Abstract
Background
Women presenting with signs and symptoms of myocardial ischemia frequently have no or non-obstructive coronary artery disease (CAD).
Objective
To investigate associations between angiographic measures and longer-term clinical outcomes among women with signs and symptoms of ischemia referred for coronary angiography.
Methods
Prospective cohort analysis of women referred for coronary angiography and enrolled in the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). An angiographic severity score was prospectively developed, assigning points for any stenosis weighted by stenosis severity, location and collaterals, and then tested for prediction for adverse outcome in 917 women over a median 9.3 years.
Setting
Referral centers.
Patients
Women, with signs and/or symptoms of myocardial ischemia, referred for coronary angiography were consecutively consented and enrolled in a prospective study.
Main Outcome Measures
First occurrence of cardiovascular death or non-fatal myocardial infarction. Hospitalization for angina was a secondary outcome.
Results
Cardiovascular death or myocardial infarction at 10 years occurred in 6.7%, 12.8% and 25.9% of women with no, non-obstructive, and obstructive CAD (p<0.0001), respectively. Cumulative 10-year cardiovascular death or MI rates showed progressive, near linear, increases for each WISE CAD severity score range of 5, 5.1–10, 10.1–20, 20.1–50, and >50. The optimal threshold in the WISE severity score classifications for predicting cardiovascular mortality was >10 (e.g. 5.0–10 vs. 10.1–89), with both a sensitivity and specificity of 0.64 and an area under the curve of 0.64 (p=0.02, 95% CI = 0.59, 0.68).
Conclusions
Among women with signs and symptoms of ischemia, non-obstructive CAD is common, and associated with adverse outcomes over the longer-term. The new WISE angiographic score appears to be useful for risk prediction in this population.
Keywords: angiography score, coronary disease, prognosis, women
Background
Patients presenting with ischemic chest pain or acute coronary syndromes (ACS) are increasingly found at angiography to have no obstructive epicardial coronary artery disease (CAD). These rates are as higher in women than men1–3, occur as often as 65%1–4, and represent 14–17% in cases of biomarker positive ACS2, 4. Prior studies using simple visual assessment of coronary angiograms revealing no obstructive stenosis have suggested excellent near and longer-term survival5–9. Although these prior reports reported few deaths, high rates of persistent symptoms were noted10–12. Many of these patients labeled as “normal” have angiographically detectable but minimal, non-obstructive (<50% diameter stenosis) CAD. However, without standardized core laboratory angiographic quantitative assessment the proportion of patients in prior reports with evidently normal coronary arteries versus those with detectable but non-obstructive CAD is unknown. Although typically managed as if they were normal, it is not known whether or not lesion severity in such subjects with detectable but non-obstructive CAD is associated with an adverse prognosis.
In order to determine whether no and non-obstructive CAD are associated with long-term adverse outcomes in women, angiograms from women undergoing clinically indicated coronary angiography were prospectively analyzed quantitatively by a core laboratory masked to all clinical characteristics, for the presence, extent, and severity of CAD. An angiographic score was prospectively developed in the WISE study and examined as a predictor of adverse outcomes.
Methods
Study
The study cohort consisted of 917 women presenting with symptoms and/or signs of ischemic heart disease undergoing coronary angiography enrolled in the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) and followed for adverse outcomes. Exclusion criteria for WISE included any contra-indication to provocative stress testing, thus enrolled subjects almost exclusively had stable symptoms including typical, atypical and non-anginal chest pain. Myocardial ischemia was defined as an ischemic response on any non-invasive tests the details of which are reported elsewhere13.
Baseline Evaluations
Initial evaluation in addition to coronary angiography included collection of demographic, medical history, symptom data, physical examination as well as blood sampling for lipids, reproductive hormones, and inflammatory markers. The design and methodology are described in detail elsewhere13.
