µg/mL), and Pseudomonas aeruginosa (200 µg/mL). ECH showed specific anti-bacterial activity against Staphylococcus aureus, Escherichia coli, Bacillus subtilis and Pseudomonas aeruginosa at a concentration higher than 225 µg/mL. Both AND and ECH were not effective against the two yeast strains, Candida albicans and Saccharomyces cerevisiae tested in this study.
Conclusion
This preliminary study showed promising anti-bacterial activity and moderate free radical scavenging activity of AND and ECH, and it may provide the scientific rationale for its popular folklore medicines.
Keywords: Andrographolides, Disk diffusion method, Folklore medicine, King of bitters, Minimum inhibitory concentration, Phenolic compounds
1. Introduction
Anti-microbial compounds are of key interest nowadays in research areas due to the outbreak of newer infectious diseases, the increase in resistance of microorganisms towards the existing microbial compounds and harmful side effects of the compounds. For this reason, there is a need for the development of compounds with less side effects and more targeted activity on the microorganisms. Research is going on to identify compounds with anti-microbial activity mainly from plant origin as they have lesser side effects[1]. Most of the researchers concentrate on screening and minimum inhibitory concentration (MIC) determination of extracts of plants rather than identifying compounds with activity, which makes most of the researches unuseful for drug discovery. In this attempt, we isolated active constituents of the plant and screened for anti-microbial activity which can be used further in research to develop anti-microbial compounds with the isolated compounds or their synthetic analogues.
Free radicals produced in the body due to oxidative processes are responsible for many diseases including cancer and cardiovascular diseases[2]. Although the human body has mechanisms to neutralize the free radicals produced, they are not sufficient enough due to the diets most people follow. It is recommended to have diets rich in anti-oxidants to increase the body's resistance to free radicals. Most of the previous researches were carried out on extracts of plants. In this research, along with anti-microbial activity of the isolated compounds, we also screened for anti-oxidant activity.
The genus Andrographis (Acanthaceae), comprising of about 40 species, is essentially distributed in southern India and in tropical Asia. Andrographis paniculata Nees (A. paniculata) is commonly known as Creat or King of bitters in English due to its bitter taste, Kalmegh in India, Hempedu bumi in Malaysia, Fah Tah Lai in Thailand, Chuan-Xin-Lin in China and Senshinren in Japan. It is a popular ethno-medicinal herb used for treating infection, inflammation, cold, fever, snake bite, diarrhoea, dysentery, jaundice, kidney diseases, and anti-oxidant agent[3]. Previous phytochemical studies on A. paniculata Nees has resulted in isolation of flavonoids and andrographolides (AND)[4], and various parts of this plant have been reported to possess multiple therapeutic properties including anti-cancer[5], anti-diabetes[6], anti-inflammatory[7], anti-malarial, antipyretic, anti-typhoid, antiviral, hepatoprotective[8], antihuman immunodeficiency virus and immunostimulatory activity[9].
The crude aqueous extract of A. paniculata has long been used for treating dysentery and diarrhoea with overall effectiveness of 91.3%[10]. Prajjal et al. have investigated the anti-microbial activity of aqueous extract of A. paniculata and showed antibacterial activity against few microbes at higher concentration (10 mg/disc)[11]. Youhong et al. have also studied the anti-microbial activity of aqueous fractions isolated from this species and found that neither the aqueous extract nor andrographolide were bacteriostatic or bactericidal against the tested microorganism[12]. Sule et al. showed the effectiveness of A. paniculata extracts in controlling bacteria that cause skin diseases[13]. According to Arash Rafat et al., the anti-oxidant activity of A. paniculata is related to the total phenolic content of the extract, and there is a correlation between the content of phenolic compounds and antioxidant activiy[14]. The ethanol extract has more phenolic content and shows stronger activity than aqueous extract, which is also supported by the research of Wasman et al[15]. Considering the importance of this species in traditional therapy against bacterial infections, wound healing and diarrhoea, the present study was therefore undertaken to evaluate the anti-bacterial and total anti-oxidant activity of the secondary metabolites - AND and echiodinin (ECH) isolated from organogenic callus culture of A. paniculata Nees. Plant cell and organ cultures are promising technologies to obtain plant-specific valuable metabolites and can be exploited successfully to meet the increasing demands of enhancing the yield of bioactive compounds and to expedite the pace of conservation and propagation of an important ethno-medicinal plants[16]. Leaf explants of A. paniculata were used for callus culture on MS medium containing different concentrations of auxins and the active constituents of the plant were isolated and identified. The isolated compounds were used to screen antimicrobial activity by modified agar-disc diffusion method and MIC determination by micro-dilution technique against four Gram positive bacteria, three Gram negative bacteria and two yeast strains. The antioxidant activity of the isolated compounds was determined by using DPPH method.
