Abstract
The volume of parcellated cortical regions is a composite measure related to both thickness and surface area. It is not clear whether volumetric decreases in medial temporal lobe (MTL) cortical regions in aging and Alzheimer's disease (AD) are due to thinning, loss of surface area, or both, nor is it clear whether aging and AD differ in their effects on these properties. Participants included 28 Younger Normals, 47 Older Normals, and 29 patients with mild AD. T1-weighted MRI data were analyzed using a novel semi-automated protocol (presented in a companion article) to delineate the boundaries of entorhinal (ERC), perirhinal (PRC), and posterior parahippocampal (PPHC) cortical regions and calculate their mean thickness, surface area, and volume. Compared to Younger Normals, Older Normals demonstrated moderately reduced ERC and PPHC volumes, which were due primarily to reduced surface area. In contrast, the expected AD-related reduction in ERC volume was produced by a large reduction in thickness with minimal additional effect (beyond that of aging) on surface area. PRC and PPHC also showed large AD-related reductions in thickness. Of all these MTL morphometric measures, ERC and PRC thinning were the best predictors of poorer episodic memory performance in AD. Although the volumes of MTL cortical regions may decrease with both aging and AD, thickness is relatively preserved in normal aging, while even in its mild clinical stage, AD is associated with a large degree of thinning of MTL cortex. These differential morphometric effects of aging and AD may reflect distinct biologic processes and ultimately may provide insights into the anatomic substrates of change in memory-related functions of MTL cortex.
Keywords: Entorhinal cortex, Perirhinal cortex, Parahippocampal cortex, Magnetic resonance imaging, Alzheimer's disease, Aging
1. Introduction
The cortex of the medial temporal lobe subserves fundamental mnemonic functions, providing critical input from heteromodal association cortices to the hippocampal formation and receiving reciprocal afferents from the hippocampal formation (Van Hoesen and Pandya, 1975a,b; Van Hoesen et al., 1975). Medial temporal cortex can be subdivided into entorhinal, perirhinal, and posterior parahippocampal regions, based on distinct cytoarchitectural features (Insausti et al., 1995; Van Hoesen, 1997). These features have been linked with macroscopic landmarks, such that the identification of landmarks in high-resolution MRI data can be used to define MTL regional boundaries (Insausti et al., 1998; Bobinski et al., 1999; Goncharova et al., 2001). Although boundaries defined by macroscopic anatomical features do not correspond perfectly to microscopic histologic subdivisions (Amunts et al., 2005), this approach has been useful in the in vivo measurement of entorhinal atrophy in early clinical and incipient phases of Alzheimer's disease (AD) (Killiany et al., 2000; Xu et al., 2000; de Leon et al., 2001; Dickerson et al., 2001; Du et al., 2001).
The entorhinal and perirhinal cortices are devastated by neurofibrillary pathology and cell loss very early in the course of AD (Hyman et al., 1984; Braak and Braak, 1991; Price et al., 1991; Gomez-Isla et al., 1996; Van Hoesen et al., 2000; Kordower et al., 2001). The posterior parahippocampal cortex caudal to these regions appears to be less affected, and demonstrates pathologic change in somewhat more advanced stages of the disease. It is less clear whether and how medial temporal cortical regions are affected by the normal aging process: although neurofibrillary pathology is more common in these regions in normal older adults, there appears to be no age-related neuronal loss (Price et al., 2001; von Gunten et al., 2005).
Over nearly the past decade, MRI techniques to measure entorhinal cortical volume have been developed (Insausti et al., 1998; Bobinski et al., 1999; Goncharova et al., 2001) and have revealed – in living individuals – reduced volume in early AD and mild cognitive impairment (Killiany et al., 2000; Xu et al., 2000; de Leon et al., 2001; Dickerson et al., 2001; Du et al., 2001). In addition, age-related loss of entorhinal volume has been reported (Du et al., 2006). Yet, as with the entire cortical mantle, the volume of specific bounded regions is related directly to both the thickness of the cortical region and its surface area, but neither of these elements of medial temporal cortical anatomy has yet been studied in aging or AD. Thus, it is not clear whether age-related and AD-related entorhinal volume loss result from similar changes in thickness and surface area, or whether distinct processes might be revealed within these component measures. Furthermore, little investigation of the perirhinal and posterior parahippocampal cortical regions has been reported (Insausti et al., 1998; Pruessner et al., 2002).
