Abstract
Objective
To investigate the suitability of citrus-press cakes, by-products of the juice industry as a source for the whitening agents for cosmetic industry.
Methods
Ethylacetate extracts of citrus-press cakes (CCE) were examined for their anti-melanogenic potentials in terms of the inhibition of melanin production and mechanisim of melanogenesis by using Western Blot analysis with tyrosinese, tyrosinase-related protein-1 (TRP-1), TRP2, and microphthalmia-associated transcription factor (MITF) proteins. To apply the topical agents, citrus-press cakes was investigated the safety in human skin cell line. Finally flavonoid analysis of CCE was also determined by HPLC analysis.
Results
Results indicated that CCE were shown to down-regulate melanin content in a dose-dependent pattern. The CCE inhibited tyrosinase, TRP-2, and MITF expressions in a dose-dependent manner. To test the applicability of CCE to human skin, we used MTT assay to assess the cytotoxic effects of CCE on human keratinocyte HaCaT cells. The CCE exhibited low cytotoxicity at 50 µg/mL. Characterization of the citrus-press cakes for flavonoid contents using HPLC showed varied quantity of rutin, narirutin, and hesperidin.
Conclusions
Considering the anti-melanogenic activity and human safety, CCE is considered as a potential anti-melanogenic agent and may be effective for topical application for treating hyperpigmentation disorders.
Keywords: Citrus-press cakes, Melanin, Melanogenesis, Tyrosinase, MITF, TRP-1, TRP-2
1. Introduction
Melanin, the end product of melanogenesis, determines the color of human skin, hair, and eyes and is synthesized within unique organelles of melanocytes called melanosomes[1],[2]. Although melanin mainly plays a photoprotective role in absorbing free radicals from the cytoplasm and shielding from UV light, the overproduction and accumulation of melanin in the skin could be a serious skin disorder and aesthetic problems, which are decreasing social functioning, reducing productivity at work or school, and lowering the self-esteem of patients[3]–[6]. Melanogenesis is regulated by various environmental, hormonal and genetic factors, including α-melanocyte stimulating hormone, theophylline, cAMP-elevating agents, and estrogen, and UV light. In the melanogenesis processes, tyrosinase is the key enzyme in the rate-limiting step in which L-tyrosine is hydroxylated to L-DOPA, which is further oxidized into the corresponding O-quinone. Tyrosinase is modulated by microphthalmia-associated transcription factor (MITF), a master transcription factor in melanogenesis. In addition, tyrosinase-related protein (TRP)-1 and TRP-2 are major targets of melanogenic enzymes induced by MITF. TRP-2 catalyzes the rearrangement of dopachrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA), whereas TRP-1 oxidizes DHICA to a carboxylated indole-quinone, which is eventually converted into melanin[6],[7]–[9]. Hence, downregulation of tyrosinase activity has been proposed to be responsible for reduced melanin production. In industrial point of view, cosmetics can help depigment skin color, which is one of the important parts among several functional cosmetic fields. Especially, development of novel whitening phytochemicals from natural sources has become new popular trends recently[10]. Actually, several studies have focused on the inhibition of melanogenesis and the prevention of abnormal pigmentation for cosmetic reasons[11]–[13].
Citrus fruits rank first in the world with respect to production among the fruits. The citrus crop is also a valuable contributor to the economy of Jeju Island, Korea, with annual yields of more than 600 000 tons. Citrus unshiu is the most cultivated compared with other species of citrus and is an economically important fruit of Jeju Island in Korea. However, the citrus-press cakes are one of the major problem of agricultural waste, with annual yields of more than 60 000 tonnes in Jeju Island alone from juice factories. This waste involves substantial costs for handling and transport to disposal location and the prices of recycled materials are often not high enough to cover operating costs[14],[15]. Because of the London Dumping Convention, it will also become impossible to dump the waste into the ocean. Therefore, research into the utilisation of the citrus-press cakes of C. unshiu, is important and urgently required in order to solve the problem of agricultural wastes in Jeju Island. The aim of this research was to elucidate the biological activities of citrus-press cakes of C. unshiu, which would facilitate the conversion of this waste into high value-added products. This would then allow it to be recycled as a component of cosmetic materials. More specifically, the present study focused on whether citrus-press cakes of C. unshiu has anti-melanogenic effects.