2. Materials and methods
2.1. In vitro callus induction
Mature leaves collected from field grown plants were used as explants; they were surface sterilized in 70% (v/v) ethanol for 30 sec followed by repeated rinsing in sterile distilled water. Then the leaf explants were treated with 0.1% HgCl2 (w/v) for 2 min, followed by washing in sterile distilled water. The cut ends of the explants were further trimmed with sharp edge sterile surgical blades. Then the explants were blotted on sterile filter paper discs before planting them on agar gelled MS medium taken in 150 mL conical flasks. Morphogenic callus induction on MS medium supplemented with various concentrations of auxins (NAA, 2,4-D and IAA) were studied. The optimum and bulk quantity of callus induction was observed in presence of 1.0 mg/L IAA and was used for characterization of bioactive compounds.
2.2. Extraction and isolation of active constituents
The dried powdered callus (750 g) was extracted with acetone and methanol in a 500 mL soxhlet apparatus for 24 h. The acetone and methanol extracts were column chromatographed on silica gel (100-200 mesh size) using hexane-ethyl acetate (3:7, v:v) and ethyl acetate-methanol (9:1, 8:2, v:v) step gradient, respectively. Fractions (50 mL each) were collected and subsequently monitored by preparative thin layer chromatography (TLC, silica glass plates of 20 cm × 20 cm) using hexane-ethyl acetate and ethyl-acetate methanol step gradient, respectively. This resulted in the isolation of two compounds previously reported from A. paniculata as echioidinin (yield 20 mg, melting point 264-265 °C; molecular formula, C16H12O5, molecular weight, [M+H]+ 285) and andrographolide (yield 70 mg, melting point 232-234 °C; molecular formula, C20H30O5, molecular weight, [M+H]+ 351). The IR, 1H NMR, 13C NMR and mass spectra of the compound were matched with previously reported in the literature[4],[17].
2.3. Tested microorganisms
The following microbial strains were employed in the screening investigation: Staphylococcus aureus (S. aureus) MTCC 96, Streptococcus thermophilus (S. thermophilus) MTCC 1938, Bacillus subtilis (B. subtilis) MTCC 736, Mycobacterium smegmatis (M. smegmatis) MTCC 6, Escherichia coli (E. coli) MTCC 739, Pseudomonas aeruginosa (P. aeruginosa) ATCC 8162, Klebsiella pneumonia (DRL, Tezpur) (K. pneumonia), Candida albicans (DRL, Tezpur) (C. albicans), and Saccharomyces cerevisiae (S. cerevisiae) MTCC 3980.