We undertook this study to determine whether AD is associated with morphometric alterations within the medial temporal cortex that are distinct from normal aging. We developed a novel method, described in a companion article (Feczko et al., 2009), for measuring the thickness, surface area, and volume of entorhinal, perirhinal, and posterior parahippocampal cortex using a semi-automated computational morphometric procedure. Since previous histologic literature employing stereologic methods indicates that AD is associated with reduced cortical thickness (Gomez-Isla et al., 1996; Regeur, 2000), we hypothesized that entorhinal and perirhinal cortical thinning would be observed in AD, while normal aging would not be associated with such thinning but would be related to loss of surface area due to non-specific global grey and white matter loss (Fotenos et al., 2005). Although previous studies have been performed of the effects of aging and AD on cortical thickness, they have been exploratory in nature, surveying the entire hemispheric thickness, without rigorous measurement protocols for defining the boundaries of MTL cortical regions (Salat et al., 2004; Lerch et al., 2005). As discussed in the accompanying article, individual variability in the morphology of the collateral sulcus makes it difficult, even with surface-based coordinate systems (Fischl et al., 1999a,b), to co-register individuals well enough to reliably identify these regions without the intervention of a manual operator, which was employed in the present methodology.
2. Participants and methods
2.1. Participants
A total of 105 adults (age 18–97) participated in this study, all of whom were paid for their participation. Data from subsets of the participants have been published in previous studies (Buckner et al., 2004, 2005; Salat et al., 2004; Fotenos et al., 2005).
Younger adults were recruited from the Washington University community. Nondemented and demented older adults were recruited exclusively from the ongoing longitudinal sample of the Washington University AD Research Center (ADRC). All procedures were approved by Washington University's human subjects committee; written informed consent was obtained from all participants and their informants. At study enrollment, participants were free of non-AD disorders that could potentially cause dementia such as major depression, clinical history of stroke, Parkinson disease, and head trauma (Berg et al., 1998).
2.2. Clinical assessment
Experienced clinicians assessed each ADRC participant for the presence and severity of dementia based on semi-structured interviews with the research participant and a knowledgeable informant (usually a spouse or adult child) followed by a neurological examination of the participant (Morris, 1993; Morris et al., 1997). The assessment protocol evaluates cognitive problems that represent a decline from a former level of function in daily life for that individual. Also included in the protocol are a health history, depression inventory, aphasia battery, and medication inventory. A psychometric battery is administered to the participants at a separate visit. The CDR staging and clinical diagnostic determinations were made by the examining clinician based solely on the clinical assessment (i.e., without reference to psychometric test results). Diagnostic criteria for AD required the gradual onset and progression of impairment in memory and in at least one other cognitive and functional domain, comparable to standard diagnostic criteria for probable AD (McKhann et al., 1984). The validated clinical criteria for AD used in this study are confirmed by the neuropathologic presence of AD in 93% of cases, including those identified in the CDR 0.5 stage (Berg et al., 1998; Morris et al., 2001).
For the purposes of the present study, the resultant clinical diagnostic categories include Normal or dementia of the Alzheimer type (referred to here as AD).
2.3. MRI data acquisition
Multiple (three or four) high-resolution structural T1-weighted magnetization-prepared rapid gradient echo (MP-RAGE) images were acquired on a 1.5T Vision scanner (Siemens, Erlangen, Germany). MP-RAGE parameters were empirically optimized for gray-white contrast (repetition time (TR) 9.7 ms, echo time (TE) 4 ms, flip angle (FA) 10, inversion time (TI) 20 ms, delay time (TD) 200 ms, 256×256 (1 mm×1 mm) in-plane resolution, 128 sagittal 1.25 mm slices without gaps, time per acquisition 6.6 min). Participants were provided cushioning, headphones, and a thermoplastic face-mask for communication and to minimize head movements. Positioning was low in the head coil (toward the feet) to center the field of view on the cerebral hemispheres.
2.4. MRI morphometric data analysis—automated cortical surface reconstruction
The multiple MP-RAGE acquisitions for each participant were motion corrected and averaged to create a single image volume with a high contrast-to-noise ratio. Cortical thickness measurements were obtained by reconstructing representations of the gray/white matter boundary and the cortical (pial) surface and then calculating the distance between those surfaces at each point across the cortical mantle (Dale et al., 1999; Fischl et al., 1999a,b; Fischl and Dale, 2000). This method uses both intensity and continuity information from the entire three-dimensional MR volume in segmentation and deformation procedures to produce representations of cortical thickness. The representations are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The representations produced are not restricted to the voxel resolution of the original data thus are capable of detecting submillimeter differences between groups (Fischl and Dale, 2000). These procedures were performed using Freesurfer software (http://surfer.nmr.mgh.harvard.edu).