Coronary Angiography Core Laboratory
All angiograms were quantitatively evaluated (WISE angiographic core laboratory) masked to all clinical findings including symptoms and results of non-invasive testing. All coronary segments with any visual abnormality (even luminal irregularities) were measured quantitatively. Cine film (rare) was measured with electronic calipers and digital recordings on CD-ROM (most) were analyzed using a computer based edge detection algorithm described previously14. An angiographic severity score was prospectively developed which assigned points according to the category of severity of percent diameter stenosis (0–19, 20–49, 50–69, 70–89, 90–98, and 99–100) adjusted for the presence of partial or complete collaterals. Scores were weighted by lesion location with more proximal lesions receiving a higher weighting factor. Individual lesion point totals were then added together to yield a final score14 (Appendix). A severity score of 5 was assigned to those women with no CAD. A severity score ≥ 50 represents severe, diffuse 3-vessel obstructive CAD14.
Definitions
“No CAD” was defined as normal appearing coronary arteries and when measured, no diameter stenosis ≧20% diameter narrowing in any epicardial coronary artery because in our experience: 1) any luminal irregularity when measured quantitatively will yield at least a 20% diameter reduction versus a completely normal reference; 2) it is common to obtain a 0–19% diameter reduction when measuring normal segments as a result of vessel tapering. Non-obstructive CAD was defined as at least 1 diameter stenosis ≧20 but <50%. Obstructive (single, double or triple vessel) CAD was defined as at least 1 diameter stenosis ≧50%.
Follow-up Procedures
Each patient gave informed consent and all centers had IRB approval for the inclusion of patients in this targeted cohort study as well as for collection of follow-up data.
Outcome data used in this report were collected in two consecutive collection phases. During the first phase, patients were contacted at 6 weeks, and at 1-year intervals following enrollment. During telephone contact, a scripted interview was completed by an experienced nurse or physician at the respective center: each patient or family member was queried for occurrence of major adverse cardiac events or hospitalizations. In the event of death, a death certificate and/or physician narrative was obtained. During the second phase, for those who were alive at last contact and had not withdrawn consent, this was later followed by a National Death Index search. All deaths were adjudicated as cardiovascular (CV) or non-CV by a committee of senior WISE investigators blinded to angiographic findings. Major adverse events included cardiovascular mortality and non-fatal myocardial infarction. A secondary outcome was hospitalization for angina.
Statistical analysis
Continuous variables were expressed as means ± standard deviation. The primary measure of interest for the prognosis analysis was time to first occurrence of either death or non-fatal myocardial infarction. Cox proportional hazards (PH) modeling was used to test associations between angiographic findings and adverse outcomes. Because of the skewed distribution of the CAD severity score it was log transformed for this analysis. Multivariate modeling was conducted in subsequent steps: a) unadjusted associations; b) adjusting for age; c) adding to the models known cardiac risk factors that were also strong predictors in this cohort, including diabetes, ever smoking, and hypertension. From the PH model, the relative risk ratio and 95% confidence intervals were calculated. Unadjusted Kaplan-Meier survival curves were also calculated. Receiver operator characteristics (ROC) analysis was used to estimate the optimal threshold among the WISE angiographic severity score classes for predicting cardiovascular mortality as well as estimating sensitivity and specificity. All tests were two-sided, and a p-value <0.05 was considered statistically significant. Data were analyzed using SAS version 9.3 (Cary, N.C).
Results
Coronary Angiography and Clinical Characteristics
A total of 917 women had angiograms acceptable for core lab analysis and follow-up data. Outcome information was collected for 883 of the women, at a median of 6.0 years (first phase). The National Death Index search resulted in outcome information on a total of 917 women and extended follow-up for mortality only, to a median of 9.3 years (median interquartile range 8.6–10 years)(second phase). The no, non-obstructive, and obstructive CAD distribution and WISE angiographic scores are depicted in Table 1. Baseline clinical characteristics, subgrouped by extent of CAD category are summarized in Table 2.
Table 1.