2. Materials and methods
2.1. Materials and solvent extraction
The citrus-press cakes of C. unshiu after juice extraction was obtained from a local food processing company (Ilhae Corporation, Jeju, Korea), frozen and stored at -20 °C until use. For extraction, the material was first ground into a fine powder and freeze-dried using a vacuum freeze-dryer. The dried powder (50 g) was extracted with 80% ethanol (EtOH; 2 L) at room temperature for 24 h and then evaporated under vacuum. The evaporated EtOH extract (5 g) was suspended in water (1 L) and fractionated with ethyl acetate (EtOAc; 500 mL). The yield and recovery of EtOAc fractions were 0.108 g and 2.16%, respectively.
2.2. Cell culture
Mouse melanocyte B16F10 was purchased from the Korean Cell Line Bank (KCLB; Seoul, Korea). Human keratinocyte HaCaT cells were acquired from the Biospectrum Inc. R&D Center, Korea. Mouse melanocyte B16/F10 and human keratinocyte HaCaT cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal bovine serum (GIBCO, Inc., NY, USA) and 1% penicillin-streptomycin at 37 °C in a humidified 95% air/5% CO2 atmosphere.
2.3. Cell viability assay
The cell viability assay was carried out as described using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Chemical Co.)[11]. Shortly thereafter, B16F10 murine melanoma cells and human keratinocyte HaCaT cells were plated in a 24-well plate. After cells were exposed separately to citrus-press cakes EtOAc fractions (CCE) at concentrations of 12.5, 25.0, and 50.0 µg/mL for 24 h, MTT solutions were added and the insoluble derivative formed by cellular dehydrogenase was solubilized with EtOH-dimethyl sulfoxide (DMSO) (1:1 mixture solution); the absorbance of each well was estimated at 560 nm using a microplate reader. Percent of cells showing cytotoxicity was determined relative to the control group.
2.4. Melanin content assay
B16F10 melanoma cells were cultured in DMEM with 10% fetal bovine serum and penicillin/streptomycin (100 IU/50 µg/mL) in a humidified atmosphere containing 5% CO2 in air at 37 °C. Intracellular melanin content was measured as previous described with some modifications[11]. The cells were treated with α-melanocyte stimulating hormone (MSH) (100 nmol/L) for 24 h, and further treated with CCE (12.5, 25, and 50 µg/mL) for another 24 h. After treatments, the cells were detached by incubation in trypsin/ethylene diamine tetraacetic acid and subsequently centrifuged at 5 000 g for 5 min, and then the cell pellets were solubilized in NaOH at 60 °C for 60 min. The melanin content was assayed at 405 nm absorbance by spectrophotometric analysis.
2.5. Analysis of the expression of proteins regulating melanogenesis by Western blotting
Measurement of tyrosinase, TRP-1, TRP-2, and MITF in B16F10 cells by Western blot was undertaken as described previously by Yoon et al. in 2010. B16F10 cells that had been stimulated by α-MSH (100 nmol/L) were treated with the CCE (12.5, 25.0, and 50.0 µg/mL) for 3 days. After treatment, the cells were collected and lysed with cell lysis buffer [50 mmol/L Tris-HCl (pH 6.8), 2% sodium dodecyl sulfonate, 6% mercaptoethanol, 1% glycerol]. Whole cell lysates were separated by 7.5% SDS-PAGE and transferred to polyvinylidene fluoride membrane. The membrane was blocked with 5% skimmed milk in PBS containing 0.05% Tween 20. Tyrosinase, TRP-1, TRP-2 and MITF bands were detected with the rabbit polyclonal anti-tyrosinase antibody (dilution 1:1 000), anti-TRP-1 antibody (dilution 1:1 000), anti-TRP-2 antibody and anti-MITF antibody (dilution 1:500), respectively, which were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and then further incubated with horseradish peroxidase-conjugated anti-rabbit IgG antibody at a 1:5 000 dilution. Bound antibodies were detected using an enhanced chemiluminescence kit (Amersham) following the manufacturer's instructions. Loading control was assessed using anti-β-actin antibody. Positive bands were analysed using a gel image analysis instrument.
