Abstract
Objective
To assess the molluscicidal and cercariacidal activities of aqueous extracts of Balanites aegyptiaca (B. aegyptiaca) against Ethiopian Biomphalaria pfeifferi (B. pfeifferi), Lymnaea natalensis (L. natalensis) and Schistosoma mansoni (S. mansoni) cercariae.
Methods
Extracts of seeds, endocarp, mesocarp, and fruit of B. aegyptiaca were tested for their activities against adult B. pfeifferi and L. natalensis. The cercariacidal activity of the seeds of the plant was also evaluated against S. mansoni. Bioassays were carried out following the methods recommended by WHO. Snail mortalities were compared between each plant part and snail species, and LC50 and LC90 values for the plant parts tested were computed. The cercariacidal activity of the plant was assessed by exposing the mice to the cercariae pre-exposed to aqueous extract of B. aegyptiaca seeds.
Results
For the molluscicidal activities of seeds, endocarp, mesocarp and whole fruit, the LC50 values against B. pfeifferi were 56.32, 77.53, 65.51 and 66.63 mg/L, respectively, while the respective LC90 values were 77.70, 120.04, 89.50 and 97.55 mg/L. Similarly, the LC50 values for the seeds, endocarp, mesocarp and whole fruit against L. natalensis were 80.33, 92.61, 83.52 and 87.84 mg/L, respectively, while the respective LC90 values were 102.30, 138.21, 115.42 and 127.69 mg/L. B. pfeifferi were found to be more susceptible to B. aegyptiaca than L. natalensis. S. mansoni cercariae exposed to 15 mg/L of extract of seeds were incapable of infecting mice. The mean egg load of tissue was reduced in mice infected with the cercariae exposed to 5 and 10 mg/L of the extract.
Conclusions
The aqueous extracts of different parts of B. aegyptiaca exhibited reasonable molluscicidal activity against B. pfeifferi and L. natalensis, as well as cercariacidal activity against S. mansoni cercariae. However, comprehensive laboratory evaluation is recommended prior to field tests of the plant parts since their impact on other aquatic biota is not known.
Keywords: Balanites aegyptiaca, Molluscicide, Biomphalaria pfeifferi, Lymnaea natalensis, Cercariacide, Schistosoma mansoni
1. Introduction
Schistosomiasis is one of the most prevalent parasitic worm infections and has significant economic and public health consequences. It affects many countries, particularly in sub-Saharan Africa[1]. It is estimated that over 200 million people are infected with Schistosoma and over 600 million people are reported to be at risk[2],[3]. The five most common species of schistosome infecting humans are Schistosoma mansoni (S. mansoni), Schistosoma japonicum, Schistosoma haematobium, Schistosoma mekongi, and Schistosoma intercalatum[4]. The snails of the genera Biomphalaria, Bulinus and Oncomelania serve as intermediate hosts of schistosomes and play a crucial role in the transmission of the disease[5].
Fascioliasis is an important helminthic infection caused by trematodes of the genus Fasciola. The distributions of etiologic agents overlap in many areas of Africa and Asia[6]. Fascioliasis is one of the most important parasitic diseases in tropical and subtropical countries[7]. The World Health Organization (WHO) has estimated that 2.4 million people are infected with Fasciola and 180 million are at risk of infection[8].
The high cost of synthetic molluscicides and their negative impacts on the environment, as well as fear for emergence of snail resistance to these compounds have given a new impetus to the study of molluscicidal plants. Several plants, such as Phytolacca dodecandra (Endod), Solanum xanthocarpum, Annona squamosa, Thuja orientalis, Stryphnodendron polyphyllum, Calotropis procera and Adenium arabicum have already been identified as useful to control the intermediate hosts of trematodes[9]–[14].
Balanites aegyptiaca (B. aegyptiaca) is a tree, classified as a member of the family Balanitaceae. B. aegyptiaca, deep rooted arid zone tree has a very wide natural range. The tree is valued for its fruits and seeds. The seed kernel is rich in oil, protein, minerals and is edible as snacks after boiling. The wide range of habitat in which this species is occurring suggests high pattern of variation among and within locations[15].
