Abstract
Objective
To evaluate antibacterial activity of the Indonesian water soluble green tea extract, Camellia sinensis, against clinical isolates of methicillin-resistant Staphylococcus aureus (S. aureus) (MRSA) and multi-drug resistant Pseudomonas aeruginosa (MDR-P. aeruginosa).
Methods
Antimicrobial activity of green tea extract was determined by the disc diffusion method and the minimum inhibitory concentration (MIC) was determined by the twofold serial broth dilutions method. The tested bacteria using in this study were the standard strains and multi-drug resistant clinical isolates of S. aureus and P. aeruginosa, obtained from Laboratory of Clinical Microbiology, Faculty of Medicine, University of Indonesia.
Results
The results showed that the inhibition zone diameter of green tea extracts for S. aureus ATCC 25923 and MRSA were (18.970±0.287) mm, and (19.130±0.250) mm respectively. While the inhibition zone diameter for P. aeruginosa ATCC 27853 and MDR-P. aeruginosa were (17.550±0.393) mm and (17.670±0.398) mm respectively. The MIC of green tea extracts against S. aureus ATCC 25923 and MRSA were 400 µg/mL and 400 µg/mL, respectively, whereas the MIC for P. aeruginosa ATCC 27853 and MDR-P. aeruginosa were 800 µg/mL, and 800 µg/mL, respectively.
Conclusions
Camellia sinensis leaves extract could be useful in combating emerging drug-resistance caused by MRSA and P. aeruginosa.
Keywords: Camellia sinensis, Green tea, Antibacterial activity, MIC, Multi-drug resistant bacteria
1. Introduction
The development of antibiotic resistance in bacteria is a major issue in the prevention of infectious diseases. Currently the spread of multi-drug resistant bacteria is not only through nosocomial infections, but also occur in the community[1]–[2]. Several multi-drug resistant bacteria that are most commonly found, especially through nosocomial infections, are Enterococcus faecium, Staphylococcus aureus (S. aureus), Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa (P. aeruginosa) and Enterobacter spp[3].
In Indonesia, the predominant multi-resistant bacteria that causes the infection such as P. aeruginosa, Klebsiella pneumoniae, Escherichia coli and methicillin resistant Staphylococcus aureus (S. aureus) (MRSA) have been found in several hospitals in Indonesia[4]–[7].
As the bacteria that cause the infection was resistant to first-line antibiotics, treatment options are usually replaced with a second or third choice of antibiotics, which are generally much more expensive. Therefore, alternative antimicrobial agents are needed to be developed and employed to control multi-drug resistant bacteria. To face this challenge, there has been growing interests to find antimicrobial compounds from medicinal plant extracts as an alternative approach to discover new antimicrobial compounds. The antimicrobial activities of some herbal medicines against different pathogens have been reported from different countries[8],[9].
Camellia sinensis (C. sinensis), which is one of the most popular beverages worldwide, has been reported to have antimicrobial activities against various pathogenic bacteria[10]–[13], including MRSA[15] and MDR-P. aeruginosa[14]–[16]. However, in Indonesia, there is no study on investigation of the antibacterial effects of C. sinensis against MRSA and MDR-P. aeruginosa. Therefore, in this study, we investigated the antibacterial activity of the extract of green tea (C. sinensis) against clinical isolates of MRSA and MDR-P. aeruginosa.
2. Materials and methods
2.1. Preparations of plant extracts
C. sinensis leaves were collected in Bogor, West Java, Indonesia, and identified at the Center for Plant Conservation Bogor Botanical Gardens, Indonesia, and the voucher specimens were deposited at the Laboratory of Phytochemistry, Faculty of Pharmacy, University of Indonesia.
The leaves of the plant were air-dried and reduced to coarse powder. About 40 g was extracted overnight with distilled water. The distillates were freeze-dried to get dried plant extracts. Certain concentration of the plant extract was prepared by dissolving it with sterile distilled water, and filtrated through a 0.2 µm membrane filter (Whatman, USA).
2.2. Antibiotic sensitivity test
Susceptibility testing of the isolated strains and the standard strains of P. aeruginosa and S. aureus was performed using disc-diffusion method. Antibiotic susceptibility test of the bacterial strains was performed using standard antimicrobial susceptibility testing discs (Oxoid). The results are interpreted in accordance with the guidelines of the Clinical and Laboratory Standards Institute[17].
2.3. Antibacterial activity test
Antibacterial activity was determined using the disc diffusion method according to the Clinical and Laboratory Standards Institute guidelines[18]. The bacteria used in this study were S. aureus ATCC 25923, P. aeruginosa ATCC 27853, clinical isolates of MRSA and MDR-P. aeruginosa, which were obtained from the Laboratory of Clinical Microbiology, Faculty of Medicine, University of Indonesia.