2.6. Determination of cellular tyrosinase activity
Cellular tyrosinase activity was measured by the method of Yen et al. with some modification. Briefly, the cells were treated with α-MSH (100 nmol/L) for 24 h, and then further treated with various concentrations of CCE (12.5, 25.0, and 50.0 µg/mL) for another 24 h. After treatments, the cells were washed twice with PBS buffer and homogenized with 50 mmol/L PBS (pH 7.5) buffer containing 1.0% Triton X-100 and 0.1 mmol/L phenylmethanesulfonyl fluoride. Cellular extracts (100 µL) were mixed with freshly prepared L-DOPA solution (5.0 mmol/L in 50 mmol/L PBS, pH 6.8) and incubated at 37 °C for 30 min. The absorbance at 490 nm was measured with a microplate reader (BIO-TEK Instrument, Winooski, VT, USA) to monitor the production of dopachrome.
2.7. HPLC fingerprint ofCCE
Chromatographic analysis of CCE performed using a HPLC with Aliance™ Waters e2695 separation module coupled to a Waters 2489 UV/Visible detector, utilizing a Capcell pak 4.6mm×250.0mm, 5§--) C18 column at a flow rate 1.0 mL/min. A binary gradient elution system consisted of methanol (A) and 1% acetic acid in water (B). The gradient program was: 0-5 min, 10%-25% A; 5-30 min, 25%-40% A; 30-35 min, 40%-100% A; 30-40 min; and finally, reconditioning the column with 10% A isocratic for 8min. The injection volume was 10 µL and UV detection was performed at 280 nm. Ethanol stock solution containing (1) rutin, (2) narirutin, (3) naringin, (4) hesperidin, (5) neohesperidin, (6) neohesperidin dihydrochalcone, (7) naringenin, (8) hesperetin, (9) aprgrnin, (10) nobiletin, (11) tangeretin, (12) auraptene was diluted with ethanol to obtain a series concentration of working solutions, and subjected to HPLC analysis.
2.8. Statistical analysis
All data were obtained in triplicate and are presented as means±standard error. Significant differences between treatments were determined with the Student's t-test in One-way analysis of variance.
3. Results
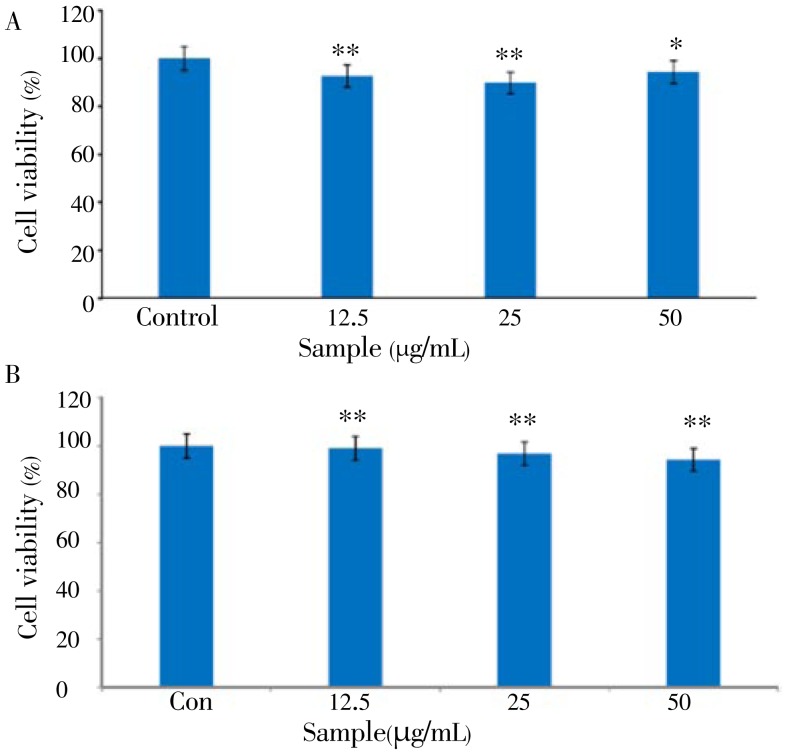
The MTT assay is a common colorimetric assay to measure the activity of enzymes that reduce MTT to formazan dyes, giving a purple color. It can also be used to determine the cytotoxicity of potential medicinal agents and toxic materials, since those agents stimulate or inhibit cell viability and growth. The viability of B16F10 cells treated with different concentrations of CCE is shown in Figure 1A.