Various parts of B. aegyptiaca tree have been used for folk medicines in many regions of Africa and Asia[16]. Its fruit, bark and other parts have lethal properties to snail intermediate hosts, schistosome miracidia and cercariae, and the cercariae of other trematodes[17]. Nevertheless, the molluscicidal and cercariacidal properties of the plant have not been studied in Ethiopia. The purpose of the present study, therefore, was to evaluate the molluscicidal and cercariacidal activity of aqueous extracts of B. aegyptiaca seeds and fruits in Ethiopia.
2. Materials and methods
2.1. Collection and preparation of the plant materials
The seeds and fruits of B. aegyptiaca were collected from Ziway and Arsi Negele areas, in the Oromia Regional State of Ethiopia. The plant was authenticated by a botanist, and voucher specimens were deposited at the National Herbarium of Addis Ababa University, (specimen No. Eshetu 001). Mature unripe B. aegyptiaca fruits (Figure 1) were washed with tap water. The epicarp (outer cover) of the fruits was removed by using sterile sharp surgical blade, and the fruits pulps/mesocarps were scraped manually and the seeds were then collected. The endocarp of the fruits was broken manually and the seeds were then collected. The prepared fruit parts (endocarp and mesocarp), the whole fruit and seeds were air dried under shade in the laboratory for 5-6 d, separately[16]. Thereafter, the dried specimens were manually ground into powder using mortar and pestle. The powder, then, was sieved through 250 microns mesh to obtain a fine material. The powdered fine plant material was then transferred into closed containers until use.
Figure 1. B. aegyptiaca, a branch with fruits.

2.2. Collection of the snails
The snails Biomphalaria pfeifferi (B. pfeifferi) and Lymnaea natalensis (L. natalensis) were collected from different areas. B. pfeifferi were collected from Chacha, Senbete and Mekele (Northern Ethiopia). L. natalensis were collected from Wondo Genet (Southern Ethiopia) and Jiga (Northwest Ethiopia). The snails were then transported to the laboratory at Aklilu Lemma Institute of Pathobiology, Addis Ababa University for maintenance. They were then left to acclimatize to standard laboratory conditions before being used in the experiments. Snails were maintained in the aquaria containing dechlorinated tap water at room temperature[7].
2.3. Preparation of the stock solution and serial dilution
The concentrations used in the bioassays were prepared from fruits (endocarp and mesocarp), seeds and the whole fruit in successive dilutions with aged water. One gram of each powdered dry plant part used in the tests was weighed using an electric balance. The weighed powdered dry part was soaked in 1 000 mL of aged water for 24 h with occasional vigorous shaking using a shaker. Then, the suspension was filtered using filter paper (Whatman No. 1).
After the preparation of the stock solution of 1 000 mg/L, successive concentrations of the aqueous extracts were prepared to obtain the final concentrations of 65, 70, 75, 80, 85, 90, 95, 100 mg/L for testing against B. pfeifferi snails. And for L. natalensis snails, 85, 90, 95, 100, 105, 110, 115 and 120 mg/L, in a final volume of 500 mL in each solution were prepared. For each test, there was a control with aged water, without plant material, with the same volume of the solution[10],[18].
2.4. Molluscicidal activity tests
Water extracts of B. aegyptiaca seeds, endocarp, mesocarp, and fruit were tested against adult B. pfeifferi and L. natalensis snails according to the method recommended by WHO[19]. To each concentration of plant parts used, 10 adult snails of both B. pfeifferi and L. natalensis were immersed. Each test concentration was set up in duplicate. Tests were carried out at room temperature. After 24 h of exposure, the suspension was decanted, the snails were rinsed thrice with aged water and transferred to another aged water and maintained there for another 24 h recovery period. Ten snails were immersed in separate aged water with the same volumes of the solvent that would serve as control. Two replicates for each test were used. All groups were observed carefully after 24 h, the number and the percentages of death in each group were calculated. Snails were considered dead if they could not move or retracted well into or hanging out of the shell, with the body and shell discolored[20].
