Abstract
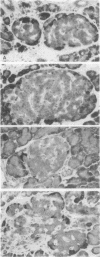
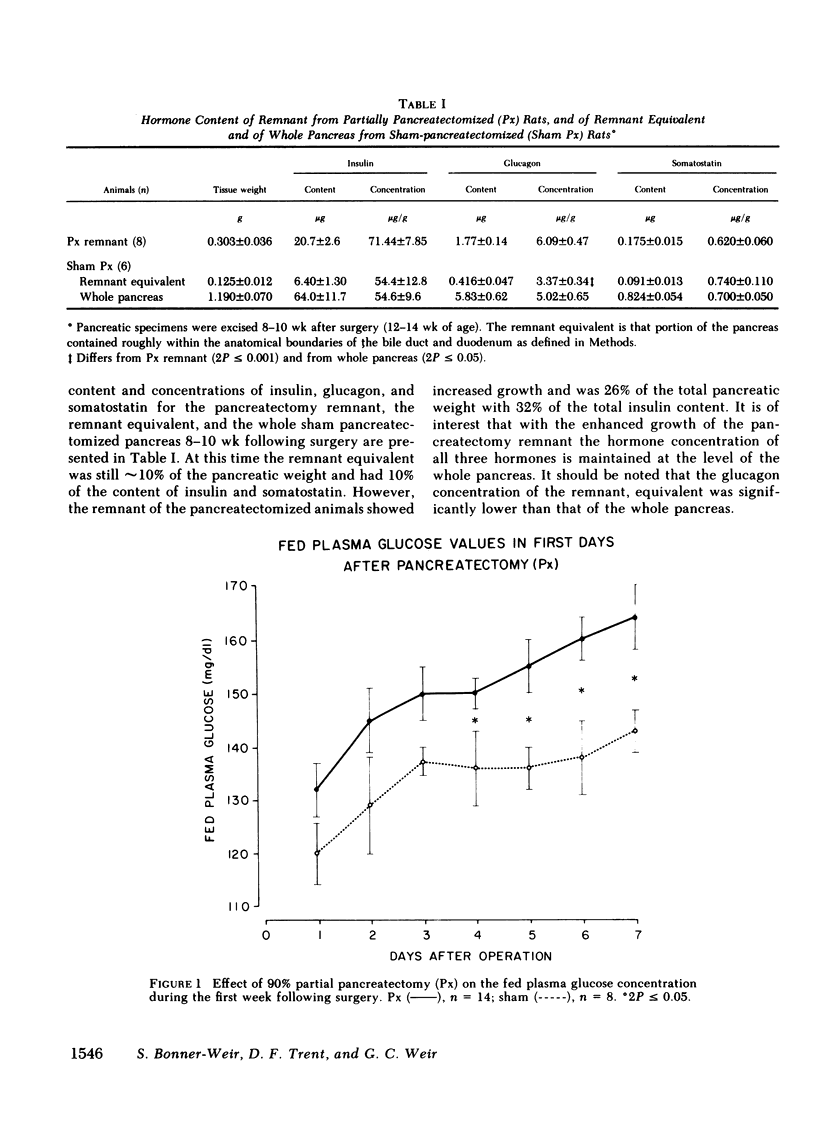
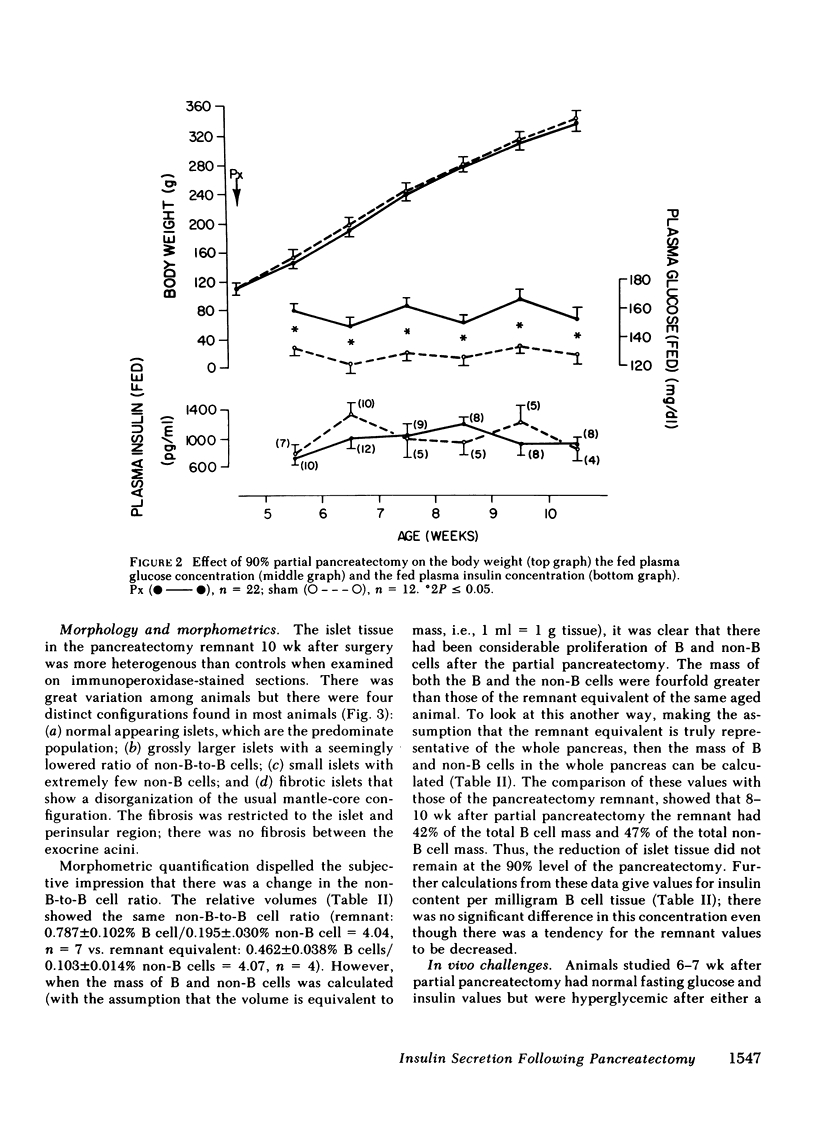
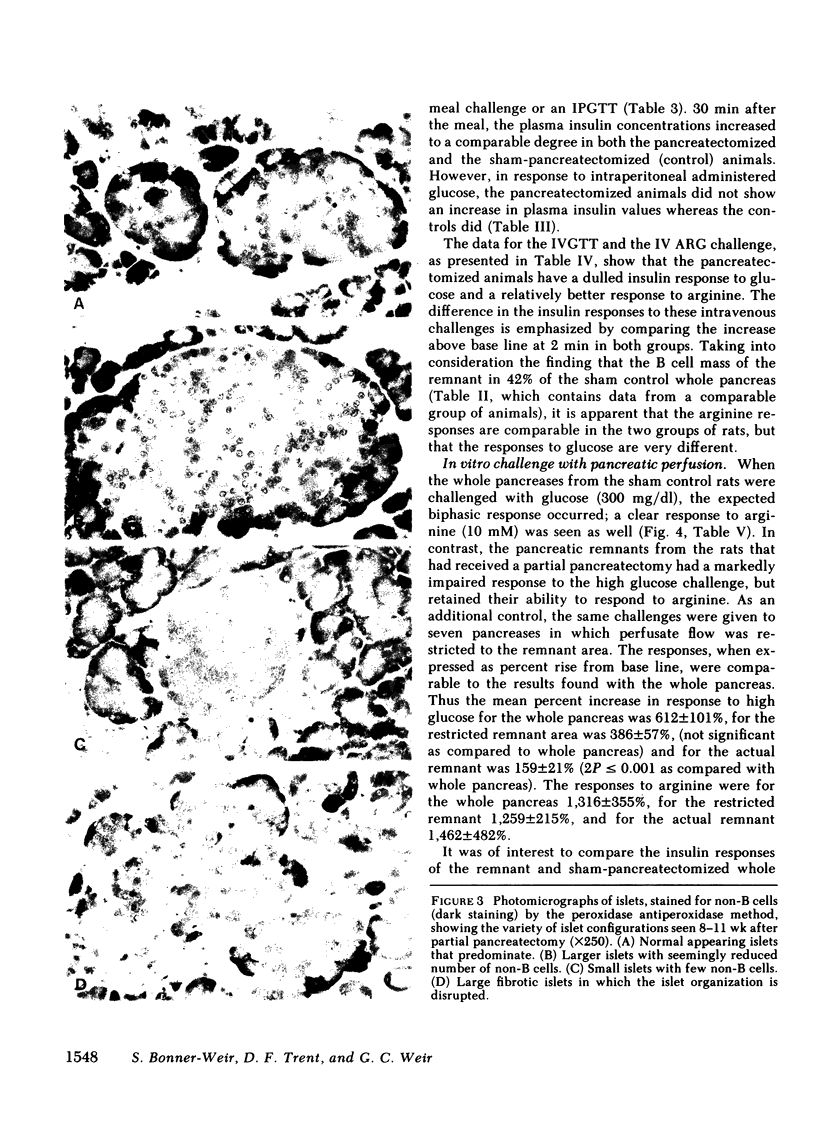
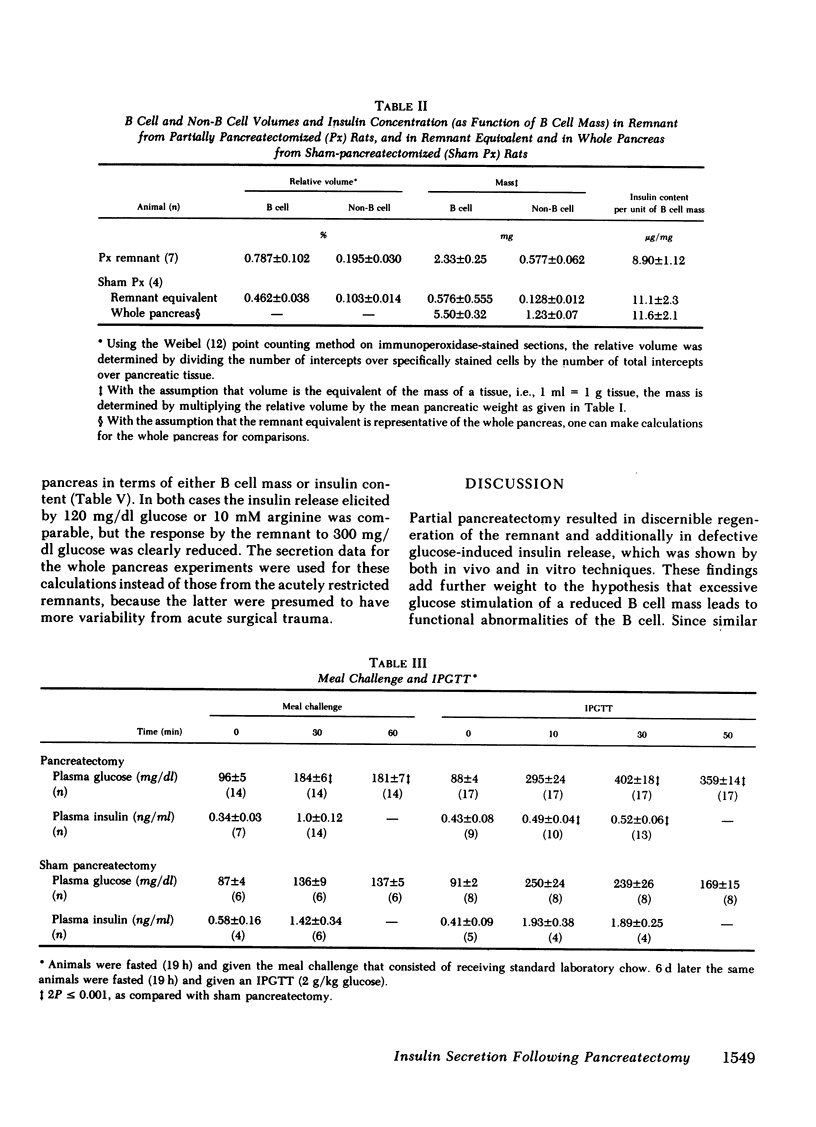
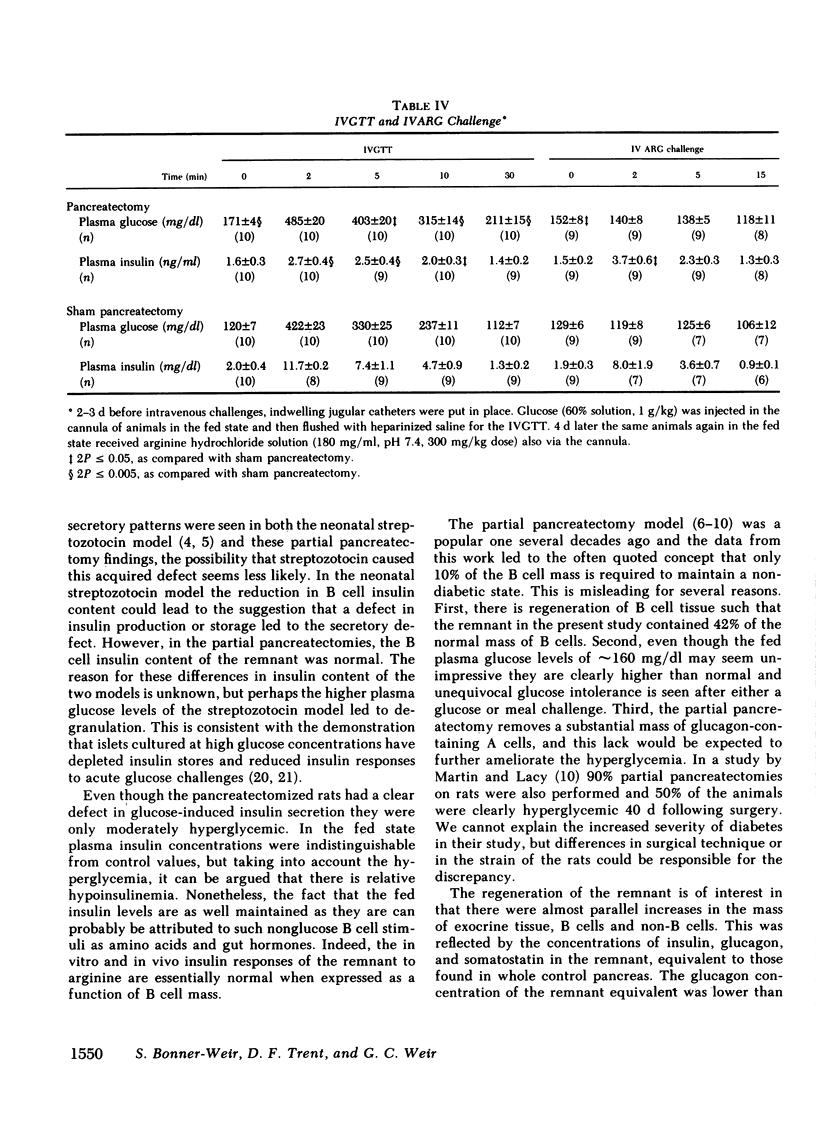
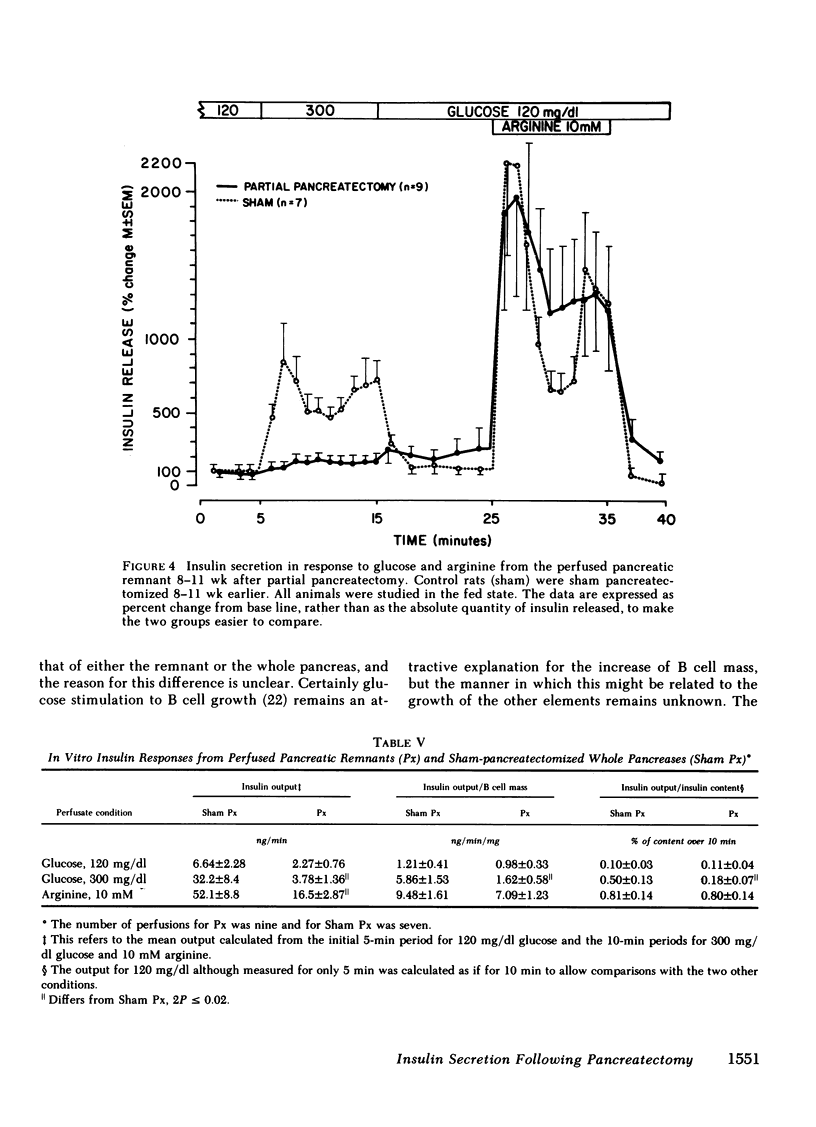
To define the consequences of a known reduction of B cell mass in rats, 90% partial pancreatectomies were performed. For the 6 wk following surgery moderate hyperglycemia was maintained in the fed state but there were no differences in body weight nor plasma insulin concentrations compared with sham-pancreatectomized controls. 8-10 wk following surgery regeneration of the remnant was evident with remnant weight being 26%, B cell mass being 42%, and non-B cell mass being 47% of values found for control whole pancreas. There were comparable increases in the remnant content of insulin, glucagon, and somatostatin. Following meal challenges, intraperitoneal and intravenous glucose tolerance tests and intravenous arginine challenge given 6-7 wk after surgery, the insulin responses to glucose were blunted or absent but the responses following the meals or arginine were intact. Similarly, when the pancreatic remnant was perfused