Figure 1. Cell viability of the citrus-press cakes on the B16F10 cells and human keratinocyte HaCaT cells.
A: B16F10 cells; B: human keratinocyte HaCaT cells. MTT assay was performed after incubation of the B16F10 cells treated with varying concentrations (12.5, 25.0, and 50 µg/mL) of the EtOAc fraction of citrus-press cakes for 24 h at 37 °C in a 5% CO2 atmosphere. The absorbance was measured at 570 nm with a spectrophotometer (Power Wave; Bio-tek, Winooski, VT). Values are the mean±SEM of triplicate experiments. *: P<0.05; **: P<0.01.
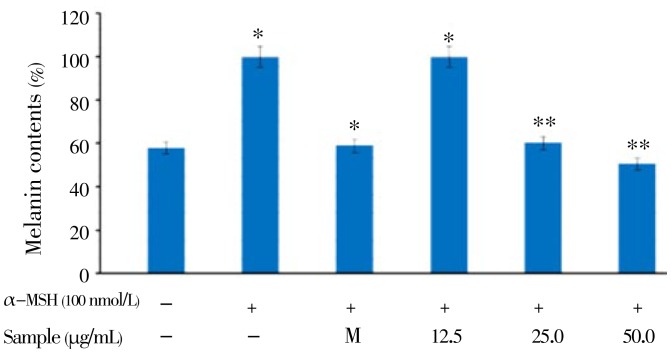
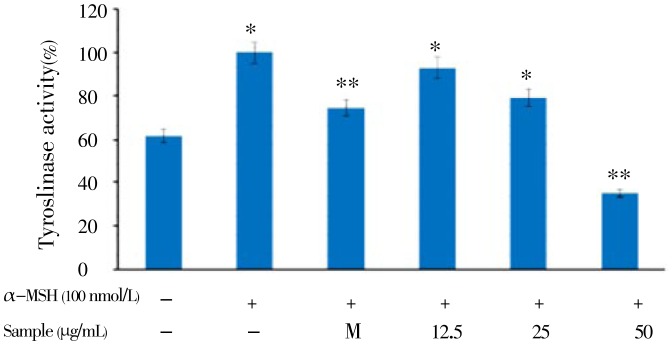
Murine B16F10 melanoma cells were treated with a serial dose of CCE (12.5 to 50.0 µg/mL), and their viability was more than 95%. In addition, the viability of human keratinocyte HaCaT cells treated with the same concentration of CCE was almost 100% (Figure 1B). These results indicated that CCE is a safe ingredient for determining the antimelanogenesis effect. Therefore, we used CCE at doses of 12.5-50.0 µg/mL to determine the cellular melanin synthesis and tyrosinase activity in B16F10 cells. Since cellular tyrosinase activity is the major factor that stimulates melanin synthesis and ultimately induces melanogenesis, we determined the cellular tyrosinase activity and melanin content for investigating the anti-melanogenesis activity of CCE on B16F10 cells. B16F10 cells were pretreated with CCE at dose of 12.5-50.0 µg/mL. As shown in Figure 2, CCE treatment significantly decreased the cellular melanin content in a dose-dependent manner compared to that in the control group (P<0.05). In addition, CCE treatment significantly reduced the cellular tyrosinase activity in a dose-dependent manner compared to the control (Figure 3).
Figure 2. Melanin content assay of the citrus-press cakes on the B16F10 cells.