2.5. Cercariacidal activity tests
Infected B. pfeifferi snails were individually placed in shedding vials containing 5 mL of aged water and exposed to artificial light for 30 min. The emerging cercariae were pooled and counted with the aid of microscope. Three glass beakers containing 500 mL of 5, 10 and 15 mg/L of water extract of B. aegyptiaca seeds prepared in aged water were set up in duplicates. Over 300 cercariae were slightly caught with pipettes and transferred to each beaker and allowed to stand for 2 or 4 h. Similarly, six glass beakers containing 500 mL aged water were set up in duplicates. After exposing the cercariae for 2 or 4 h to these concentrations, 200 cercariae (pre-determined infective dose) were again transferred from beaker to another beaker containing aged water. In each of the beakers one mouse was placed and allowed to stay for 40 min for exposure by the paddling method (through skin penetration of the legs). After 40 min of exposure to cercariae the mice were returned to their cages and maintained in the animal house under standard conditions. Control mice were exposed to the same numbers of cercariae that were not exposed to the seeds extract[21].
After 45 d post exposure to the cercariae, the faecal samples of mice were collected and checked for the presence of schistosome eggs. Each infected mice were sacrificed to determine the mean egg load in each tissue (liver, small and large intestines). Tissue egg load was determined by digesting known weights of liver, small intestine and large intestine by mechanical grinder. After grinding each tissue, about 0.10 g of each tissue was observed in microscope and the eggs were counted. The number of eggs obtained were then used to extrapolate for the total weight of each tissue and finally converted to number of eggs per gram of tissue[21]. The mean of numbers of egg loads for the total mice perfused for each concentration was calculated by geometric mean, using the formula:
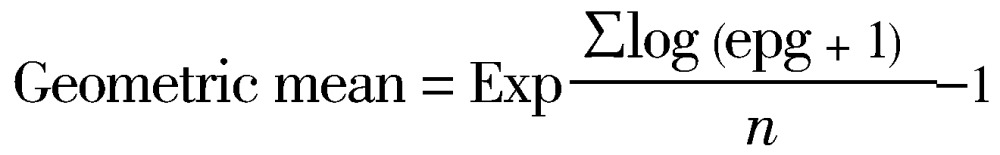 |
Where,
epg=the number of eggs per gram of tissue;
Exp=exponential or antilogarithm;
log (epg + 1)=the sum of the logarithm of each mouse epg;
n=the number of mice perfused in each concentration.
2.6. Data analysis
Probit regression analysis using SPSS program version 13.0 was carried out for all tested parts of the plant to determine the LC50 and LC90 values against both snail species with 95% confidence intervals (CI). Analysis for variance (One-way ANOVA) was used to determine the significant reduction in tissue egg load in the mice.
3. Results
3.1. Molluscicidal activity
The molluscicidal potency of all tested parts of B. aegyptiaca against both B. pfeifferi and L. natalensis were concentration dependent. Generally, mortality increased with the increase in concentration of the extracts.
The lethal concentrations for aqueous extracts of B. aegyptiaca seeds, endocarp, mesocarp and the whole fruit that killed 50% (LC50) of adult B. pfeifferi were 56.32, 77.53, 65.51 and 66.63 mg/L, respectively, while the respective LC90 values were 77.70, 120.04, 89.50 and 97.55 mg/L (Table 1).
Table 1. Effect of aqueous extract of B. aegyptiaca on mortality rates of B. pfeifferi after 24 h of exposure.
| Plant parts | LC50 (mg/L) | LC50 (CI) | LC90 (mg/L) | LC90 (CI) |
| Seeds | 56.32 | (36.99-63.61) | 77.70 | (71.97-86.63) |
| Endocarp | 77.53 | (70.15-83.11) | 120.04 | (104.18-178.95) |
| Mesocarp | 65.51 | (56.31-70.35) | 89.50 | (83.81-102.16) |
| Fruit | 66.63 | (55.64-72.09) | 97.55 | (89.42-120.35) |
The lethal concentrations for aqueous extracts of B. aegyptiaca seeds, endocarp, mesocarp and the whole fruit that killed 50% (LC50) of adult L. natalensis were, 80.33, 92.61, 83.52 and 87.84 mg/L, respectively, while the respective LC90 values were 102.30, 138.21, 115.42 and 127.69 mg/L (Table 2). Comparing the LC50 and LC90 values of the plant parts, seeds showed the highest molluscicidal activity, followed by mesocarp, whole fruit and then the endocarp against both snail species.