in vitro, insulin release after challenge with 300 mg/dl glucose was markedly reduced whereas intact responsiveness to 10 mM arginine was retained. These data suggest that the chronic stimulation of a reduced B cell mass can lead to a selective loss of glucose-induced insulin secretion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Andersson A. Long-term effects of glucose on insulin release and glucose oxidation by mouse pancreatic islets maintained in tissue culture. Biochem J. 1974 Jun;140(3):377–382. doi: 10.1042/bj1400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A., Westman J., Hellerström C. Effects of glucose on the ultrastructure and insulin biosynthesis of isolated mouse pancreatic islets maintained in tissue culture. Diabetologia. 1974 Dec;10(6):743–753. doi: 10.1007/BF01219536. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Honey R. N., Weir G. C. Responses of neonatal rat islets to streptozotocin: limited B-cell regeneration and hyperglycemia. Diabetes. 1981 Jan;30(1):64–69. doi: 10.2337/diab.30.1.64. [DOI] [PubMed] [Google Scholar]
- Chick W. L. Beta cell replication in rat pancreatic monolayer cultures. Effects of glucose, tolbutamide, glucocorticoid, growth hormone and glucagon. Diabetes. 1973 Sep;22(9):687–693. doi: 10.2337/diab.22.9.687. [DOI] [PubMed] [Google Scholar]