Presence, Extent, and Severity of Angiographically-Defined Coronary Disease in Women with Suspected Myocardial Ischemia
| N=917 | |
|---|---|
|
| |
| Extent of Coronary Disease | |
| No or <20% stenosis | 37% |
| Non-Obs (Minimal or Stenosis ≥20 <50%) | 25% |
| Obstructive or ≥50% stenosis | 38% |
|
| |
| Number of Vessels with ≥50% Stenosis | |
| 0 | 62% |
| 1 Vessel | 17% |
| 2 Vessel | 11% |
| 3 Vessel | 11% |
|
| |
| By Coronary Artery | |
| Left Main Stenosis ≥50% | 2% |
| Left Anterior Descending ≥50% | 27% |
| Left Circumflex Stenosis ≥50% | 18% |
| Right Coronary Artery ≥50% | 24% |
|
| |
| WISE Severity Score* | 14.8± 14.7 |
| Anterior (LAD) Severity Score | 7.0± 8.3 |
| Inferior (RCA) Severity Score | 4.5± 6.4 |
| Lateral (LCX) Severity Score | 3.8± 5.6 |
Severity score measures were calculated as mean ± standard deviation. All other values are noted as percentages.
Table 2.
Pertinent Clinical Characteristics by Extent of CAD
| Characteristic | CAD Severity | p-value | |||
|---|---|---|---|---|---|
|
| |||||
| None n=339 | Minimal n=228 | Obstructive n=350 | None vs. Minimal (age-adj.) | Minimal vs. obstr. (age-adj.) | |
|
| |||||
| Age (mean ± SD) | 54 ± 10 | 59 ± 11 | 63 ± 12 | <0.0001 | 0.0001 |
|
| |||||
| Post-menopausal (%) | 65 | 80 | 84 | 0.0001 | 0.18 |
|
| |||||
| BMI (mean ± SD) | 30.4 ± 7.0 | 29.3 ± 6.3 | 29.3 ± 6.2 | 0.30 | 0.57 |
|
| |||||
| Rest or nitrate responsive angina (%) | 69 | 76 | 77 | 0.027 | 0.82 |
|
| |||||
| Angina with effort or stress (%) | 55 | 50 | 61 | 0.32 | 0.013 |
|
| |||||
| Chest pain within 6 weeks of study entry(%) | 94 | 97 | 91 | 0.043 | 0.06 |
|
| |||||
| Prior MI (%) | 5 | 17 | 37 | <0.0001 | <0.0001 |
|
| |||||
| Hypertension (%) | 49 | 60 | 68 | 0.10 | 0.06 |
|
| |||||
| Ever smoker (%) | 46 | 58 | 56 | 0.002 | 0.73 |
|
| |||||
| Current smoker (%) | 17 | 24 | 20 | 0.0005 | 0.82 |
|
| |||||
| Diabetes (%) | 15 | 18 | 39 | 0.39 | <0.0001 |
|
| |||||
| Insulin-dependent (%) | 39 | 60 | 47 | 0.037 | 0.25 |
|
| |||||
| Insulin-dependent diabetes (population) (%) | 6 | 11 | 18 | 0.30 | 0.013 |
|
| |||||
| Hypercholesterolemia (%) | 36 | 62 | 69 | <0.0001 | 0.21 |
|
| |||||
| Family hx of premature CAD (%) | 65 | 66 | 66 | 0.52 | 0.60 |
|
| |||||