Table 2. Effect of aqueous extract of B. aegyptiaca on mortality rates of L. natalensis after 24 h of exposure.
| Plant parts | LC50 (mg/L) | LC50 (CI) | LC90 (mg/L) | LC90 (CI) |
| Seeds | 80.33 | (66.38-86.38) | 102.30 | (97.35-111.92) |
| Endocarp | 92.61 | (79.88-98.76) | 138.21 | (121.97-212.11) |
| Mesocarp | 83.52 | (68.15-90.03) | 115.42 | (107.53-139.06) |
| Fruit | 87.84 | (72.93-94.19) | 127.69 | (115.59-174.56) |
3.2. Cercariacidal activity
The infectivity of the cercariae to mice was evaluated by exposing the mice to the cercariae pre-exposed to aqueous extract of B. aegyptiaca seeds. The infection was completely inhibited at 15 mg/L, at 2 or 4 hours of exposure of cercariae to the seeds extract. Concentrations lower than 15 mg/L significantly reduced tissue egg load (P<0.05) (Table 3).
Table 3. The in vivo observation on S. mansoni cercarial infectivity of aqueous extract of B. aegyptiaca seeds.
| Exposure time | Concentration (mg/L) | No. of mice | GM |
||
| SI | Liver | LI | |||
| 2 h | Control | 2 | 80.44 | 52.94 | 48.00 |
| 5 | 2 | 50.40 | 35.55 | 39.09 | |
| 10 | 2 | 37.60 | 26.70 | 25.93 | |
| 15 | 2 | 0.00 | 0.00 | 0.00 | |
| 4 h | Control | 2 | 62.18 | 59.74 | 47.46 |
| 5 | 2 | 27.46 | 32.50 | 29.29 | |
| 10 | 2 | 9.32 | 15.82 | 10.94 | |
| 15 | 2 | 0.00 | 0.00 | 0.00 | |
LI=Large intestine; SI=Small intestine; GM=Geometric mean of egg counts per gram of tissue.
4. Discussion
The present study showed that aqueous extracts of B. aegyptiaca seeds and fruits possess molluscicidal and cercariacidal properties. Their activities are time and concentration-dependent. Between the test snail hosts of trematodes, B. pfeifferi were more susceptible to the plant extract than L. natalensis. Aqueous extracts of B. aegyptiaca seeds showed the highest molluscicidal activity followed by mesocarp, whole fruit and endocarp on both test snail species after 24 h of exposure period. The seeds and fruits showed high molluscicidal activity against both snails. Previous investigators also reported similar observations[20].
The varying potencies of each plant part may be due to the differences in concentration and/or the type of the active ingredient(s) present in each part[22]. In this observation, water extracts of seeds and mesocarps were more potent than the water extract of endocarps, perhaps due to the more concentrations of saponins in the mesocarps and seeds, and also presence of high amount of deltonin in the seeds.
The LC50 values of aqueous extracts of the seeds and fruit observed against L. natalensis in this study were higher than the values reported by Vijay[20]. Vijay obtained LC50 values of 60 mg/L after the exposure of L. acuminata to aqueous extracts of B. aegyptiaca fruits for 24 h. In the present study, the LC50 values against L. natalensis were 80.33 mg/L for seeds, 92.61 mg/L for endocarp, 83.52 mg/L for mesocarp, and 87.84 mg/L for fruit. On the other hand, the LC50 value of the seeds against B. pfeifferi was 56.32 mg/L, but for other plant parts the LC50 values were above 60 mg/L (77.53 mg/L for endocarp, 65.51 mg/L for mesocarp and 66.63 mg/L for whole fruit). In another study, Anto et al. reported that extracts of B. aegyptiaca fruits have molluscicidal activity with LC95 values of 19.7 mg/L and 12.0 mg/L against adult Bulinus globosus and Bulinus truncatus, respectively[23].