- Clark A., Bown E., King T., Vanhegan R. I., Turner R. C. Islet changes induced by hyperglycemia in rats. Effect of insulin or chlorpropamide therapy. Diabetes. 1982 Apr;31(4 Pt 1):319–325. doi: 10.2337/diab.31.4.319. [DOI] [PubMed] [Google Scholar]
- Deckert T., Lauridsen U. B., Madsen S. N., Mogensen P. Insulin response to glucose, tolbutamide, secretin, and isoprenaline in maturity-onset diabetes mellitus. Dan Med Bull. 1972 Oct;19(7):222–226. [PubMed] [Google Scholar]
- HOUSSAY B. A., RODRIGUEZ R. R., CARDEZA A. F. Prevention of experimental diabetes with adrenal steroids. Endocrinology. 1954 May;54(5):550–552. doi: 10.1210/endo-54-5-550. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Nagulesparan M., Klimes I., Clark R., Sasaki H., Aronoff S. L., Vasquez B., Rubenstein A. H., Unger R. H. Improvement of insulin secretion but not insulin resistance after short term control of plasma glucose in obese type II diabetics. J Clin Endocrinol Metab. 1982 Feb;54(2):217–222. doi: 10.1210/jcem-54-2-217. [DOI] [PubMed] [Google Scholar]