2.4. Anti-microbial assay
The in vitro anti-bacterial and anti-fungal assays were carried out by adopting the modified agar-disc diffusion method[18]. Mueller-Hinton Agar (MHA, Sigma) was inoculated with (overnight, 12 h) bacterial cell suspension (200 µL in 20 mL medium, 5×105 CFU/mL). Sterile filter paper discs of 6 mm diameter were impregnated with 20 µL purified compounds - AND and ECH (concentration 1 mg/disc). After complete evaporation, the discs were placed on the surface of the inoculated agar plate. Gentamicine (30 µg/disk) and myconazole (1 mg/disc) were used as positive controls, and negative controls were done using paper discs loaded with 20 µL of the solvent (dimethyl sufoxide, DMSO). The plates were incubated at 37 °C for 18 h. Similarly, Sabouraud dextrose agar was inoculated with yeast overnight cell suspension and incubated at 28 °C for 48 h. At the end of the incubation period, the anti-microbial activities were evaluated by measuring the zone of inhibition. An inhibition zone of 14 mm or greater (including diameter of the disc, 6 mm) was considered as high anti-microbial activity. All determinations were carried out in triplicate and the results were averaged.
2.5. Determination of MIC using the micro-dilution technique on the different strains of bacteria and yeast
The in vitro antibacterial and antifungal assays were carried out by adopting the micro-dilution techniques according to the National Committee for Clinical Laboratory Standards procedures for aerobic testing. Briefly, each of the microbes tested were sub-cultured twice on Mueller-Hinton agar, and the colonies (5-7) were then transferred aseptically into individual tubes containing sterile nutrient broth (10 mL). The tubes were incubated for a period of 8-12 h at 37 °C to attain growth at log phase. Subsequently, these inoculates were diluted with sterile distilled water to obtain a density corresponding approximately to 0.5 McFarland standard turbidity scale (1×106 CFU/mL). Stock solutions of test compounds - AND and ECH were prepared in 100% DMSO at a concentration of 1 mg/mL. A known volume (100 µL) of each solution was placed in the first well of a 96-well microplate and 2-fold serially diluted with sterile distilled water until the least concentration is achieved. A known volume of inoculum (100 µL) was then added to each well. The plates were then incubated at 37 °C for 24 h. After incubation, 40 µL of MTT (0.2 mg/mL) was added to each well and incubated for further 10-15 min. Bacterial growth is denoted by a blue coloration of the wells. The well of the lowest concentration in which no blue coloration observed was taken as the MIC.
2.6. Evaluation of anti-oxidant (antiradical) activity