The dried plant extracts were dissolved in sterile distilled water to a final concentration of 0.8 mg/mL, and sterilized by filtration through a 0.2 µm membrane filter (Whatman, USA).
Pre-warmed Mueller-Hinton agar (Oxoid) plates were seeded with 106 CFU suspension of tested bacteria. An aliquot of plant extract (0.8 mg/mL) were pipetted (20 µL) onto sterile paper discs (6 mm diameter, Oxoid) and placed onto the surface of inoculated agar plates. Plates were incubated at 37 °C for 24 h. Antibacterial activity was expressed as the diameter of the inhibition zone (mm) produced by the extracts around the disc. All tests were carried out in triplicates.
2.4. Determination of minimum inhibitory concentration (MIC)
The MIC assay was determined by the twofold serial broth dilutions method in sterile tubes, according to Clinical and Laboratory Standards Institute[18], with slight modifications. The dried plant extracts were dissolved in sterile distilled water to a final concentration of 3.2 mg/mL, and filtrated through a 0.2 µm membrane filter (Whatman, USA).
Overnight culture of each test organisms (approximately 106 CFU) was seeded into the tubes containing nutrient broth (Oxoid) and the plant extracts were tested at concentration from 1.600 to 0.025 mg/mL. The tubes were incubated for 24 h at 37 °C. MIC was determined as the lowest concentration of the plant extract that inhibited the growth of the tested bacteria.
3. Results
Determination of antibiotic sensitivity against clinical isolates used in this study exhibited that MRSA was resistant to some antibiotics such as amoxicillin+clavulanic acid (30 µg), oxacillin (1 µg), sulbenicillin (100 µg), gentamicin (10 µg), tetracycline (30 µg), erythromycin (15 µg), ofloxacin (5 µg), and clarithromycin (15 µg), while MDR-P. aeruginosa was resistant to amoxicillin+clavulanic acid (30 µg), sulbenicillin (100 µg), carbenicillin (100 µg), kanamycin (30 µg), chloramphenicol (30 µg), sulphamethoxazole+trimethoprim (25 µg), and ampicillin+sulbactam (20 µg). The results have proved that either MRSA or MDR-P. aeruginosa isolates were resistant to many classes of antibiotics.
The inhibition zone diameter of green tea extracts against selected bacterial strains is shown in Table 1. The MIC of green tea extracts for laboratory strain S. aureus ATCC 25923 and MRSA were 400 µg/mL and 400 µg/mL, respectively, whereas the MIC for laboratory strain P. aeruginosa ATCC 27853 and MDR-P. aeruginosa were 800 µg/mL and 800 µg/mL, respectively.
Table 1. Antimicrobial activity of green tea extracts on selected bacterial strains (Mean±SD).
| Bacterial strains | Zone of inhibition (mm)* |
| S. aureus ATCC 25923 | 18.970±0.287 |
| MRSA | 19.130±0.250 |
| P. aeruginosa ATCC 27853 | 17.550±0.393 |
| MDR-P. aeruginosa | 17.670±0.398 |
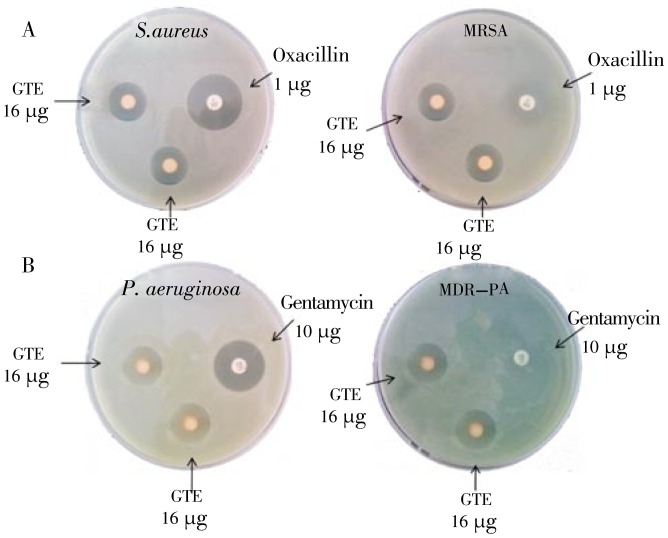
Figure 1 describes the killing activities of green tea extract against laboratory strain S. aureus ATCC 25923, MRSA, laboratory strain P. aeruginosa ATCC 27853, and MDR-P. aeruginosa, compared with standard antibiotic oxacillin (1 µg) and gentamicin (10 µg),
Figure 1. Antimicrobial activity of green tea extracts (GTE).