- Owerbach D., Nerup J. Restriction fragment length polymorphism of the insulin gene in diabetes mellitus. Diabetes. 1982 Mar;31(3):275–277. doi: 10.2337/diab.31.3.275. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Benson J. W., Walter R. M., Ensinck J. W. Arginine-stimulated acute phase of insulin and glucagon secretion in diabetic subjects. J Clin Invest. 1976 Sep;58(3):565–570. doi: 10.1172/JCI108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel Y. C., Reichlin S. Somatostatin in hypothalamus, extrahypothalamic brain, and peripheral tissues of the rat. Endocrinology. 1978 Feb;102(2):523–530. doi: 10.1210/endo-102-2-523. [DOI] [PubMed] [Google Scholar]
- Robertson R. P., Porte D., Jr The glucose receptor. A defective mechanism in diabetes mellitus distinct from the beta adrenergic receptor. J Clin Invest. 1973 Apr;52(4):870–876. doi: 10.1172/JCI107251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotwein P., Chyn R., Chirgwin J., Cordell B., Goodman H. M., Permut M. A. Polymorphism in the 5'-flanking region of the human insulin gene and its possible relation to type 2 diabetes. Science. 1981 Sep 4;213(4512):1117–1120. doi: 10.1126/science.6267694. [DOI] [PubMed] [Google Scholar]