2.5. MRI morphometric data analysis—surface-volume MTL cortical protocol
MTL cortical morphometric measures were quantified from anatomic regions of interest (ROI) using a new protocol developed in our laboratory, the complete technical details of which are described in an accompanying manuscript (Feczko et al., 2009). Briefly, this method involves the use of a nearly fully automated algorithm for identifying the grey–white cortical surface boundary and the pial cortical surface boundary and calculating the thickness of the cortical mantle (Fischl and Dale, 2000).
To enable parcellation of the three MTL ROIs (ERC, PRC, and PPHC), boundaries are traced on each individual subject's cortical surface model generated by the aforementioned procedure, as shown in Fig. 1, while simultaneously visualizing landmarks from traditionally oriented slices of MRI data (e.g., coronal, sagittal, or axial). This procedure enables ROI boundaries within the MTL to be identified using both cortical and subcortical landmarks in a reliable fashion, and enables the calculation of the thickness, surface area, and volume of each ROI (Feczko et al., 2009). All morphometric measurements were performed by a single operator (E.F.) who was blind to clinical data.
Fig. 1.
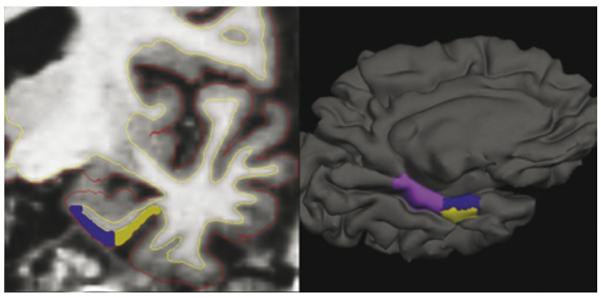
Illustration of novel semi-automated medial temporal cortical morphometric measurement protocol. Slices of greyscale MRI volume data are used to manually identify landmarks for rostrocaudal boundary definitions (left). Surface reconstruction models are used to manually identify sulcal and gyral landmarks for mediolateral boundary definitions (right). Grey–white and pial surface boundaries generated using automated processing (yellow and red lines, respectively, in left image) are checked for accuracy and then used to measure surface area, mean thickness, and volume of cortical regions of interest. Dark purple = ERC, yellow = PRC, light purple = PPHC. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2.6. Relationship of MTL morphometric measures to memory performance
For the purposes of this study, a single memory performance measure was selected from the psychometric battery for analysis with respect to MTL morphometric measures. We chose, a priori, to focus on the Wechsler Memory Scale Logical Memory II measure since it is a measure of delayed verbal recall, which is known to be compromised early in the course of AD. This measure is also commonly used in clinical studies, including clinical trials of AD and mild cognitive impairment. Additional investigation of MTL morphometric measures and other memory performance measures will be the subject of future studies.
2.7. Statistical analysis
Group comparisons were performed using three-way analyses of variance (ANOVA) and covariance (ANCOVA), with planned post hoc comparisons: the effects of age were examined through post hoc comparisons between Normal Older and Normal Younger groups, and those of AD were examined by comparing Mild AD and Normal Older groups. Pearson correlations and partial correlations (adjusting for covariates) were performed to examine relationships among the primary variables of interest. Stepwise multiple linear regression models were also performed, with independent variables accepted if the p value <0.05. These statistical analyses were performed using SPSS 11.0 (Chicago, IL).
3. Results
3.1. Subject characteristics
The Normal Younger group consisted of right-handed volunteers (n = 29, age range 18–31, mean age = 23.6±3.7; 20 females (f)/9 males (m)) with normal cognition. The Normal Older group (n = 47, age range 66–90, mean age = 76.2±6.8; 30f/17m) demonstrated normal cognition, with overall CDR Ratings of 0 and MMSE scores of 25–30. The Mild AD group (n = 29, age range 56–90; mean age = 74.3±10.2; 18f/11m) included individuals with very mild (CDR = 0.5) to mild (CDR = 1) levels of impairment, with MMSE scores ranging from 16–28 (see Table 1 of Feczko et al. (2009) for details).