| Systolic blood pressure (mean mmHg ± SD) | 135 ± 20 | 136 ± 21 | 139 ± 23 | 0.33 | 0.48 |
|
| |||||
| Diastolic blood pressure (mean mmHg ± SD) | 77 ± 10 | 77 ± 11 | 76 ± 12 | 0.81 | 0.83 |
|
| |||||
| Racial subsets (%): | |||||
| African American | 17 | 15 | 19 | ||
| Caucasian | 82 | 84 | 79 | ||
| Other | 1 | 1 | 2 | 0.79* | 0.06* |
|
| |||||
| Ischemia (any non-invasive test) (%) | 43 | 33 | 62 | 0.09 | <0.0001 |
|
| |||||
| Duke Activity Status Index (mean ± SD) | 22.0 ± 16.2 | 22.1 ± 14.7 | 16.5 ± 12.4 | 0.98 | <0.0001 |
| Estimated METS (mean ± SD) | 6.3 ± 4.6 | 6.3 ± 4.2 | 4.7 ± 3.5 | 0.98 | <0.0001 |
| Medications used(%): | |||||
| Aspirin | 43 | 61 | 76 | 0.0009 | 0.0009 |
| ACE inhibitor | 17 | 27 | 34 | 0.044 | 0.14 |
| ARB | 3 | 2 | 3 | 0.16 | 0.25 |
| Beta Blocker | 29 | 39 | 49 | 0.046 | 0.012 |
| Statin | 10 | 31 | 36 | <0.0001 | 0.59 |
|
| |||||
| Prior CABG (%) | 0 | 1 | 16 | 0.95 | <0.0001 |
|
| |||||
| Prior PTCA (%) | 1 | 18 | 27 | <0.0001 | 0.010 |
|
| |||||
| Indications for Cath(%) | |||||
| Chest pain | 93 | 93 | 91 | 0.57 | 0.96 |
| Stress test result | 52 | 44 | 40 | 0.045 | 0.27 |
| Shortness of breath | 60 | 67 | 50 | 0.15 | 0.0003 |
| Syncope | 10 | 14 | 7 | 0.08 | 0.018 |
p-value comparing white vs. non-white
Adverse Events
At 10 years, 161 (18%) women had died of which 103 (64%) were cardiovascular deaths, and 30 (3%) women had nonfatal myocardial infarctions (MIs). Because 11 of the 30 women with nonfatal MIs subsequently died later, there were a total of 122 women with either nonfatal MI or cardiovascular death.
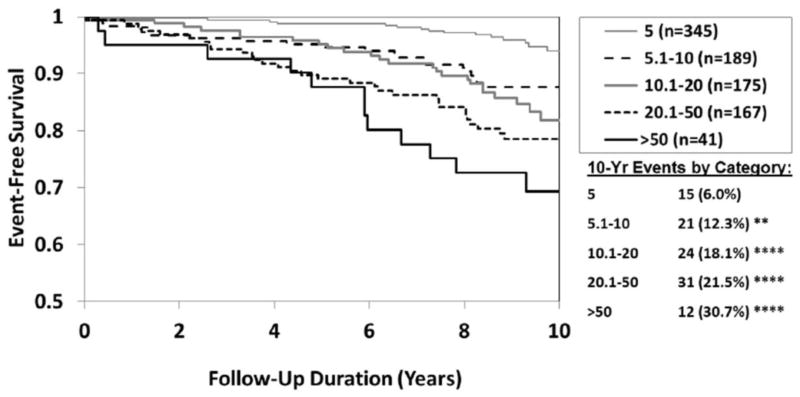
The overall rate of cardiovascular death or nonfatal myocardial infarction, at 10 years, doubled when comparing women with no CAD (6.7%) to women with non-obstructive (12.8%) and doubled again (25.9%) for women with obstructive CAD (p<0.01 none versus minimal and p<0.0001 for none versus obstructive). At 10 years, cardiovascular death rates also showed this approximate rate doubling when comparing women with no (7.1%), minimal (13.6%) and obstructive (30.3%) CAD respectively (Figure 1).
Figure 1.
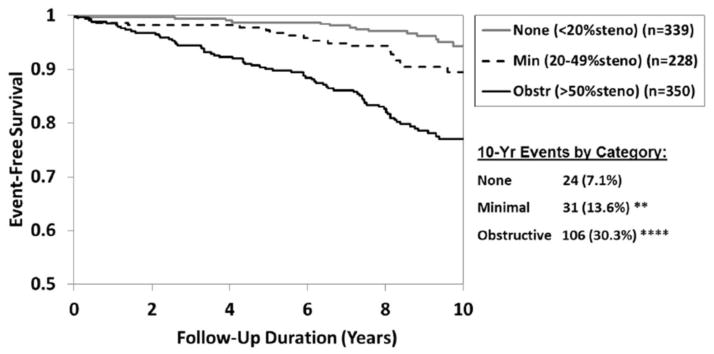
10-Year Freedom from CV Death by Coronary Artery Disease Category (CAD). N=917 (103 events)
(Min = minimal or non-obstructive CAD; obstr = obstructive CAD).