- Saito K., Yaginuma N., Takahashi T. Differential volumetry of A, B and D cells in the pancreatic islets of diabetic and nondiabetic subjects. Tohoku J Exp Med. 1979 Nov;129(3):273–283. doi: 10.1620/tjem.129.273. [DOI] [PubMed] [Google Scholar]
- Shah J. H., Stevens B., Sorensen B. J. Dissociation of the effects of vincristine on stimulated insulin release and the pancreatic beta-cell microtubular structures in the intact rat. Diabetes. 1981 Jul;30(7):539–544. doi: 10.2337/diab.30.7.539. [DOI] [PubMed] [Google Scholar]
- Shah J. H., Wongsurawat N., Aran P. P., Motto G. S., Bowser E. N. A method for studying acute insulin secretion and glucose tolerance in unanesthetized and unrestrained rats. The effect of mild stress on carbohydrate metabolism. Diabetes. 1977 Jan;26(1):1–6. doi: 10.2337/diab.26.1.1. [DOI] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Turner R. C., McCarthy S. T., Holman R. R., Harris E. Beta-cell function improved by supplementing basal insulin secretion in mild diabetes. Br Med J. 1976 May 22;1(6020):1252–1254. doi: 10.1136/bmj.1.6020.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vague P., Moulin J. P. The defective glucose sensitivity of the B cell in non insulin dependent diabetes. Improvement after twenty hours of normoglycaemia. Metabolism. 1982 Feb;31(2):139–142. doi: 10.1016/0026-0495(82)90125-1. [DOI] [PubMed] [Google Scholar]
- WEIBEL E. R. Principles and methods for the morphometric study of the lung and other organs. Lab Invest. 1963 Feb;12:131–155. [PubMed] [Google Scholar]
- Weir G. C., Clore E. T., Zmachinski C. J., Bonner-Weir S. Islet secretion in a new experimental model for non-insulin-dependent diabetes. Diabetes. 1981 Jul;30(7):590–595. doi: 10.2337/diab.30.7.590. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Goltsos P. C., Steinberg E. P., Patel Y. C. High concentration of somatostatin immunoreactivity in chicken pancreas. Diabetologia. 1976 May;12(2):129–132. doi: 10.1007/BF00428977. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Knowlton S. D., Martin D. B. Glucagon secretion from the perfused rat pancreas. Studies with glucose and catecholamines. J Clin Invest. 1974 Dec;54(6):1403–1412. doi: 10.1172/JCI107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G. C. Non-insulin-dependent diabetes mellitus: interplay between B-cell inadequacy and insulin resistance. Am J Med. 1982 Oct;73(4):461–464. doi: 10.1016/0002-9343(82)90321-7. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978 Nov;15(5):417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]