The free radical scavenging activity was measured by using 2,2-diphenyl-2-picryl-hydrazyl (DPPH) method of Nihal Turkmen et al.[19], with minor modifications. Each purified compounds - AND and ECH were precisely diluted in methanol and methanol was used as a control. The reaction mixture contained 500 µL of test compounds and 125 µL of DPPH in methanol. Different concentrations of test compounds (10, 20, 40, 80 and 160 µg/mL) were prepared in methanol while the concentration of DPPH was 0.1 mmol/L in the reaction mixture. These reaction mixtures were taken in microfuge tubes, vortexed and incubated at 37 °C for 30 min. The absorbance was measured at 517 nm using a spectrophotometer with methanol as blank. Ascorbic acid was used as positive control.
3. Results
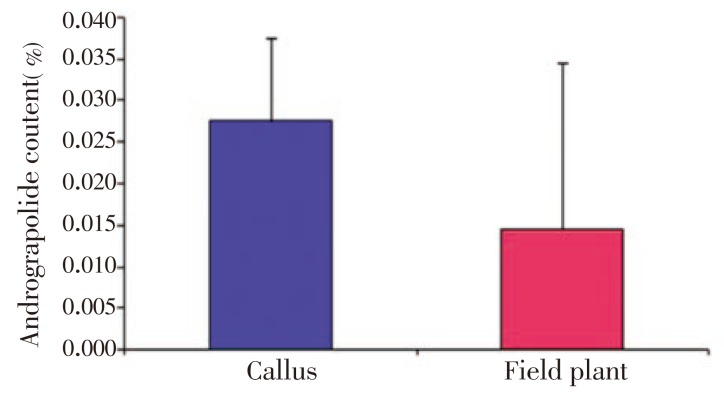
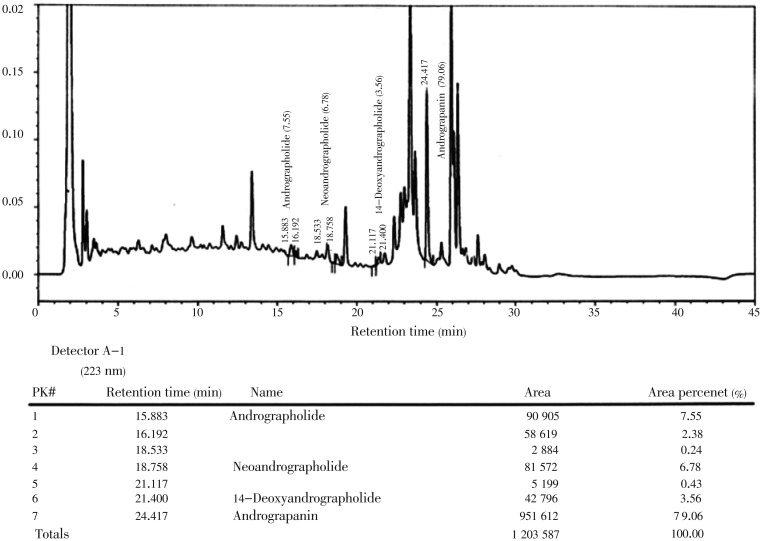
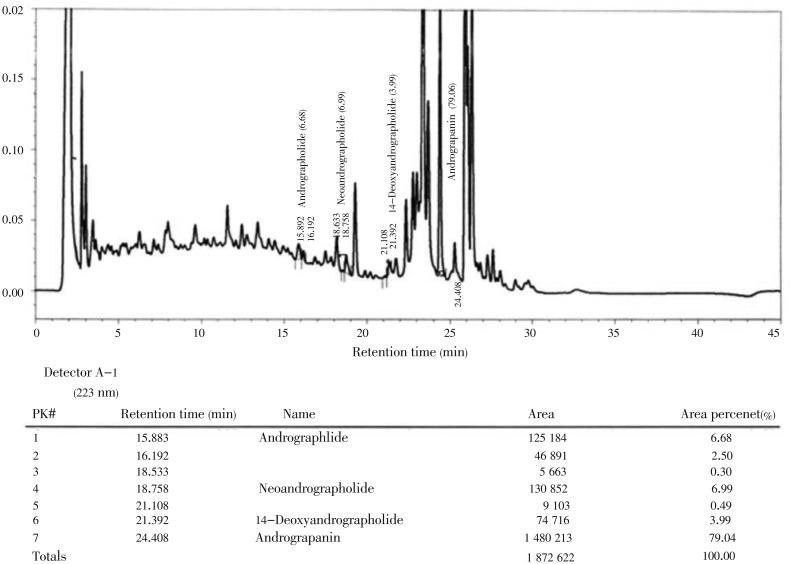
In this study, we investigated the anti-bacterial and anti-oxidant activity of AND and ECH isolated and purified from in vitro callus cultures of A. paniculata. The profile of andrographolide content in organogenic callus and leaf extracts from field grown plant of A. paniculata (Figure 1) showed variable content of AND, 0.027 5% and 0.014 5%, respectively, as analyzed by high performance liquid chromatography (HPLC) (Figures 2 and 3), which indicates the establishment of secondary metabolite production by callus culture is better than extracting from field grown plants with many practical problems related to quality and quantity of AND. Anti-bacterial activities of AND and ECH are presented in Table 1. Among the two compounds, the AND showed a broad spectrum of anti-bacterial activity against Gram (+) and Gram (-) bacteria, while ECH was only effective against S. aureus, B. subtilis, E. coli and P. aeruginosa among the bacteria tested. Both AND and ECH were inactive against C. albicans and S. cerevisiae at given concentrations. B. subtilis, S. aureus, E. coli, K. pneumonia, P. aeruginosa, and M. smegmatis were the most sensitive microorganisms (zone of inhibition above 14 mm in this study) to the concentration of purified AND used in this assay. The MIC value of each microbe was determined and the lowest concentration at which no visible microbial growth observed was defined as MIC. As shown in Table 2, the AND showed MIC value as low as 50 µg/mL against enteric bacteria E. coli and S. aureus at 100 µg/mL, while the ECH revealed specific growth inhibition activity against S. aureus, B. subtilis, E. coli, P. aeruginosa at a concentration higher than 225 µg/mL. Further, the isolated compounds from A. paniculata - AND and ECH were subjected to free radical scavenging activity by DPPH assay. The results showed the free superoxide anion scavenging activity ranging from 10% to 22% for AND, from 12% to 30% for ECH at a given concentrations of 10-160 µg/mL (Table 3).