Event rates (%) estimated by Kaplan-Meier analysis
*p<0.05 **p<0.01 ***p<0.001 ****p<0.0001 (compared to No)
Cumulative 10-year cardiovascular death or MI rates showed progressive, near linear, increases from 6.9%, 14.1%, 20.1%, 24.6% to 37.9% for each WISE CAD severity score range of 5, 5.1–10, 10.1–20, 20.1–50, and >50 respectively (p<0.001 for 5.1–10 versus 5, and p<0.0001 for all of the other categories versus 5). This pattern was again seen when cardiovascular death rates were compared in isolation to angiographic severity score at 10 years of follow-up (Figure 2). By ROC analysis, the optimal threshold in the WISE severity score classifications for predicting cardiovascular mortality was >9 with both a sensitivity and specificity of 0.64 and an area under the curve of 0.71 (p<0.0001, 95% CI = 0.66, 0.76).
Figure 2.
10-Year Freedom from CV Death by Angiographic Severity Score Category. N=917 (103 events)
Event rates (%) estimated by Kaplan-Meier analysis
*p<0.05 **p<0.01 ***p<0.001 ****p<0.0001 (compared to lowest severity [5])
Recurrent symptoms occurred frequently, as more than a quarter (28%) of the women were re-hospitalized with chest pain over the initial phase follow-up observation period (median 6.0 years). Five-year re-hospitalization for worsening or refractory chest pain occurred in 15.9%, 22.3% and 33.3% for those women with no, non-obstructive and obstructive CAD (p<0.0001). By the WISE angiographic severity score, 5-year chest pain re-hospitalization rates demonstrated a significant overall relationship of increasing score corresponding to increased rates, however the observation was inconsistent when comparing individual score groupings directly (Figure 3).
Figure 3.
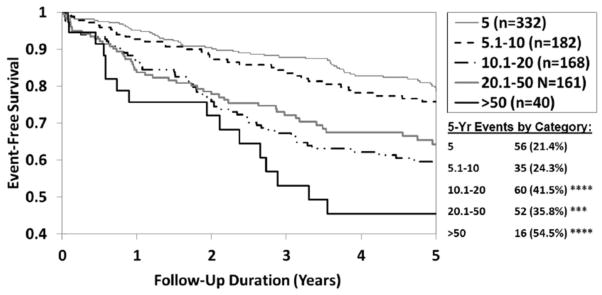
5-Year Freedom from Hospitalization for Angina by Angiographic Severity Score Category.
N=883 (214 events)
Event rates (%) estimated by Kaplan-Meier analysis
*p<0.05 **p<0.01 ***p<0.001 ****p<0.0001 (compared to lowest severity [5])
Therapy During Follow-up
The likelihood of receiving a statin at 1-year follow-up ranged from 12% to 33% to 53% (p=<0.0001) for women with no, non-obstructive and obstructive CAD respectively. Similar trends were noted for receiving Aspirin (41%, 61%, 74% respectively, p=0.003), a beta blocker (24%, 44%, 56% respectively, p<0.0001) and ACE inhibitor (17%, 27%, 35% respectively, p< 0.0001). Repeat coronary angiography was performed in 9%, 19%, and 34% (p=0.0002) for women with no, non-obstructive and obstructive CAD respectively. Similar trends were noted for PCI (0%, 12%, 27% respectively, p<0.0001) and CABG (0%, 3%, 16% respectively, p=0.0004). All listed medications and both percutaneous and surgical revascularization were significantly more likely to be utilized among women with both obstructive and non-obstructive versus those with no CAD.