Table 1.
Quantitative morphometric data on thickness, surface area, and volume of medial temporal cortical regions in Normal Younger, Normal Older, and mild AD participant groups
| Thickness |
Surface area |
Volume |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Younger | Older | Mild AD | Younger | Older | Mild AD | Younger | Older | Mild AD | |
| ERC | |||||||||
| LH | 2.88 (0.26) | 2.75 (0.32) | 2.35 (0.39)** | 187 (48) | 173 (56)* | 152 (44) | 892 (190) | 773 (254)* | 553 (194)** |
| RH | 2.66 (0.39) | 2.54 (0.32) | 2.11 (0.36)** | 199 (46) | 167 (52)* | 154 (54) | 847 (195) | 676 (223)* | 495 (218)** |
| PRC | |||||||||
| LH | 2.12 (0.46) | 2.15 (0.45) | 1.87 (0.41)** | 248 (81) | 219 (72) | 249 (90) | 601 (197) | 532 (185) | 521 (212) |
| RH | 2.09 (0.42) | 2.08 (0.35) | 1.85 (0.36)** | 219 (70) | 220 (66) | 210 (85) | 519 (135) | 525 (163) | 459 (170) |
| PPHC | |||||||||
| LH | 1.96 (0.21) | 1.94 (0.26) | 1.83 (0.29)** | 539 (68) | 478 (81)* | 481 (81) | 1263 (226) | 1075 (242)* | 989 (231) |
| RH | 1.86 (0.20) | 1.92 (0.21) | 1.77 (0.26)** | 502 (59) | 445 (84)* | 461 (100) | 1078 (173) | 970 (200)* | 916 (271) |
Values represent mean (standard deviation). ERC: entorhinal cortex; PRC: perirhinal cortex; PPHC: posterior parahippocampal cortex; AD: Alzheimer's disease; LH: left hemisphere; RH: right hemisphere.
Normal Older group is different from Normal Younger group, p < 0.05.
Mild AD group is different from Normal Older group, p < 0.05.
3.2. Main effects of three-way ANOVA on MTL cortical thickness, volume, and surface area
Mean thickness, surface area, and volume data for all three MTL cortical regions are plotted in Figs. 2–4; all morphometric measures are presented in Table 1. Group differences in each measure were initially assessed with separate three-way repeated measures ANCOVAs with group and hemisphere as the two factors. Head size, as estimated by total intracranial volume (eTIV) measurements (Buckner et al., 2004), was used as a covariate in all analyses, as eTIV measurements correlate with regional volume and surface area (note that head size does not correlate with cortical thickness in most regions of the cortex, including MTL, but the same covariates were used to maintain formal statistical equivalency of all analyses). Main effects of group were found in ERC volume [F(2, 101) = 25.3, p < 0.001], surface area [F(2, 101) = 6.1, p < 0.004], and thickness [F(2,102) = 26.3, p < 0.001]; PRC thickness [F(2, 102) = 6.0, p < 0.005]; and PPHC volume [F(2, 101) = 9.5, p < 0.001], surface area [F(2,101) = 7.0, p < 0.002], and thickness [F(2,102) = 3.7, p < 0.03]. No significant group-by-hemisphere interactions were observed. Main group effects were further examined using planned post-hoc comparisons to first examine AD-related effects and then age-related effects.
Fig. 2.
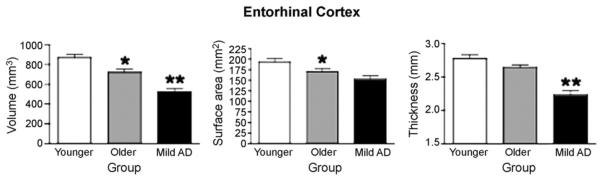
Bar graph illustrating morphometric differences of ERC volume, surface area, and cortical thickness between Normal Younger, Normal Older, and Mild AD groups. Measurements displayed were obtained from the average of both hemispheres. Error bars represent standard error. *p < 0.05 compared to Normal Younger group; **p < 0.05 compared to Normal Older group.
Fig. 4.