In Sudan, Ragab et al. studied the molluscicidal activity of this plant and found out that B. aegyptiaca saponins (seeds) showed significant effect on Bulinus truncatus at different concentrations[24]. They also observed that the mortality of snails increased with increasing concentrations and exposure period.
Compared to some other plants tested for their molluscicidal activities, B. aegyptiaca has high toxicity to snail hosts of trematodes for some while for others it has low molluscicidal property. The LC50 values for Cymbopogon nervatus against B. pfeifferi are less than 213.099 mg/L[25]. Abdalla et al. observed that the LC50 and LC90 values for ethanolic extract of Euphorbia aphylla against Biomphalaria alexandrina were 87.6 mg/L and 142.5 mg/L, respectively[26]. Hassan et al. also observed that the LC50 and LC90 values of butanol extracts of Meryta denhamii fruits against Lymnaea natalensis were 26.4 mg/L and 70.8 mg/L, respectively[27], which is lower than the present study against the same snail species. The LC50 and LC90 of Ziziphus spinachristi ethanolic extract against L. natalensis snails were 311 mg/L and 500 mg/L respectively, which is higher as compared to the current study[26].
In order for a plant to be considered as candidate molluscicide, its crude extract should be active at 100 mg/L or lower when 90% of the snails are killed after 24 h exposure[28]. The results of the current observation indicated that aqueous extracts of the seeds, mesocarp and whole fruit caused 90% B. pfeifferi mortality after 24 h exposure time at 77.70, 89.50 and 97.55 mg/L, respectively. Hence, this plant could be a potential molluscicide for B. pfeifferi. On the other hand, the LC90 values for B. aegyptiaca against L. natalensis were above 100 mg/L, that is, 102.30, 115.42 and 127.69 mg/L for seeds, mesocarps and fruits, respectively, indicating that aqueous extract of this plant parts are less potent against L. natalensis.
The present in vivo observation on cercariacidal properties of B. aegyptiaca showed that water extract of B. aegyptiaca seeds made S. mansoni cercariae less infective to mice at lower concentrations. S. mansoni cercariae exposed to 15 mg/L of aqueous extract of seeds were incapable of infecting the mice. Furthermore, at 5 and 10 mg/L, the mean tissue egg load was significantly reduced. This observation is in agreement with that of others who used different plant species as schistosomicide. Birrie et al. showed that pre-treatment of the cercariae with 12 mg/L of the endod berries completely inhibited infection of mice and significantly reduce egg deposition in tissue even below a concentration of 12 mg/L[21]. Abozeid et al. also demonstrated that tannins extracted of pomegranate (Punica granatum) have a remarkable antischitosomal activity particularly on miracidia[29]. Both molluscicidal and cercariacidal activities of extracts of Punica granatum were demonstrated. Rind methanol and water extracts were lethal to 100% of cercariae at concentrations of 25 and 30 mg/L, respectively, after 24 h[29].
From the present study, it can be concluded that aqueous extracts of seeds and fruits of B. aegyptiaca have reasonable molluscicidal activity against B. pfeifferi and L. natalensis, and cercariacidal activity against S. mansoni cercariae. The aqueous extracts of B. aegyptiaca seeds were more toxic to the cercariae of S. mansoni than to B. pfeifferi, snail intermediate hosts of S. mansoni.
Acknowledgments
The study was financially supported by School of Graduate Studies, Addis Ababa University (Grant No. GSR/2830/02). The authors would also like to thank the Aklilu Lemma Institute of Pathobiology, Addis Ababa University, for permission to use laboratory and all the necessary facilities for the study.
Comments
Background
Trematode infection is one of the major problems leading to enormous health and economic losses in Ethiopia. Commercial molluscicidal or cercariacidal drugs are relatively expensive and this is further complicated by the development of drug resistance and toxicity to aquatic life. Screening and proper evaluation of medicinal plants could offer possible alternative for sustainable and affordable use. The results of this preliminary study are interesting and warrant further studies.