B16F10 cells (2.0×104 µg/mL) were pre-incubated for 18 h, and the melanin content was assayed after incubation of the B16F10 cells treated with α-MSH (100 nmol/L), melasolv (40 µmol/L), and the CCE (12.5, 25.0, and 50.0 µg/mL) for 72 h at 37 °C in a 5% CO2 atmosphere. The absorbance was measured at 405 nm by an ELISA.
Figure 3. Inhibitory effect on tyrosinase activity of citrus-press cakes in B16F10 cells.
B16F10 cells (2.0×104 µg/mL) were pre-incubated for 18 h and tyrosinase activity was performed after incubation of B16F10 cells treated with α-MSH (100 nmol/L), melasolv (40 µmol/L) and EtOAc fraction of citrus-press cakes (12.5, 25.0, and 50.0 µg/mL) for 72 h at 37 °C in a 5% CO2 atmosphere. Absorbance was measured at 405 nm with a ELISA. Values are mean±SEM of triplicate experiments. *: P<0.05; **: P<0.01.
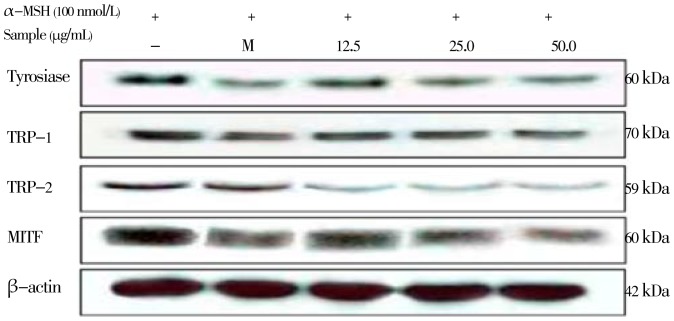
To investigate whether CCE can influence melanogenic protein expression, Western blotting analysis was carried out using the lysate of B16/F10 melanoma cells treated with CCE and stimulated by α-MSH (100 nmol/L) (Figure 4). When cells were stimulated by α-MSH, a significant increase in tyrosinase protein was observed, and TRP-1 and TRP-2 expression also increased. The other way around, the CCE inhibited α-MSH-stimulated tyrosinase and TRP-1, TRP-2 expression of B16/F10 melanoma cells in a dose-dependent manner. The protein level of β-actin, a housekeeping protein that was used as an internal control, also showed no change. Taken together, these observations suggest that the CCE reduced the expression of tyrosinase, TRP-1, and TRP-2, in a concentration-dependent manner.
Figure 4. Inhibitory effect of the citrus-press cakes on the protein level related to melanogenic factors in the B16F10 cells.
B16F10 cells (1.0 × 105 cells/mL) were pre-incubated for 18 h and were stimulated with α-MSH (100 nmol/l) in the presence of melasolv (40 µmol/L) and the EtOAc fraction of citrus-press cakes (12.5, 25.0, and 50.0 µg/mL) for 24 h. The protein level was determined by immunoblotting.
MITF is the major regulator of the synthesis of TRPs such as TYR, TRP-1, and TRP-2 during the process of melanogenesis in mammalian cells. Therefore, the effect of the CCE on MITF expression have to be evaluated. As shown in Figure 4, based on the Western blot analysis, the upstream transcription factor MITF was down-regulated in a dose-dependent manner. The present results suggest that MITF protein levels are reduced by the CCE. The hypopigmentation effect of the CCE may be the result of down-regulation of MITF gene expression, which would then repress the protein and gene expressions of tyrosinase, TRP-1, and TRP-2.
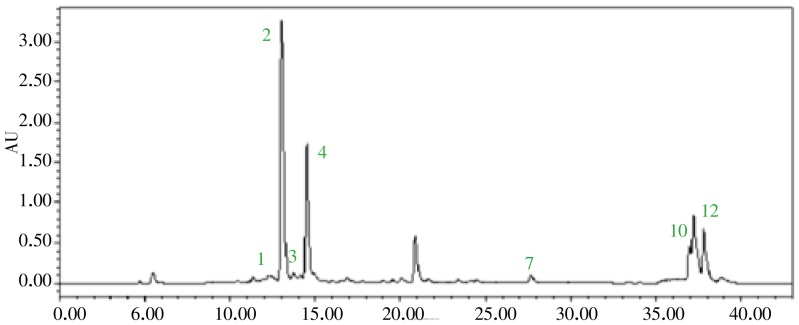
Finally, in other to apply CCE for whitening agents in cosmetic industry, Standard and/or functional materials have to be investigated. Therefore, the flavonoid constituents of the CCE were also investigated using HPLC analysis in the present study. As shown in Figure 5, a total of 12 citrus flavonoids were identified on the basis of the compared to those in our HPLC library. The CCE is a predominantly composed of rutin (12 mg/g), narirutin (206 mg/g), and hesperigin (86 mg/g).