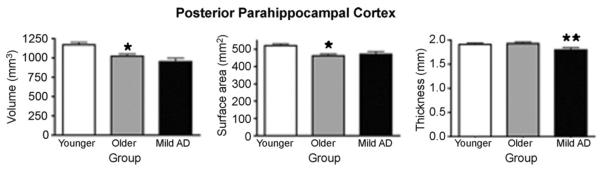
Bar graph illustrating morphometric differences of PPHC volume, surface area, and cortical thickness between Normal Younger, Normal Older, and Mild AD groups. Measurements displayed were obtained from the average of both hemispheres. Error bars represent standard error. The legend used is identical to the one used in Fig. 2.
3.3. Effects of Alzheimer's disease on MTL cortical thickness, surface area, and volume
We first focused on analyses of the effects of AD in comparison to normal aging. For ERC volume, an effect of group (p < 0.001, Cohen's d = 1.05) was found, indicating that ERC volume is smaller in AD than Normal Older individuals, as expected. With respect to ERC thickness, a group effect (p < 0.001, d = 1.32) was found, whereas for ERC surface area, a group trend (p = 0.08, d = 0.41) was observed. Thus, although atrophy of the ERC in AD can be detected through volumetric measures, a stronger effect of AD (as measured by the Cohen's d effect size) is present in the thickness measure.
For PRC, AD also has an effect on thickness (p < 0.002, d = 0.82). No effects were identified in volume or surface area (p values >0.28).
For the PPHC, again, there was a group effect on thickness (p < 0.01, d = 0.57), indicating thinning in AD. There were no volume or surface area effects for the PPHC (p values >0.16).
Effect sizes for the three regions show that, even at very mild-to-mild clinical stages, AD is associated with a large degree of thinning in ERC and PRC, with a moderate degree of thinning in PPHC.
3.4. Effects of aging on MTL cortical thickness, surface area, and volume
We next analyzed the effects of aging on MTL measures by comparing the Normal Older group to the Normal Younger group. For ERC volume, a group (p < 0.001, d = 0.80) effect was found. For ERC surface area, a similar group (p < 0.05, d = 0.51) effect was observed. A trend was present for ERC thickness (p = 0.07, d = 0.46). These findings indicate that age-related loss of ERC volume is in large part a product of reduced ERC surface area.
For the PPHC, there was group effect on volume (p < 0.003, d = 0.82). Similarly, a group effect was observed for PPHC surface area (p < 0.001, d = 0.92) with no effect on PPHC thickness, indicating that age-related reduction in PPHC volume is driven by reduced PPHC surface area.
No effects due to aging were found in the PRC (p values >0.4).
Thus, in contrast to AD, the major effects of normal aging on ERC and PPHC volume were attributable to reductions in surface area rather than thickness.
3.5. Relationship between MTL cortical thickness and memory performance
The relationship between MTL morphometric measures and memory performance was investigated for the older normal and AD groups separately using correlation and regression analyses. The Wechsler Memory Scale (WMS) Logical Memory II test score was used as the dependent variable in the following analyses. Again, as above, analyses were performed using morphometric measures with adjustment for head size.
In the AD group, memory performance correlated with mean ERC thickness (r = 0.38, p < 0.05) and mean PRC thickness (r = 0.46, p < 0.02). There was also a correlation between memory performance and the volumes of ERC (r = 0.42, p < 0.03) and PRC (r = 0.39, p < 0.04). By comparison, the memory performance did not correlate with measures of surface area (all p values >0.13). Thus, the correlations between Logical Memory performance and volumes of ERC and PRC were driven solely by thickness. A stepwise linear regression analysis identified mean PRC thickness as the best predictor of memory performance [F(1,28) = 7.06, p < 0.02, R2 = 0.21].
For the Normal Older group, memory performance did not correlate with most morphometric measures (−0.17 < r < 0.18, p > 0.25). A trend toward a correlation was observed between mean ERC thickness and memory performance (r = 0.28, p = 0.054). These relationships are illustrated in Fig. 5.
Fig. 5.