Discussion
The current study represents a prospective, contemporary large cohort of women presenting with signs and symptoms of ischemia for coronary angiography with quantitative coronary angiographic analysis by core laboratory and long-term follow-up. Several observations are worthy of emphasis. First, almost two-thirds (62%) of the women did not have a significant, obstructive stenosis (≧50% diameter reduction), a rate similar to that recently reported in very large retrospective study identifying a non-obstructive rate of 65% among women with angina3. Prior studies have described coronary angiographic findings in women referred for coronary angiography, and have also noted a lower prevalence of obstructive CAD when compared with men1–4.
Secondly, 10-year adverse outcome rates (cardiovascular death or MI rate) in the women with non-obstructive CAD were almost double (12.8% versus 6.7%) than that observed in women with angiographically normal coronary arteries. These results extend our prior WISE findings that women with signs and symptoms of ischemia but no obstructive CAD have a twofold elevated major adverse event rate compared to asymptomatic community control women. These results are in contrast to prior smaller series which have suggested that women without significant stenosis have an excellent prognosis5–11, 15. These prior studies used visual interpretation of the angiogram and did not include or stratify between women with normal versus non-obstructive coronary disease. Thus, our finding of adverse outcomes in the non-obstructive groups may be due to prior reports including a higher percentage of women with truly normal coronary arteries and possible non-cardiac chest pain.
Third, in this clinically stable population of women referred for coronary angiography, adverse outcomes were directly related to the WISE angiographic severity score with 10 -year cardiovascular death or MI rate ranging from 6 to over 30% for women with scores of 5 through ≧50, respectively. By ROC analysis, a threshold in severity score was identified with modest sensitivity and specificity to predict adverse cardiovascular outcome. It is evident however, from the very smooth shape of the curve that the “severity-response relationship” is continuous (higher score = worse outcome). Combining individual lesion point totals into one final severity score is consistent with the concept of “global” coronary vulnerability related to components of the atherosclerosis process (e.g. inflammation, endothelial dysfunction, etc.)16. Specifically, each individual lesion, no matter how minimal, has the potential to contribute to an individuals’ risk for adverse outcome. Therefore, as a measure of global atherosclerosis burden our WISE severity score appears to provide important additional prognostic information when compared with traditional, single, “culprit” lesion based characterizations of extent of CAD (none, minimal, single, double and triple vessel disease).
Our observation that the relationship between WISE angiographic score and cardiovascular death/MI, was attenuated with addition of known cardiovascular risk covariates suggests that the risk associated with non-obstructive CAD is at least partially related to atherosclerosis. Our prior work demonstrating elevated cardiovascular events, including non-fatal stroke, among WISE women with normal and non-obstructive coronary angiography vs. reference asymptomatic women underscores this concept17.
Whether or not non-obstructive CAD, per se, is responsible for the ischemic symptoms and/or abnormal non-invasive test results leading to the coronary angiogram is unclear. Most importantly however, finding no “culprit” stenosis often leads to these women with non-obstructive coronary disease being labeled as “normal” and not targeted for aggressive risk factor modification for their CAD18. In WISE, there was increased medical therapy utilization when compared with women with no CAD however, women with non-obstructive CAD remained significantly less likely versus women with obstructive CAD to be treated during follow-up with aspirin, beta blockers and statins. Future trials should prospectively test this. The pathophysiology of the signs and symptoms of ischemia among women without obstructive CAD is unknown, although prior studies have suggested possible mechanisms9, 19–21. These include endothelial and microvascular coronary dysfunction as well as sex differences in plaque morphology22. Microvascular coronary dysfunction, as assessed by coronary flow reserve, has been shown to be associated with an increased risk for major adverse outcomes in women23. In support of this concept, we noted even in our women with angiographically normal coronary arteries, that 5% had a history of prior MI and 43% had ischemia identified in a non-invasive test. It is interesting to note that 1% of these same women had a prior PTCA. Whether this was performed for coronary vasospasm or for obstructive CAD which then appeared normal on the subsequent angiogram analyzed by the core laboratory at WISE entry, is unclear.