A: The activity of GTE against S.aureus ATCC 25923 and MRSA, compared with standard antibiotic oxacillin 1 µg; B: The activity of GTE against P. aeruginosa ATCC 27853, and MDR-P. aeruginosa, compared with standard antibiotic gentamicin 10 µg.
MRSA that was resistant to oxacillin (1 µg), showed sensitive to green tea extracts (16 µg), The inhibition zone diameter of green tea extract (16 µg) for MRSA was 19.13 mm, while MDR-P. aeruginosa that has proven resistant to gentamicin (10 µg), was still sensitive to green tea extracts (16 µg).
4. Discussion
Control of infections acquired in hospitals and communities caused by multi-drug resistant Gram-positive and Gram-negative bacteria has become a major problem not only in developing countries but also in developed countries. In the past few decades, MRSA and MDR-P. aeruginosa become an increasingly important pathogen in both hospitals and community settings[4],[19], MRSA and MDR-P. aeruginosa play an important role in the colonization and infection of hospitalized patients. These bacteria are often implicated in a variety of nosocomial infections including bacteremia, urinary tract infections, and nosocomial pneumonia. Treatment of these infections is often very difficult due to cross-resistance of these bacteria with a large group of antibiotics, so it seems reasonable to explore new sources of natural compounds with antibacterial activity against them. Recently, natural products and herbal medicines with anti-microbial effects have been recognized with increasing interest by clinical pharmacologists. C. sinensis has been proved to possess medicinal and health promotion properties, including the ability to inhibit the growth of some types of pathogenic bacteria[20].
In this study we found that the MIC of green tea extract against MRSA was 400 µg/mL, while the MIC for MDR-P. aeruginosa was 800 µg/mL. The anti-bacterial activity of green tea extract is comparable to standard antibiotic. The activity of 16 µg green tea extract against laboratory strain S. aureus ATCC 25923 was comparable to that of commercially available oxacillin (1 µg), whereas the activity of 16 µg green tea extract was comparable to that of commercially available gentamicin (10 µg) against laboratory strain P. aeruginosa ATCC 27853, even though green tea extract was slightly less effective. Green tea extract showed good activity against MRSA and MDR-P. aeruginosa, although both of these bacteria have been resistant to multiple classes of antibiotics.
The result of this study is consistent with other studies that have previously been reported that green tea has anti-bacterial activity against resistant bacteria strains such as vancomycin-resistant enterococci, MRSA, and MDR-P. aeruginosa. Several previous studies have shown that green tea extract showed activity against both MRSA and methicillin-sensitive Staphylococcus aureus[21], and against MDR-P. aeruginosa[11],[16].
The properties of green tea which inhibit bacterial growth are mainly related to their polyphenolic components including epicatechin, epicatechin gallate, epigallocatechin, and epigallocatechin gallate against various Gram-positive and Gram-negative bacteria[22],[23]. Green tea was also reported to have a synergistic effect with β-lactam antibiotics against MRSA[24]–[28], It was also reported that the main component of tea polyphenols, epigallocatechin gallate can reverse methicillin resistance of MRSA by inhibiting the synthesis of PBP2[29], Epigallocatechin gallate not only increases the activity of β-lactams but also increases the activity of non-β-lactam cell wall biosynthesis inhibitors[26].
Mechanism of action of green tea leaves extract has been proposed that green tea can prevent the attachment of pathogenic bacteria on the host cell membrane. Thus, green tea extract inhibits the adhesion of bacteria on host cell surface membranes and acts as a potential anti-adhesive agent[30]. Epigallocatechin gallate, which is a type of proanthocyanidin from green tea has also been reported to interact with the outer membrane bacterial and may prevent the adhesion to mammalian epithelial cells (HEp-2), and probably without alteration in mammalian epithelial cells[31],[32].
Another possible mechanism is green tea extract may affect the activity of dihydrofolate reductase, an enzyme that is needed by pathogenic bacteria to synthesize purine and pyrimidine as well as increase the thickness of the epidermis[33].
It can be concluded that C. sinensis leaves extract can be used as complementary medicine in treating diseases caused by multidrug resistant strains of S. aureusand P. aeruginosa. However, further investigation is needed to determine the bioavailability of the active compounds and to determine the dose and toxicity before it can be used as therapeutic agents.
Acknowledgments
We would like to acknowledge the funding received from Collaborative Project Research, Faculty of Pharmacy and Department of Microbiology, Medical Faculty, University of Indonesia, 2012/0806327660.