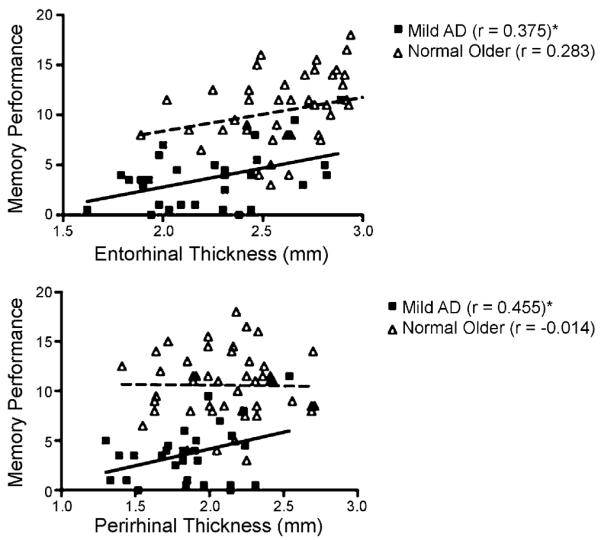
Scatterplots illustrating relationships between WMS Logical Memory II performance and entorhinal and perirhinal thickness for Mild AD and Normal Older subjects. Thickness measurements for each subject were calculated as an average of both hemispheres. Regression lines are displayed for Mild AD (*p < 0.05) in bold and for Normal Older subjects as dashed lines.
4. Discussion
We developed and applied a new protocol for measuring the volume, thickness, and surface area of the ERC, PRC, and PPHC to investigate these morphometric properties in mild AD in comparison to normal older and younger groups. Both AD and aging are associated with smaller volumes of all three MTL cortical regions, although volume loss is greater in AD than in aging. However, aging and AD appear to have differential effects on the fundamental component morphometric properties that make up cortical volume: surface area and thickness. While aging was associated with diminished MTL cortical surface area, thinning of these regions was found primarily in AD. Thickness of PRC and PPHC was preserved in aging, and ERC thickness was minimally affected by age.
The thickness of the cerebral cortex likely represents a host of cytoarchitectural features, probably not limited to neuron numbers but also including many other components of the neuropil, including intracortical axons, dendrites, synaptic elements, and glia. Although the classic corticographers specifically discussed regional variation in the thickness of the cortical mantle and laminae (Bailey and von Bonin, 1951; Brodmann, 2006), surprisingly little quantitative work links stereologic measures of cytologic features with thickness. These bridges will be easier to build as new imaging technology is developed to directly visualize the cytoarchitectonic features of the ERC (Augustinack et al., 2005). It is clear, however, that the cortical ribbon is thinner in AD than in age-matched controls (Regeur, 2000), and that even very mild AD is characterized by striking laminar thinning within the EC, along with prominent loss of neurons in specific laminae and the presence of pathologic features (Gomez-Isla et al., 1996). In this study, compared to the normal older group, we observed mean thinning in the mild AD group of 0.42 mm in the ERC, 0.25 mm in the PRC, and 0.13 mm in the PPHC, or 16%, 12%, and 7%, respectively. In the only other study of cortical thinning in AD that reported specifically on MTL, a probabilistically defined ERC region showed 0.5–1.2 mm thinning in AD; the larger effect is likely related in part to the inclusion of more severely impaired AD patients (Lerch et al., 2005).
In contrast, normal aging appears to have little effect on MTL cortical thickness, as has been previously observed (Salat et al., 2004). Compared to the younger group, the normal older individuals showed no thinning in PRC and PPHC, and only 0.13 mm (5%) in the ERC. Even the oldest-old cognitively normal subjects in this study had MTL cortical thickness measures that were greater than 57% of the AD patients. Scatterplots of individual subject data show that there is much less overlap in thickness measures than there is in volume measures. These observations suggest that, in this context, MTL cortical thinning may be an important morphometric imaging marker of AD, reflecting, at least in part, the early laminar thinning known to occur in these regions in the disease. It will be of interest to determine whether the individuals who are currently cognitively intact but with the thinnest ERC volume will begin to demonstrate a decline in memory function in the coming few years.
The biologic correlates of cortical surface area are less clear, and any attempt to perform regionally focused analyses of cortical surface area must take into account the global effects of head size and brain size. Unlike cortical thickness, which is not correlated with head size measures in most regions, gyral surface area is larger in individuals with larger heads and those with larger brains. Thus, as with volumetric measures, between-group comparisons can only be made after adjusting for head size (e.g., total intracranial volume). But since both aging and AD are associated with reduced whole-brain volume, attempts to identify specific effects on MTL cortical surface area need to account for whole-hemisphere surface area, as well (which correlates with brain volume). In this study, we found that ERC and PPHC surface area were reduced in aging after adjusting for head size, but no additional effects were present (beyond that of aging) in AD. Furthermore, these age-related effects hold up after covarying whole hemisphere surface area, suggesting that they are not merely a reflection of global brain (grey matter and white matter) atrophy. It is possible that the loss of fiber bundles in the parahippocampal white matter could be reflected in specific loss of MTL gyral surface area, but this possibility deserves further investigation.