Figure 5. HPLC fingerprinting analysis of the CCE.
The wavelength of flavonoid absorbance is 280 nm. The injection volume was 10 µL. Ethanol stock solution containing (1) rutin, (2) narirutin, (3) naringin, (4) hesperidin, (5) neohesperidin, (6) neohesperidin dihydrochalcone (NHDC), (7) naringenin, (8) hesperetin, (9) aprgrnin, (10) nobiletin, (11) tangeretin, (12) auraptene was diluted with ethanol to obtain a series concentration of working solutions, and was subjected to HPLC analysis.
4. Discussion
Careful consideration should be given to issues of safety to formulate the plant extract or phytochemicals as functional food and cosmetic agents. For example, hydroquinone is a commonly used skin-whitening agent for treating and preventing hyperpigmentation disorders in cosmetic industry, however it has been associated with a lot of side effects, including skin irritation, contact dermatitis, and exogenous ochronosis in dark-skinned people[16]. Therefore, the use of cosmetics containing hydroquinone is prohibited in the European Union and is strictly controlled in the United States by the Food and Drug Administration[16],[17]. For that reason, we firstly performed the cell viability of CCE for estimating the in vitro safety, including murine B16F10 melanoma cells and human keratinocyte HaCaT cells in this study. The results in the present study indicated that even higher concentration of CCE had no cytotoxic effect on B16F10 melanoma cell and human keratinocyte HaCaT cell viability.
Melanin is synthesized by an enzymatic cascade that is controlled by tyrosinase TRP1, and TRP2. Accordingly, the inhibition of melanogenesis-related gene and protein expression plays an important role for the efficacy of cosmetics and depigmenting agents, which are utilized in the treatment of hyperpigmentation. To elucidate the true inhibitory effect of the CCE on melanogenesis, B16F10 melanin content and intracellular tyrosinase activity were assayed at the same concentration range. The results in Figure 2 indicated that the CCE present a stronger inhibitory effect on melanin formation in B16F10 cells than arbutin does (data not shown). The data provided evidence that CCE blocks melanogenesis in B16F10 melanoma cells. The results shown in Figure 3 were in accordance with the results indicated in Figure 2, which means the CCE inhibited B16F10 intracellular tyrosinase activity and then decreased the melanin content in a dose-dependent manner. In mammalian melanocytes, melanogenesis is controlled at least by three regulatory proteins, tyrosinase, and TRP1 and TRP2. In contrast, hyperpigmentation is by overactivity of TRPs, but not all skin-whitening agents can simultaneously inhibit TYR, TRP-1, and TRP-2, such as Arthrophytum scoparium, Glechoma hederacea and nicotinic acid hydroxamat[18]–[20]. However, our Western blotting assay showed that CCE treatment reduced the expression of all rate-limiting enzymes, including tyrosinase, TRP-1, and TRP-2 protein, and prevented abnormal accumulation of melanin in the process of melanogenesis. These data suggested that the decrease in melanin content by CCE treatment was because of inhibition of TRPs; therefore, CCE could be used as a skin-whitening agent against hyperpigmentation. In summary, the present study is firstly demonstrated that CCE inhibits melanogenesis by downregulating MITF protein and finally reduces the synthesis of tyrosinase and production of melanin. Thus, on the basis of the molecular biological mechanism of CCE, we suggest that CCE can be safely used as be a skin-whitening agent. Further investigations will focus on the in vivo assessment of the biological activity of citrus-press cakes for anti-melanogenesis activity. In conclusion, to the best of our knowledge, this is the first report demonstrating the in vitro anti-melanogenesis activity of the citrus-press cakes and providing a scientific basis for its cosmetic application.