Figure 1. Analysis of andrographolide content in organogenic callus and leaf extracts from field grown plant of A. paniculata by HPLC.
Figure 2. Analysis of andrographolide content from leaf extracts from field grown plant of A. paniculata Nees by HPLC.
Figure 3. Analysis of andrographolide content in organogenic callus of A. paniculata Nees by analytical HPLC.
Table 1. Anti-bacterial properties of andrographolide and echiodinin against various microorganisms using agar-disc diffusion method.
| Test organisms | Zone of inhibition (mm) |
|||
| Andrographolide (1 mg/disc) | Echiodinin (1 mg/disc) | Gentamicine (30 µg/ disc) | Myconazole (1 mg/disc) | |
| Staphylococcus aureus | 17.0±0.10 | 14.0±0.25 | 22.3±1.08 | - |
| Streptococcus thermophilus | 9.8±0.22 | x | 17.6±1.73 | - |
| Bacillus subtilis | 16.2±0.25 | 13.0±0.12 | 21.7±1.51 | - |
| Escheria coli | 18.5±0.40 | 12.0±0.25 | 23.4±0.76 | - |
| Mycobacterium smegmatis | 16.0±0.32 | x | 17.0±0.55 | - |
| Klebsiella pneumonia | 16.5±0.12 | x | 21.6±1.08 | - |
| Pseudomonas aeruginosa | 15.0±0.00 | 12.0±0.25 | 20.3±1.52 | - |
| Candida albicans | x | x | - | 16.7±3.5 |
| Saccharomyces cerevisiae | x | x | - | 17.0±1.5 |
“x” indicates no activity. The values for zone of growth inhibition are presented as mean±SD.
Table 2. Minimal inhibitory concentrations (MIC) of andrographolide and echiodinin using micro-dilution technique (µg/mL).
| Test organisms | Andrographolide | Echiodinin |
| Staphylococcus aureus | 100 | 225 |
| Streptococcus thermophilus | 350 | x |
| Bacillus subtilis | 100 | 325 |
| Escheria coli | 50 | 350 |
| Mycobacterium smegmatis | 200 | x |
| Klebsiella pneumonia | 100 | x |
| Pseudomonas aeruginosa | 200 | 300 |
| Candida albicans | x | x |
| Saccharomyces cerevisiae | x | x |
“x” indicates no activity.
Table 3. Free radical scavenging activities of andrographolide (AND) and echiodinin (ECH) determined by DPPH assay (%).
| Compound | Radical scavenging activity at different concentrations (µg/mL) |
||||
| 10 | 20 | 40 | 80 | 160 | |
| AND | 10±0.34 | 11±0.00 | 15±0.22 | 20±0.12 | 22±0.22 |
| ECH | 12±0.33 | 17±0.00 | 23±0.33 | 25±0.02 | 30±0.04 |
| Ascorbic acid | ND | 61± 0.33 | ND | ND | ND |
ND: not determined.