Previous work has shown a relationship between entorhinal volume and memory performance in older, MCI, and AD subjects (De Toledo-Morrell et al., 2000; Du et al., 2003; Rosen et al., 2003; Rodrigue and Raz, 2004; Stoub et al., 2006), but, to our knowledge, there have been no investigations of the thickness or surface area of ERC or of other MTL cortical regions in relation to memory performance. We found here that the ERC and PRC thinning in AD relates to poorer delayed verbal recall performance, but that surface area of MTL regions does not relate to memory performance. Further investigations will be required to better understand the histologic correlates of these MRI-based morphometric measures, but it is possible that cortical thinning in AD relates more specifically than surface area to the loss of neurons, synapses, and dendritic branching, all of which likely contribute to the decline in memory function in AD. If anything, only a trend-level correlation between ERC thickness and memory performance is present in the normal older group. This is likely a reflection of pre-clinical AD pathology, and is congruent with a recent clinicopathologic study showing that poorer memory performance – even in clinically normal older individuals – may relate to the presence of AD pathology (Barnes et al., 2006).
In summary, this novel morphometric approach takes advantage of automated software tools for detecting the grey–white and pial-cerebrospinal fluid boundaries of the cortical surface, which enable very precise measurement of cortical thickness. This precision can be combined with the accuracy offered by highly skilled manual operators for identifying sulcal (i.e., collateral sulcus) and subcortical (e.g., lateral geniculate nucleus) anatomical features for defining boundaries in the tradition of manual MRI morphometry. Together, this surface-volume hybrid approach enables regional cortical volumes within the MTL to be analyzed for their component parts—thickness and surface area. ERC, PRC, and PPHC surface area is diminished in aging, while thinning of these regions is predominantly seen in AD. Given the reliability of thickness measures (Han et al., 2006), which enable small absolute effects to be robustly detected, it will be of great interest to try to improve the reliability of rostrocaudal and mediolateral boundary identification using automated algorithms to make the process more efficient, which may enable measures of MTL cortical thickness to be translated into high-throughput in vivo biomarkers useful for early diagnosis.
Fig. 3.
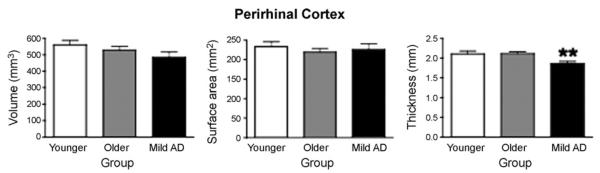
Bar graph illustrating morphometric differences of PRC volume, surface area, and cortical thickness between Normal Younger, Normal Older, and Mild AD groups. Measurements displayed were obtained from the average of both hemispheres. Error bars represent standard error. The legend used is identical to the one used in Fig. 2.
Acknowledgements
This study was supported by grants from the NIA (K23-AG22509, P50-AG05681, P01-AG03991), the NCRR (P41-RR14075 R01 RR16594-01A1, the NCRR BIRN Morphometric Project BIRN002, U24 RR021382 & U24-RR021382), and the Mental Illness and Neuroscience Discovery (MIND) Institute. Additional support was provided by the National Institute for Biomedical Imaging and Bioengineering (R01 EB001550), the National Institute for Neurological Disorders and Stroke (R01 NS052585-01) and the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149.
We thank Drs. Leyla deToledo-Morrell and Gary Van Hoesen for helpful discussions, and the faculty and staff of the Washington University ADRC. We express special appreciation to the participants in this study for their valuable contributions, without which this research would not have been possible.
Footnotes
Conflict of interest There are no actual or potential conflicts of interest.