Acknowledgments
Foundation Project: Supported by the Next-Generation BioGreen 21 Program, Rural Development Administration, Republic of Korea. Grant No. PJ009583002013.
Comments
Background
Overproduction and accumulation of melanin in the skin could be a serious skin disorder and aesthetic problems, which are decreasing social functioning, reducing productivity at work or school, and lowering the self-esteem of patients. Hence, production of cheap, affordable, effective and less toxic anti-melanogenic agent from natural resources are timely needed. The aim of this research was to elucidate the biological activities of citrus-press cakes of C. unshiu as a new anti-melanogenic agent.
Research frontiers
Current research was conducted to study the potential C. unshiu as anti-melanogenic agent. The finding of this study may help to developed natural products based anti-melanogenic agent which is less toxic.
Related reports
Bae et al., reported protective effects of fermented C. unshiu peel extract against ultraviolet-a-induced photoageing in human dermal fibrobolasts in Phytotherapy Research, 2012. The photoprotective potential of fermented C. unshiu peel extract with S. commune (S-CPE) was tested in human dermal fibroblasts (HDFs) exposed to UVA. It was revealed that S-CPE had an inhibitory effect on human interstitial collagenase (matrix metalloproteinase, MMP-1) expression in UVA-irradiated HDFs. The treatment of UVA-irradiated HDFs with S-CPE resulted in a dose-dependent decrease in the expression level of MMP-1 mRNA. The UVA irradiation raised the proportion of senescence-associated β-galactosidase (SA-β-gal) positive cells in comparison with the normal control group. The treatment of UVA-irradiated HDFs with S-CPE was shown to decrease the level of SA-β-gal (by approximately 45% at an S-CPE concentration 0.1%, w/v) compared with the UVA-irradiated HDFs. It was found that S-CPE containing hesperetin has notable collagen biosynthetic activity for fibroblasts, indicating that S-CPE can be promising cosmetic ingredients.
Innovations and breakthroughs
The citrus-press cakes are one of the major problems of agricultural waste, with annual yields of more than 60 000 tonnes in Jeju Island alone from juice factories. This waste involves substantial costs for handling and transport to disposal location and the prices of recycled materials often are not high enough to cover operating costs. Interestingly, the authors were attempted to convert the agro waste into useful products such as anti-melanogenic agent.
Applications
A citrus-press cake from C. unshiu has great potential in cosmetic application and cosmetic products development.
Peer review
This is a good study in which the author investigates the suitability of citrus-press cakes, by-products of the juice industry as a source for the whitening agents or anti-melanogenic agents for cosmetic industry. The results are interesting and can be applied in the development of cosmetic products especially the cosmetic products dealing with skin disorders.
Footnotes
Foundation Project: Supported by the Next-Generation BioGreen 21 Program, Rural Development Administration, Republic of Korea, Grant No. PJ009583002013.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Regad T. Molecular and cellular pathogenesis of melanoma initiation and progression. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1324-2. Epub 2013 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88:76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandya AG, Guevara IL. Disorders of hyperpigmentation. Dermatol Clin. 2000;18:91–98. doi: 10.1016/s0733-8635(05)70150-9. [DOI] [PubMed] [Google Scholar]
- 4.Balkrishnan R, McMichael AJ, Camacho FT, Saltzberg F, Housman TS, Grummer S, et al. et al. Development and validation of a health-related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572–577. doi: 10.1046/j.1365-2133.2003.05419.x. [DOI] [PubMed] [Google Scholar]