4. Discussion
Plant secondary metabolites such as flavonoids, terpenoids, glycosides, and steroids have gained considerable attention in recent years due to their diverse pharmacological properties including antibacterial and antioxidant activities. In the present study, we investigated the anti-bacterial and anti-oxidant activities of AND and ECH isolated and purified from in vitro callus cultures of A. paniculata. In the present study, the AND showed a broad spectrum of anti-bacterial activity against Gram (+) and Gram (-) bacteria including avirulent M. smegmatis, indicating the need of further research of the compound on virulent M. tuberculi. A previous study on crude methanol and chloroform extract of A. paniculata has shown significant anti-microbial activity only against B. subtilis and C. albicans at a concentration of 10 mg/disc[11]. Youhong et al. reported that neither the aqueous extract nor AND of A. paniculata were active against both Gram (+) and Gram (-) bacteria except that the ethanol fractions showed direct anti-bacterial activity against only two human pathogens like Legionella pneumophila and Bordetella pertussis at 5 mg/disc[12]. Sule et al. reported that dichloromethane, methanol and aqueous extracts have shown activity on all microbes tested at 1 mg/disc[13]. On contrary to the previous observation, AND showed a broad spectrum of growth inhibition activity, while ECH showed specific anti-bacterial activity against only S. aureus, E. coli, B. subtilis and P. aeruginosa at a concentration higher than 225 µg/mL. The differences in cell wall structure between Gram (+) and Gram (-) bacteria and polarity nature of compounds might be the probable reasons for their differential susceptibility to the bioactive compounds tested in this study. However, either of the isolated compounds did not show any anti-fungal activity against C. albicans and S. cerevisiae carried out in the present study, which does not support the antifungal activity of aqueous extracts and the fractions that were positive for arabinogalactan proteins and andrographolides reported previously[11]. This finding may be related with inability of the isolated compounds to penetrate the hydrophobic and hydrophilic lipid bilayers of cell wall architecture of yeast. Inhibition of non-pathogenic strain of M. smegmatis growth in this study is highly predictive of activity against human pathogenic M. tuberculosis[20].
Previous studies on crude extract of A. paniculata showed potent free radical activity with scavenging rate ranging from 48.0% to 66.8% at concentrations of 1-50 µg/mL[21]. In this study, the isolated compounds from A. paniculata - AND and ECH showed a moderate increase in the free superoxide anion scavenging activity ranging from 10% to 22% for AND and from 12% to 30% for ECH at a given concentrations of 10-160 µg/mL. These results suggest that the purified compounds possess potent free radical scavenging activity. As expected, the ECH showed relatively higher total antioxidant activity in a given tested concentration as compared to AND, which could be explained partially with respect to the number and position of free hydroxyl group attached to phenol rings[22]. The free hydroxyl group of ECH is more acidic in nature and can readily donates the phenolic hydrogens or electron to the acceptor molecules. This observation on total anti-oxidant activity of isolated compounds correlates well with a recent report[21]. Thus the observed weak and moderate free radical scavenging activity could be due to the absence of other potent anti-oxidant compounds present in the crude extracts of A. paniculata.
In conclusions, this study may provide pharmacological evidence for folklore medicinal uses of A. paniculata Nees against the treatment of microbial related ailments such as respiratory tract infection, acute diarrhoea, wound healing, skin inflammations and bacterial infections. The anti-bacterial and anti-oxidant efficacy of AND and ECH isolated from callus culture of A. paniculata Nees, an ethno-medicinal species of India, are reported for the first time, to our knowledge. Further studies are under progress in the laboratory to understand precise pharmacological properties of other isolated bioactive compounds.
Acknowledgments
The authors are highly grateful to UMK for their logistical support under grant No: R/SGJP/A07.00/00710A/001/2012/000081 and Indian Institute of Chemical Technology (IICT), Hyderabad is duly acknowledged for providing spectra of the compounds.
Comments
Background
From antiquity, medicinal herbs represent a rich source of antioxidants and antimicrobial agents. Unfortunately, in recent years, antimicrobial resistance, age related diseases and life style have become major public health concern issues. Therefore, there is a surge for novel antimicrobial and antioxidant agents from plant sources owing to its low cost and less side effects. Hence, the present research is a constructive step to fulfill the current need of the society.