References
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berl.) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, van der Kouwe AJ, Blackwell ML, Salat DH, Wiggins CJ, Frosch MP, Wiggins GC, Potthast A, Wald LL, Fischl BR. Detection of entorhinal layer II using 7Tesla [correction] magnetic resonance imaging. Ann. Neurol. 2005;57:489–494. doi: 10.1002/ana.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P, von Bonin G. The Isocortex of Man. University of Illinois Press; Urbana, IL: 1951. [Google Scholar]
- Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67:1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr., Miller JP, Storandt M, Rubin EH, Morris JC, Baty J, Coats M, Norton J, Goate AM, Price JL, Gearing M, Mirra SS, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch. Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Convit A, De Santi S, Wegiel J, Tarshish CY, Saint Louis LA, Wisniewski HM. MRI of entorhinal cortex in mild Alzheimer's disease. Lancet. 1999;353:38–40. doi: 10.1016/s0140-6736(05)74869-8. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Brodmann's Localization in the Cerebral Cortex: The Principles of Comparative Localisation in the Cerebral Cortex based on Cytoarchitectonics. third ed. Springer; New York: 2006. (translation of 1909 manuscript) [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I: Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Leon M, Bobinski M, Convit A, Wolf O, Insausti R. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2001;56:820–821. doi: 10.1212/wnl.56.6.820. [DOI] [PubMed] [Google Scholar]
- De Toledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: in vivo detection of entorhinal cortex atrophy. Ann. N. Y. Acad. Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol. Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Zhu XP, Jagust WJ, Miller BL, Reed BR, Kramer JH, Mungas D, Yaffe K, Chui HC, Weiner MW. Atrophy rates of entorhinal cortex in AD and normal aging. Neurology. 2003;60:481–486. doi: 10.1212/01.wnl.0000044400.11317.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol. Aging. 2006;27:733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feczko E, Augustinack JC, Fischl B, Dickerson BC. An MRI-based method for measuring volume, thickness and surface area of entorhinal, perirhinal, and posterior parahippocampal cortex. Neurobiol. Aging. 2009;30:420–431. doi: 10.1016/j.neurobiolaging.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr., Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J. Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova II, Dickerson BC, Stoub TR, deToledo-Morrell L. MRI of human entorhinal cortex: a reliable protocol for volumetric measurement. Neurobiol. Aging. 2001;22:737–745. doi: 10.1016/s0197-4580(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Pacheco J, Albert M, Killiany R, Maguire P, Rosas HD, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Insausti R, Tunon T, Sobreviela T, Insausti AM, Gonzalo LM. The human entorhinal cortex: a cytoarchitectonic analysis. J. Comp. Neurol. 1995;355:171–198. doi: 10.1002/cne.903550203. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am. J. Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann. Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann. Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC. Focal decline of cortical thickness in Alzheimer's disease identified by computational neuroanatomy. Cereb. Cortex. 2005;15:995–1001. doi: 10.1093/cercor/bhh200. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, Thal LJ, Woodbury P. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer's disease Cooperative Study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol. Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch. Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kohler S, Crane J, Pruessner M, Lord C, Byrne A, Kabani N, Collins DL, Evans AC. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb. Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Regeur L. Increasing loss of brain tissue with increasing dementia: a stereological study of post-mortem brains from elderly females. Eur. J. Neurol. 2000;7:47–54. doi: 10.1046/j.1468-1331.2000.00017.x. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J. Neurosci. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, Gabrieli JD, Stoub T, O'Hara R, Friedman L, Yesavage JA, deToledo-Morrell L. Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behav. Neurosci. 2003;117:1150–1160. doi: 10.1037/0735-7044.117.6.1150. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb. Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10041–10045. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I: Temporal lobe afferents. Brain Res. 1975a;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II: Frontal lobe afferents. Brain Res. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. Ventromedial temporal lobe anatomy, with comments on Alzheimer's disease and temporal injury. J. Neuropsychiatry Clin. Neurosci. 1997;9:331–341. doi: 10.1176/jnp.9.3.331. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III: Efferent connections. Brain Res. 1975b;95:39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R. The parahippocampal gyrus in Alzheimer's disease Clinical and preclinical neuroanatomical correlates. Ann. N. Y. Acad. Sci. 2000;911:254–274. doi: 10.1111/j.1749-6632.2000.tb06731.x. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Kovari E, Rivara CB, Bouras C, Hof PR, Giannakopoulos P. Stereologic analysis of hippocampal Alzheimer's disease pathology in the oldest-old: evidence for sparing of the entorhinal cortex and CA1 field. Exp. Neurol. 2005;193:198–206. doi: 10.1016/j.expneurol.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jack CR, Jr., O'Brien PC, Kokmen E, Smith GE, Ivnik RJ, Boeve BF, Tangalos RG, Petersen RC. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2000;54:1760–1767. doi: 10.1212/wnl.54.9.1760. [DOI] [PubMed] [Google Scholar]