Limitations
WISE centers may see a higher percentage of women referred for coronary angiography without obstructive CAD due to a tertiary care referral bias. Thus, our prevalence of non-obstructive CAD in women may be higher than observed rates in non-tertiary referral centers. However, the observed prevalence and prognostic implications of non-obstructive CAD are similar to a recent large study from Denmark3. Conversely, the WISE cohort of women have moderate rates of aspirin, statin, ACE-I and ARB medication use that may have minimized relationships between our coronary angiographic variables and outcomes and therefore underestimated the observed relationships. Additionally, the 10-year cumulative cardiovascular death or MI rates are an underestimate since data for MI were only obtained during phase 1 follow-up which had a median duration of 6.0 years. Finally, without a comparative male cohort, it is unknown if our findings would apply to men. Unlike our women, more men undergoing coronary angiography are more frequently labeled as having CAD, and therefore targeted for therapy to prevent atherosclerosis progression. Future investigation should be directed at testing these concepts in men.
Implications
Among the almost 2/3 of women undergoing coronary angiography without obstructive CAD, many (40%) had visually discernable and angiographically measurable but non-obstructive CAD. Thus, these findings suggest that as many as 1 in every 2.5 women with non-obstructive CAD could be risk stratified by this angiographic risk indicator. The documented risk rates in this population may represent an attractive new preventive target, with event rates substantially higher than treatment targets in existing guidelines24, 25. Given the 422,000–581,000 women referred for coronary angiography in 2007 in the US26, 27, with an estimated $25 billion in hospital charges for women with angiographically non-obstructive CAD, this is an issue of both relevance and cost27.
Conclusions
Non-obstructive CAD is common and associated with adverse cardiac outcomes in women referred for coronary angiography for signs and symptoms of ischemia. A new WISE angiographic severity score appears useful for risk prediction. These results suggest that clinical trials designed to evaluate interventions in women with measurable, but non-obstructive CAD should be considered. Further research is also needed to understand the pathophysiologic mechanisms involved, and to assess the relevance of these findings to men.
Acknowledgments
Funding: This work was supported by contracts from the National Heart, Lung and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, R01-HL090957,1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles and The Society for Women’s Health Research (SWHR), Washington, D.C.
The authors wish to thank Joanne Waskiel for her secretarial help in preparation of this manuscript.
Footnotes
Trial Registration: ClinicalTrials.gov NCT00000554, http://www.clinicaltrials.gov
Disclosure/Conflicts of Interest: None
Author Contributions
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. Authors Sharaf, Kelsey, Pepine and Bairey Merz conceived, designed, acquired and analyzed the data, and obtained funding for the study; authors Shaw, Johnson, and Kelsey had full accesss to all data in the study and take responsibility for the integrity of the data and the accuracy of the analyses, authors Wood and Anderson acquired and analyzed the data, while all authors participated in the drafting of the article and final approval of the submitted manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barry Sharaf, Rhode Island Hospital, Providence, Rhode Island.
Todd Wood, Lancaster General Hospital, Lancaster, Pennsylvania.
Leslee Shaw, Emory School of Medicine, Atlanta Georgia.
B. Delia Johnson, University of Pittsburgh, Pittsburgh, Pennsylvania.
Sheryl Kelsey, University of Pittsburgh, Pittsburgh, Pennsylvania.
R. David Anderson, University of Florida, Gainesville, Florida.
Carl J. Pepine, University of Florida, Gainesville, Florida.
Noel Bairey Merz, Cedars Sinai Heart Institute, Los Angeles, California.