Research frontiers
The isolation of secondary metabolites and their evaluation for biological potential are the starting point for pharmaceutical development. At the same time, the introduction of tissue culture technique for obtaining specific metabolite opens new avenue for further research and large scale production if required. Thus, the present study has demonstrated the amalgamation of above statements and reported the biological activities of andrographolide and echiodinin obtained from in vitro callus cultures of A. paniculata Nees for the first time.
Related reports
The isolated compounds exhibited only antibacterial activity and no antifungal activity in contrary to previously reported studies of Youhong et al. (2006) and Prajjal et al (2003). The activity level of each compound may be attributed to the extent of compound interaction with the cell wall of tested organisms. However, the moderate antioxidant activity of compounds is in accordance with previous findings of Lin et al. (2009) on andrographolide. The better antioxidant potential of echiodinin may correspond to the greater free radical scavenging tendency of phenolic OH which is lacking in andrographolide structure.
Innovations and breakthroughs
The data concerning biological activities of pure compounds from A. paniculata callus culture are scarce. The present study provides adequate evidence for the traditional use of A. paniculata by evaluating the antimicrobial and antioxidant potential of andrographolide and echiodinin. The reported observations related to antimicrobial activity of isolated compounds and antioxidant activity of echiodinin are novel to the best of our knowledge.
Applications
The isolation and identification of potential bioactive compounds are a vital step towards future pharmaceutical development. The isolated compounds can be further explored for other pathogenic bacterial and fungal stains. In order to obtain efficient activity of the compounds, their structural analogues can also be studied.
Peer review
This is an important novel research on active principles of A. paniculata in vitro callus cultures. The present study succeeded in providing supporting scientific evidence for its ethnopharmacological uses. Furthermore, isolation of andrographolide and echiodinin from callus cultures gave new dimensions to the study which may be helpful in mass production and exploitation of metabolites for future studies.
Footnotes
Foundation project: Financially supported by Universiti Malaysia Kelantan (Grant No. R/SGJP/A07.00/00710A/001/2012/000081).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Mishra BB, Tiwari VK. Natural products in drug discovery: Clinical evaluations and investigations. Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. 2011:p. 1–62. [Google Scholar]
- 2.Thirumalai T, Viviyan Therasa S, Elumalai EK, David E. Hypolipidaemic and antioxidant effect of Enicostemma littorale Blume. Asian Pac J Tropical Biomed. 2011;1:381–385. doi: 10.1016/S2221-1691(11)60084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valdiani A, Mihdzar AK, Tan SG, Talei D, Puad MA, Nikzad S. Nain-e Havandi (Andrographis paniculata) present yesterday, absent today: A plenary review on underutilized herb of Iran's pharmaceutical plants. Mol Biol Rep. 2012;39:5409–5424. doi: 10.1007/s11033-011-1341-x. [DOI] [PubMed] [Google Scholar]
- 4.Koteswara YR, Vimalamma G, Venkata Rao C, Yew-Min T. Flavonoids and andrographolides from Andrographis paniculata. Phytochem. 2004;65:2317–2321. doi: 10.1016/j.phytochem.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Sirisha Mulukuri NVL, Monda NB, Raghu Prasad M, Renuka S, Ramakrishna K. Isolation of diterpenoid lactones from the leaves of Andrographis paniculata and its anticancer activity. Int J Pharmacog Phytochem Res. 2011;3(3):39–42. [Google Scholar]