Research frontiers
Various studies are currently underway in many developing countries to assess the effects of crude plant products, by applying different extraction techniques and using in vitro and in vivo techniques. Active ingredients demonstrating promising results separated from plant products should undergo acute and chronic toxicity studies both on the hosts and environment.
Related reports
A number of in vitro and in vivo studies of medicinal plans are currently underway to examine the antiparasitic and antibiotic properties based on the very promising work on schistosomiasis control using medicinal plant products. Such studies should be encouraged and supported.
Applications
The results of this study can make a significant contribution in the control of fluke infections in humans and animals provided further in vitro and in vivo studies. Most products having molluscicidal proporties often produce toxic effect on aquatic life. Hence possible undesirable effects of this plant product should be assessed.
Peer review
This is a useful study which is adequately organized and written. The preliminary findings of the molluscicidal and cercariacidal properties of the crude extract of B. aegyptiaca have demonstrated encouraging results for further studies.
Footnotes
Foundation Project: Financially supported by School of Graduate Studies, Addis Ababa University (Grant No. GSR/2830/02).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Mbata T, Orji M, Oguoma VM. The prevalence of urinary schistosomiasis in Ogbadibo local government area of Benue State, Nigeria. The Int J Epidemiol. 2008;6(1) doi: 10.5580/25fd. [DOI] [Google Scholar]
- 2.Ibrahim MYA. Khartoum: University of Khartoum; 2009. Studies on molluscicidal activity of some plants from Darfur against B. truncatus with emphasis on Alternanthera nodiflora (Amaranthaceae) [dissertation] [Google Scholar]
- 3.WHO Schistosomiasis epidemiological record. Bull World Health Organ. 2010;85:157–164. [Google Scholar]
- 4.McManus P, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abadi RN. Khartoum: University of Khartoum; 2010. Chemical constituents and molluscicidal activity of Azadirachta indica A. Juss (Neem) leaf extract [dissertation] [Google Scholar]
- 6.Sultan K, Desouky AY, Elsiefy MA, El-bahy NM. An abattoir study on the prevalence of some gastrointestinal helminths of sheep in Gharbia Governorate, Egypt. Glob Vet. 2010;5:84–87. [Google Scholar]
- 7.Singh KL, Singh DK, Singh VK. Toxicity of Bauhinia variegata and Mimusops elengi with plant molluscicides against Lymnaea acuminata. J Biol Earth Sci. 2012;2:B76–B82. [Google Scholar]
- 8.Degheidy NS, Al-Malki JS. Epidemiological studies of fasciolosis in human and animals at Taif, Saudi Arabia. World Appl Sci J. 2012;19:1099–1104. [Google Scholar]
- 9.Karunamoorthi K, Bishaw D, Mulat T. Laboratory evaluation of Ethiopian local plant Phytolacca dodecandra extract for its toxicity effectiveness against aquatic macroinvertebrates. Eur Rev Med Pharmacol Sci. 2008;12:381–386. [PubMed] [Google Scholar]
- 10.Changbunjong T, Wongwit W, Leemingsawat S, Tongtokit Y, Deesin V. Effect of crude extract of Solanum xanthocarpum against snails and mosquito larvae. Southeast Asian J Trop Med Public Health. 2010;41:320–325. [PubMed] [Google Scholar]
- 11.Tiwari F. Bait formulation toxicity of plant derived molluscicides in attractant food pellets against vector snail, Lymnaea acuminate. World J Zool. 2012;7:54–59. [Google Scholar]
- 12.Singh A, Singh VK. Molluscicidal activity of Saraca asoca and Thuja orientalis against the fresh water snail Lymnaea acuminata. Vet Parasitol. 2009;164:206–210. doi: 10.1016/j.vetpar.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Vinaud MC, Junior RSL, Bezerra JCB. Activity of Stryphnodendron polyphyllum, a plant from the Brazilian savannah, against hemocytes of Biomphalaria glabrata, an intermediate host of Schistosoma mansoni. Rev Patol Trop. 2008;37:237–246. [Google Scholar]