References
- 1.Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114(9):894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 2.O’Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. Jama. 2008;300(1):71–80. doi: 10.1001/jama.300.1.71. [DOI] [PubMed] [Google Scholar]
- 3.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
- 4.Glaser R, Herrmann HC, Murphy SA, Demopoulos LA, DiBattiste PM, Cannon CP, et al. Benefit of an early invasive management strategy in women with acute coronary syndromes. Jama. 2002;288(24):3124–9. doi: 10.1001/jama.288.24.3124. [DOI] [PubMed] [Google Scholar]
- 5.Faxon DP, McCabe CH, Kreigel DE, Ryan TJ. Therapeutic and economic value of a normal coronary angiogram. Am J Med. 1982;73(4):500–5. doi: 10.1016/0002-9343(82)90328-x. [DOI] [PubMed] [Google Scholar]
- 6.Day LJ, Sowton E. Clinical features and follow-up of patients with angina and normal coronary arteries. Lancet. 1976;2(7981):334–7. doi: 10.1016/s0140-6736(76)92591-5. [DOI] [PubMed] [Google Scholar]
- 7.Cannon CR, Miller TD, Christian TF, Bailey KR, Gibbons RJ. Prognosis with abnormal thallium images in the absence of significant coronary artery disease. Am J Cardiol. 1992;70(15):1276–80. doi: 10.1016/0002-9149(92)90761-m. [DOI] [PubMed] [Google Scholar]
- 8.Bemiller C, Pepine CJ, Rogers A. Long-term observations in patients with angina and normal coronary arteriograms. Circ Res. 1979;47(1):36–43. doi: 10.1161/01.cir.47.1.36. [DOI] [PubMed] [Google Scholar]
- 9.Cannon RO, 3rd, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol. 1988;61(15):1338–43. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 10.Gurevitz O, Jonas M, Boyko V, Rabinowitz B, Reicher-Reiss H. Clinical profile and long-term prognosis of women < or = 50 years of age referred for coronary angiography for evaluation of chest pain. Am J Cardiol. 2000;85(7):806–9. doi: 10.1016/s0002-9149(99)00871-1. [DOI] [PubMed] [Google Scholar]
- 11.Olson MB, Kelsey SF, Matthews K, Shaw LJ, Sharaf BL, Pohost GM, et al. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI-sponsored WISE Study. Eur Heart J. 2003;24(16):1506–14. doi: 10.1016/s0195-668x(03)00279-3. [DOI] [PubMed] [Google Scholar]
- 12.Johnson BD, Shaw LJ, Pepine CJ, Reis SE, Kelsey SF, Sopko G, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27(12):1408–15. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 13.Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, et al. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33(6):1453–61. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 14.Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;87(8):937–41. A3. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan A, Mullins PA, Thuraisingham SI, Petch MC, Schofield PM. Clinical presentation and functional prognosis in syndrome X. Br Heart J. 1993;70(4):346–51. doi: 10.1136/hrt.70.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343(13):915–22. doi: 10.1056/NEJM200009283431303. [DOI] [PubMed] [Google Scholar]
- 17.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169(9):843–50. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henkin Y, Abuful A, Yosefi C, Elis A, Ilia R, Gidron Y. Preventive therapy in patients with insignificantly narrowed coronary arteries: evaluation of physician attitude and practice. Clin Cardiol. 2004;27(6):328–32. doi: 10.1002/clc.4960270605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoyama I, Ohtake T, Momomura S, Nishikawa J, Sasaki Y, Omata M. Reduced coronary flow reserve in hypercholesterolemic patients without overt coronary stenosis. Circulation. 1996;94(12):3232–8. doi: 10.1161/01.cir.94.12.3232. [DOI] [PubMed] [Google Scholar]
- 20.Quyyumi AA, Cannon RO, 3rd, Panza JA, Diodati JG, Epstein SE. Endothelial dysfunction in patients with chest pain and normal coronary arteries. Circulation. 1992;86(6):1864–71. doi: 10.1161/01.cir.86.6.1864. [DOI] [PubMed] [Google Scholar]
- 21.Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, et al. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81(2):491–7. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 22.Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia G, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;82(3):269–72. doi: 10.1136/hrt.82.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–32. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123(11):1243–62. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases.American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106(3):388–91. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 26.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report. 2010;(29):1–20. 24. [PubMed] [Google Scholar]
- 27.Facts and Figures. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2007. [PubMed] [Google Scholar]