- 6.Nugroho AE, Andrie M, Warditiani NK, Siswanto E, Pramono S, Lukitaningsih E. Antidiabetic and antihiperlipidemic effect of Andrographis paniculata (Burm. f.) Nees and andrographolide in high-fructose-fat-fed rats. Indian J Pharmacol. 2012;44(3):377–381. doi: 10.4103/0253-7613.96343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levita J, Nawawi A, Mutalib A, Ibrahim S. Andrographolide: A review of its anti-inflammatory activity via inhibition of NF-kappaB activation from computational chemistry aspects. Int J Pharmacol. 2010;6:569–576. [Google Scholar]
- 8.Devaraj S, Jegathambigai R, Kumar P, Sivaramakrishnan S. A study on the hepatoprotective effect of Andrographis paniculata (Burm.F) Nees on mice. J Phytol. 2010;2(11):25–30. [Google Scholar]
- 9.Radhika P, Annapurna A, Nageswara Rao S. Immunostimulant, cerebroprotective & nootropic activities of Andrographis paniculata leaves extract in normal & type 2 diabetic rats. Indian J Med Res. 2012;135:636–641. [PMC free article] [PubMed] [Google Scholar]
- 10.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. New Delhi: CSIR; 1980. p. 18. [Google Scholar]
- 11.Singha PK, Roy S, Dey S. Antimicrobial activity of Andrographis paniculata. Fitoterapia. 2003;74:692–694. doi: 10.1016/s0367-326x(03)00159-x. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Marshal RL, Mukkur TKS. An investigation on the antimicrobial activity of Andrographis paniculata extracts and Andrographolide in vitro. Asian J Plant Sci. 2006;5:527–530. [Google Scholar]
- 13.Sule A, Ahmed QU, Samah OA, Omar MN. Screening for antibacterial activity of Andrographis paniculata used in Malaysian folkloric medicine: A possible alternative for the treatment of skin infections. Ethnobotanical Leaflets. 2010;14:445–456. [Google Scholar]
- 14.Rafat A, Koshy P, Sekaran M. Antioxidant potential and content of phenolic compounds in ethanolic extracts of selected parts of Andrographis paniculata. J Med Plants Res. 2010;4(3):197–202. [Google Scholar]
- 15.Wasman SQ, Mahmood AA, Chua LS, Alshawah MA, Hamdan S. Antioxidant and gastroprotective activities of Andrographis paniculata (Hempedu Bumi) in Sprague Dawley rats. Indian J Exp Biol. 2011;49:767–772. [PubMed] [Google Scholar]
- 16.Kishore KC, Arifullah M, Gayathri D, Gopal RG, Satish CR. Phytochemical and antimicrobial studies of methyl angolensate and luteolin-7-O-glucoside isolated from callus culture of Soymida febrifuga. Int J Biomed Sci. 2007;4:269–278. [PMC free article] [PubMed] [Google Scholar]
- 17.Hari Kishore P, Vijaya MBR, Kesava MR, Gunasekar D, Cristelle C, Bernard B. Flavonoids from Andrographis lineata. Phytochem. 2003;63:457–461. doi: 10.1016/s0031-9422(02)00702-1. [DOI] [PubMed] [Google Scholar]
- 18.Bauer AW, Kirby MM, Sheriss JC, Turik M. Susceptibility testing by standardised single method. Am J Clini Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 19.Turkmen N, Ferda S, Velioglu YS. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005;93:713–718. [Google Scholar]
- 20.McGaw LJ, Lall N, Hlokwe TM, Michel AL, Meyer JJM, Eloff JN. Purified compounds and extracts from Euclea species with antimycobacterial activity against Mycobacterium bovis and fast-growing mycobacteria. Biol Pharm Bull. 2008;31:1429–1433. doi: 10.1248/bpb.31.1429. [DOI] [PubMed] [Google Scholar]
- 21.Lin FL, Wu SJ, Lee SC, Ng LT. Antioxidant, antioedema and analgesic activities of Andrographis paniculata extracts and their active constituents Andrographolide. Phytother Res. 2009;23:958–964. doi: 10.1002/ptr.2701. [DOI] [PubMed] [Google Scholar]
- 22.Arteaga JF, Ruiz-Montoya M, Palma A, Alonso-Garrido G, Pintado S, Rodríguez-Mellado JM. Comparison of the simple cyclic voltammetry (CV) and DPPH assays for the determination of antioxidant capacity of active principles. Molecules. 2012;17(5):5126–5138. doi: 10.3390/molecules17055126. [DOI] [PMC free article] [PubMed] [Google Scholar]