- 14.Al-Sarar A, Hussein H, Abobakr Y, Bayoumi A. Molluscicidal activity of methomyl and cardenolide extracts from Calotropis procera and Adenium arabicum against the land snail Monacha cantiana. Molecules. 2012;17:5310–5318. doi: 10.3390/molecules17055310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elfeel AA. Variability in Balanites aegyptiaca var. aegyptiaca seed kernel oil, protein and minerals contents between and within locations. Agr Biol J N Am. 2010;1:170–174. [Google Scholar]
- 16.Chapagain BP, Wiesman Z. Larvicidal effects of aqueous extracts of Balanites aegyptiaca (desert date) against the larvae of Culex pipiens mosquitoes. Afr J Biotechnol. 2005;4:1351–1354. [Google Scholar]
- 17.Chothani DL, Vaghasiya HU. A review on Balanites aegyptiaca Del (desert date): phytochemical constituents, traditional uses, and pharmacological activity. Pharmacogn Rev. 2011;5:55–62. doi: 10.4103/0973-7847.79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng F, Liu L, Huang Q, Liu N, Yang H, Sun H, et al. Molluscicidal effect of Eomecon chionantha alkaloids against Oncomelania hupensis snails. Southeast Asian J Trop Med Public Health. 2011;42:289–296. [PubMed] [Google Scholar]
- 19.WHO . Geneva: WHO; 1983. Report of scientific working group on plant molluscicide and guidelines for evaluation of plant molluscicide, (TDR/SCH – SWE (4)/ 83.3) [Google Scholar]
- 20.Vijay P. Evaluation of molluscicidal activity of some Indian medicinal plants against Lymnaea acuminate. Int J Appl Biol Pharm Technol. 2010;1:308–311. [Google Scholar]
- 21.Birrie H, Balcha F, Erko B, Bezuneh A, Gemeda N. Investigation into the cercariacidal and miracidiacidal properties of Endod (Phytolacca dodecandra) berries (type 44) East Afr Med J. 1998;75:311–314. [PubMed] [Google Scholar]
- 22.Brimer L, Elsheik S, Furu P. Preliminary investigation of the disposition of the molluscicidal saponin deltonin from Balanites aegyptiaca in a snail species (Biomphalaria glabrata) and in mice. J Pestic Sci. 2007;32:213–221. [Google Scholar]
- 23.Anto F, Aryeetey M, Anyorigiya T, Asoala V, Kpikpi J. The relative susceptibilities of juvenile and adult Bulinus globosus and Bulinus truncatus to the molluscicidal activities in the fruit of Ghanaian Blighia sapida, Blighia unijugata and Balanites aegyptiaca. Ann Trop Med Parasitol. 2005;99:211–217. doi: 10.1179/136485905X24229. [DOI] [PubMed] [Google Scholar]
- 24.Ragab SA, Elmalik KH, Adam SE. Khartoum: Sudan University of Science and Technology; 2005. Susceptibility of Bulinus truncatus snails to various concentrations of Balanites aegyptiaca saponin: Short communication. [Google Scholar]
- 25.EL-Kamali HH, EL-Nour RO, Khalid SA. Molluscicidal activity of the essential oils of Cymbopogon nervatus leaves and Boswellia papyrifera resins. Curr Res J Biol Sci. 2010;2:139–142. [Google Scholar]
- 26.Hassan AA, Mahmoud AE, Attia RAH, Huseein EAM. Evaluation of the ethanolic extracts of three plants for their molluscicidal activities against snails intermediate hosts of Schistosoma mansoni and Fasciola. Int J Basic and Appl Sci. 2012;1:235–249. [Google Scholar]
- 27.Hassan SE, Rahman EHA, El Monem ARA. Molluscicidal activity of butanol fraction of Meryta denhamii flowers against Lymnaea natalensis and Biomophalaria alexandrina. Global Veterinaria. 2010;4:15–21. [Google Scholar]
- 28.Mott K. Plant molluscicides. USA: John Wiley and Sons Ltd; 1987. p. 326. [Google Scholar]
- 29.Abozeid K, Shohayeb M, Ismael A. In vitro tests for efficacy of tannins extracted from pomegranate (Punica granatum) against Schistosoma mansoni miracidia. J Sci Tech. 2012;13:531–538. [Google Scholar]