- 5.Balkrishnan R, McMichael AJ, Hu JY, Camacho FT, Shew KR, Bouloc A, et al. et al. Correlates of health-related quality of life in women with severe facial blemishes. Int J Dermatol. 2006;45:111–115. doi: 10.1111/j.1365-4632.2004.02371.x. [DOI] [PubMed] [Google Scholar]
- 6.Yen FL, Wang MC, Liang CJ, Ko HH, Lee CW. Melanogenesis inhibitor(s) from Phyla nodiflora extract. Evid Based Complement Alternat Med. 2012;2012:867494. doi: 10.1155/2012/867494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tachibana M. MITF: a stream flowing for pigment cells. Pigment Cell Res. 2000;3:230–240. doi: 10.1034/j.1600-0749.2000.130404.x. [DOI] [PubMed] [Google Scholar]
- 8.Kadono S, Manaka I, Kawashima M, Kobayashi T, Imokawa G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J Invest Dermatol. 2001;116:571–577. doi: 10.1046/j.1523-1747.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- 9.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 10.Oh MJ, Hamid MA, Ngadiran S, Seo YK, Sarmidi MR, Park CS. Ficus deltoidea (Mas cotek) extract exerted anti-melanogenic activity by preventing tyrosinase activity in vitro and by suppressing tyrosinase gene expression in B16F1 melanoma cells. Arch Dermatol Res. 2011;303:161–170. doi: 10.1007/s00403-010-1089-5. [DOI] [PubMed] [Google Scholar]
- 11.Yoon WJ, Kim MJ, Koh HB, Lee WJ, Lee MH, et al. et al. Effect of Korean red sea cucumber (Stichopus japonicus) on melanogenic protein expression in murine B16 melanoma. Int J Pharmacol. 2010a;6:37–42. [Google Scholar]
- 12.Yoon WJ, Kim MJ, Moon JY, Kang HJ, Kim GO, Lee NH, et al. et al. Effect of palmitoleic acid on melanogenic protein expression in murine B16 melanoma. J Oleo Sci. 2010b;59:315–319. doi: 10.5650/jos.59.315. [DOI] [PubMed] [Google Scholar]
- 13.Kim SS, Hyun CG, Choi YH, Lee NH. Tyrosinase inhibitory activities of the compounds isolated from Neolitsea aciculata (Blume) Koidz. J Enzyme Inhib Med Chem. 2012 doi: 10.3109/14756366.2012.670806. [DOI] [PubMed] [Google Scholar]
- 14.Kim SS, Lee JA, Kim JY, Lee NH, Hyun CG. Citrus peel wastes as functional materials for cosmeceuticals. J Appl Biol Chem. 2008;51:7–12. [Google Scholar]
- 15.Yang EJ, Kim SS, Oh TH, Baik JS, Lee NH, Hyun CG. Essential oil of citrus fruit waste attenuates LPS-induced nitric oxide production and inhibits the growth of skin pathogens. Int J Agri Biol. 2009;11:791–794. [Google Scholar]
- 16.Lima LL, Lima RM, da Silva AF, do Carmo AM, da Silva AD, Raposo NR. Azastilbene analogs as tyrosinase inhibitors: new molecules with depigmenting potential. Sci World J. 2013;2013:274643. doi: 10.1155/2013/274643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheth VM, Pandya AG. Melasma: a comprehensive update: part II. J Am Acad Dermatol. 2011;65:699–714. doi: 10.1016/j.jaad.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Chao HC, Najjaa H, Villareal MO, Ksouri R, Han J, Neffati M, et al. et al. Arthrophytum scoparium inhibits melanogenesis through the down-regulation of tyrosinase and melanogenic gene expressions in B16 melanoma cells. Exp Dermatol. 2013;22:131–136. doi: 10.1111/exd.12089. [DOI] [PubMed] [Google Scholar]
- 19.Qiao Z, Koizumi Y, Zhang M, Natsui M, Flores MJ, Gao L, et al. et al. Anti-melanogenesis effect of Glechoma hederacea L. extract on B16 murine melanoma cells. Biosci Biotechnol Biochem. 2012;76:1877–1883. doi: 10.1271/bbb.120341. [DOI] [PubMed] [Google Scholar]
- 20.Lin YS, Chuang MT, Chen CH, Chien MY, Hou WC. Nicotinic acid hydroxamate downregulated the melanin synthesis and tyrosinase activity through activating the MEK/ERK and AKT/GSK3β signaling pathways. J Agric Food Chem. 2012;60:4859–4864. doi: 10.1021/jf301109p. [DOI] [PubMed] [Google Scholar]