Comments
Background
MDR-P. aeruginosa, and MRSA have emerged and become a worldwide public health problem including in Indonesia. As the bacteria that cause the infection are resistant to first-line antibiotics, treatment options are usually replaced with a second or third choice of antibiotics, which are generally much more expensive. This study, will investigate the antibacterial activity of the extract of Indonesian green tea (C. sinensis) against clinical isolates of MRSA and MDR-P. aeruginosa.
Research frontiers
This study was conducted to find alternative compounds that can be used to develop antimicrobial compounds. A compound that can be used as a raw materials of antimicrobial agents is expected to be found.
Related reports
Research related to this study has been done by several other researchers in various countries, but to my knowledge the activities of green tea against multi-resistant bacteria is a new research conducted in Indonesia.
Innovations and breakthroughs
Data on the activities of green tea against MRSA and MDR-P. aeruginosa in Indonesia is still very rare. This study has shown that green tea can be further developed for use in the treatment of infectious diseases.
Applications
Research results can be utilized further in developing green tea as an alternative therapy to treat infectious diseases caused by multi-drug resistant bacteria.
Peer review
This is a pretty good study in which the authors evaluated the activity of green tea extracts that are effective against MRSA and MDR-P. aeruginosa isolated from clinical specimens obtained from one of the largest hospitals in Indonesia. The results are interesting and suggest that green tea can be used as alternative therapy for infections caused by multi-drug resistant bacteria.
Footnotes
Foundation Project: Supported by Collaborative Project Research, Faculty of Pharmacy and Department of Microbiology, Medical Faculty, University of Indonesia, Grant No. 2012/0806327660.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42(5):657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 2.Gould IM. The epidemiology of antibiotic resistance. Int J Antimicrob Agents. 2008;32(Suppl 1):S2–S9. doi: 10.1016/j.ijantimicag.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197(8):1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 4.Radji M, Fauziah S, Aribinuko N. Antibiotic sensitivity pattern of bacterial pathogens in the intensive care unit of Fatmawati Hospital, Indonesia. Asian Pac J Trop Biomed. 2011;1(1):39–42. doi: 10.1016/S2221-1691(11)60065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winarto Prevalence of extended-spectrum β-Lactamases (ESBL)-bacteria of blood isolates in Dr. Kariadi Hospital Semarang 2004-2005. Media Medika Indosiana. 2009;43(5):260–267. [Google Scholar]
- 6.Lestari ES, Severin JA, Filius PMG, Kuntaman K, Duerink DO, Hadi U, et al. et al. Antimicrobial resistance among commensal isolates of Escherichia coli and Staphylococcus aureus in the Indonesian population inside and outside hospitals. Eur J Clin Microbiol Infect Dis. 2008;27:45–51. doi: 10.1007/s10096-007-0396-z. [DOI] [PubMed] [Google Scholar]
- 7.Duerink DO, Lestari ES, Hadi U, Nagelkerke NDJ, Severin JA, Verbrugh HA, et al. et al. Determinants of carriage of resistant Escherichia coli in the Indonesian population inside and outside hospitals. J Antimicrob Chemother. 2007;60:377–384. doi: 10.1093/jac/dkm197. [DOI] [PubMed] [Google Scholar]
- 8.Rios JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Tomoko N, Takashi A, Hiromu T, Yuka I, Hiroko M, Munekaju I, et al. et al. Antibacterial activity of extracts prepared from tropical and subtropical plants on methicillin-resistant Staphylococcus aureus. J Health Sci. 2002;48:273–276. [Google Scholar]
- 10.Kim SC, Ruengwilysup, Fung DY Antibacterial effect of water-soluble tea extracts on food borne pathogens in laboratory medium and in a food model. J Food Prot. 2004;67:2608–2612. doi: 10.4315/0362-028x-67.11.2608. [DOI] [PubMed] [Google Scholar]
- 11.Lee YL, Cesario T, Wang Y, Shanbrom E, Thrupp L. Antibacterial activity of vegetables and juices. Nutrition. 2003;19:994–996. doi: 10.1016/j.nut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Taguri T, Tanaka T, Kouno I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol Pharm Bull. 2004;27:1965–1969. doi: 10.1248/bpb.27.1965. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari TP, Bharti SK, Kaur HD, Dikshit RP, Hoondal GS. Synergistic antimicrobial activity of tea and antibiotics. Indian J Med Res. 2005;122(1):80–84. [PubMed] [Google Scholar]
- 14.Stapleton PD, Shah S, Anderson JC Hara Y, Hamilton-Miller JMT, Taylor PW. Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. Int J Antimicrob Agents. 2004;23(5):462–467. doi: 10.1016/j.ijantimicag.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Shibata H, Kondo K, Katsuyama R, Kawazoe K, Sato Y, Murakami K, et al. et al. Alkyl gallates, intensifiers of β-Lactam susceptibility in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49(2):549–555. doi: 10.1128/AAC.49.2.549-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jazani NH, Shahabi S, Abdi-Ali A. Antibacterial effects of water soluble green tea extracts on multi-antibiotic resistant isolates of Pseudomonas aeruginosa. Pak J Biol Sci. 2007;10(9):1544–1546. doi: 10.3923/pjbs.2007.1544.1546. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute . Performance standard antimicrobial susceptibility testing; seventeenth informational supplement. Wayne, Pennsylvania, USA: CLSI; 2007. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard-seventh edition. Wayne, Pennsylvania, USA: CLSI; 2006. [Google Scholar]
- 19.Kollef MH, Micek ST. Methicillin-resistant Staphylococcus aureus: a new community-acquired pathogen? Curr Opin Infect Dis. 2006;19:161–168. doi: 10.1097/01.qco.0000216627.13445.e2. [DOI] [PubMed] [Google Scholar]
- 20.McKay DL, Blumberg JB. The role of tea in human health: an update. J Am Coll Nutr. 2002;21(1):1–13. doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton-Miller JMT, Shah S. Activtiy of the component epicatechin gallate and analogues against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2002;46:847–863. doi: 10.1093/jac/46.5.852. [DOI] [PubMed] [Google Scholar]
- 22.Blanco AR, Sudano-Roccaro A, Spoto GC, Nostro A, Rusciano D. Epigallocatechin gallate inhibits biofilm formation by ocular staphylococcal isolates. Antimicrob Agents Chemother. 2005;49:4339–4343. doi: 10.1128/AAC.49.10.4339-4343.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao WH, Hu ZQ, Hara Y, Shimamura T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:2266–2268. doi: 10.1128/AAC.46.7.2266-2268.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu ZQ, Zhao WH, Hara Y, Shimamura T. Epigallocatechin gallate synergy with ampicillin/sulbactam against 28 clinical isolates of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2001;48:361–364. doi: 10.1093/jac/48.3.361. [DOI] [PubMed] [Google Scholar]
- 25.Hu ZQ, Zhao WH, Asano N, Yoda Y, Hara Y, Shimamura T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:558–560. doi: 10.1128/AAC.46.2.558-560.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao WH, Hu ZQ, Okubo S, Hara Y, Shimamura T. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45(6):1737–1742. doi: 10.1128/AAC.45.6.1737-1742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stapleton PD, Shah S, Hamilton-Miller JMT, Hara Y, Nagaoka Y, Kumagai A, et al. Anti-Staphylococcus aureus activity and oxacillin resistance modulating capacity of 3-O-acyl-catechins. Int J Antimicrob Agents. 2004;24:374–380. doi: 10.1016/j.ijantimicag.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Cho YS, Schiller NL, Oh KH. Antibacterial effects of green tea polyphenols on clinical isolates of methicillin-resistant Staphylococcus aureus. Curr Microbiol. 2008;57:542–546. doi: 10.1007/s00284-008-9239-0. [DOI] [PubMed] [Google Scholar]
- 29.Yam TS, Hamilton-Miller JMT, Shah S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2' synthesis and β-lactamase production in Staphylococcus aureus. J Antimicrob Chemother. 1998;42:211–216. doi: 10.1093/jac/42.2.211. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Shim JS, Chung MS, Lim ST, Kim KH. In vitro anti-adhesive activity of green tea extract against pathogen adhesion. Phytother Res. 2009;23:460–466. doi: 10.1002/ptr.2609. [DOI] [PubMed] [Google Scholar]
- 31.Sharma A, Gupta S, Sarethy IP, Dang S, Gabrani R. Green tea extract: Possible mechanism and antibacterial activity on skin pathogens. Food Chem. 2012;135:672–675. doi: 10.1016/j.foodchem.2012.04.143. [DOI] [PubMed] [Google Scholar]
- 32.Janecki A, Kolodziej H. Anti-adhesive activities of flavan-3-ols and proanthocyanidins in the interaction of group A-streptococci and human epithelial cells. Molecules. 2010;15:7139–7152. doi: 10.3390/molecules15107139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, et al. et al. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J. 2003;17(13):1913–1915. doi: 10.1096/fj.02-0914fje. [DOI] [PubMed] [Google Scholar]