Abstract
The cingulate cortex is regarded as the backbone of structural and functional connectivity of the brain. While its functional connectivity has been intensively studied, little is known about its effective connectivity, its modulation by behavioral states, and its involvement in cognitive performance. Given their previously reported effects on cingulate functional connectivity, we investigated how eye-closure and sleep deprivation changed cingulate effective connectivity, estimated from resting-state high-density electroencephalography (EEG) using a novel method to calculate Granger Causality directly in source space.
Effective connectivity along the cingulate cortex was dominant in the forward direction. Eyes-open connectivity in the forward direction was greater compared to eyes-closed, in well-rested participants. The difference between eyes-open and eyes-closed connectivity was attenuated and no longer significant after sleep deprivation. Individual variability in the forward connectivity after sleep deprivation predicted subsequent task performance, such that those subjects who showed a greater increase in forward connectivity between the eyes-open and the eyes-closed periods also performed better on a sustained attention task. Effective connectivity in the opposite, backward, direction was not affected by whether the eyes were open or closed or by sleep deprivation.
These findings indicate that the effective connectivity from posterior to anterior cingulate regions is enhanced when a well-rested subject has his eyes open compared to when they are closed. Sleep deprivation impairs this directed information flow, proportional to its deleterious effect on vigilance. Therefore, sleep may play a role in the maintenance of waking effective connectivity.
Keywords: Effective connectivity, EEG, cingulate cortex, resting state, sleep deprivation, vigilance
Introduction
Efficient communication between distant cortical areas is an essential characteristic of the healthy brain and is necessary for proper cognitive functioning (Deco and Corbetta, 2011; Deco et al., 2011). Long-distance communication can be investigated by quantifying the degree of correlation between region-specific activity, as a measure of functional connectivity (Friston, 1994; Deco et al., 2011). Functional connectivity is particularly strong between regions belonging to the default-mode network (Damoiseaux et al., 2006; Buckner et al., 2008), whose core includes regions of the cingulate cortex (Fox and Raichle, 2007; van den Heuvel et al., 2008; Buckner et al., 2009; van den Heuvel and Sporns, 2011). In addition, the cingulate cortex represents the structural backbone of the brain network (Vogt and Pandya, 1987; Schmahmann and Pandya, 2006; Hagmann et al., 2008; Greicius et al., 2009; Honey et al., 2009) and is involved in multiple cognitive functions (Vogt et al., 1992), including efficient executive control (Agam et al., 2011; Kantarci et al., 2011), conscious sensory perception (Portas et al., 2000), the state of consciousness (Sämann et al., 2011; Barrett et al., 2012), and sleep (Murphy et al., 2009; Piantoni et al., 2013).
Yet, functional connectivity does not reveal the directed influence of one region onto another (Friston, 1994). This form of connectivity is harder to assess from resting-state fMRI signals because quantification of effective connectivity is confounded by the interregional variability of the hemodynamic response (David et al., 2008; Smith et al., 2011). High-density EEG studies, on the other hand, have identified a preferential flow of information from posterior towards anterior regions during resting wakefulness at the scalp level (Kamiński et al., 1997; Baccala et al., 2001; De Gennaro et al., 2004). The most challenging limitation in EEG studies, however, is the poor spatial resolution and the potentially spurious effects caused by volume conduction (Astolfi et al., 2007; Supp et al., 2007; Gómez-Herrero et al., 2008). This limitation has traditionally been circumvented by methods which estimate, first, activity at the source level and, subsequently, the effective connectivity between those sources (Babiloni et al., 2005; Astolfi et al., 2007; Ding et al., 2007; Hui et al., 2010). Unfortunately, this two-step approach requires very high signal-to-noise ratio (SNR) to be effective. Noise in the EEG data results in correlation between the noise components of the estimated source signals that introduces bias into the estimated connectivity values. The SNR requirements are greatly reduced using a novel approach we recently introduced and validated (Cheung et al., 2010, 2012). In brief, this approach jointly solves the equations modeling connectivity in source space and the physics of measuring the source activity with EEG by means of a state-space formulation and maximum-likelihood estimation techniques. The resulting multivariable autoregressive model for the interactions in source space is used to determine conditional Granger causality as a measure of directed connectivity (Chen et al., 2006).
In the present high-density EEG study, we applied this novel technique to investigate effective connectivity along the cingulate cortex, its modulation by behavioral states, and its functional relevance for cognitive performance. The experimental manipulation involved two factors known to affect the brain’s activity and functional connectivity: the opening versus closing of the eyes (Marx et al., 2004; Barry et al., 2007; Bianciardi et al., 2009) and sleep deprivation (Gujar et al., 2010; Chee et al., 2011; Kar et al., 2011; De Havas et al., 2012). Effective connectivity within the cingulate cortex has been proven sensitive to various experimental manipulations, such as motor execution (De Vico Fallani et al., 2007) and decision making (De Vico Fallani et al., 2010).
In addition, we hypothesized that the alterations in effective connectivity induced by sleep deprivation might be associated with performance in the cognitive domain that is most sensitive to sleep deprivation: vigilance, i.e. the ability to sustain attention to a task over time (Oken et al., 2006; Lim and Dinges, 2008, 2010). Sleep deprivation increases reaction times and the frequency of lapses (Dinges, 1995), which has been attributed to less efficient communication between brain areas (Chee et al., 2008; Sadaghiani et al., 2010) and to changes in activity in the cingulate cortex (Choo et al., 2005; Tomasi et al., 2009; Gujar et al., 2010). Vigilance has been proposed as the cognitive process most sensitive to sleep deprivation in adults (Lim and Dinges, 2010) and the culprit of the performance degradation on other tasks (Philibert, 2005; Lim and Dinges, 2008, 2010). Of note, there are considerable trait-like individual differences in the effect of sleep deprivation on vigilance (Van Dongen et al., 2004, 2012; Rocklage et al., 2009). Pursuing the brain mechanisms involved in these individual differences in vulnerability is thus of considerable importance. We therefore investigated whether individual differences in cingulate activity changes elicited by sleep deprivation were predictive of subsequent performance on a vigilance task.
In summary, we tested whether: (a) Directed connectivity along the cingulate cortex changes when eyes are open compared to when they are closed; (b) Sleep deprivation impairs this response; (c) The impairment in this response predicts vigilance decrements.
Materials and Methods
Participants
Eight healthy adults (five male, age range 20–26, all right-handed) participated in a two-day experimental protocol, after giving written, informed consent. The protocol was approved by the Medical Ethics Committee of the VU University Medical Center, in accordance with the Declaration of Helsinki. All participants met the following criteria (1) no self-reported sleep complaints, assessed using validated questionnaires (Buysse et al., 1989; Johns, 1991; Soldatos et al., 2000), (2) nonsmoking, (3) no use of medication, including hormonal contraceptives, and (4) no neurological or psychiatric disorders. All had normal or corrected-to-normal vision, had a regular sleep wake rhythm assessed by actigraphy (Actiwatch, Cambridge Neuro-Technology Ltd., Cambridge, UK) during the week before the experiments, and refrained from caffeine and alcohol on the day before and during the experiment.
Experimental Protocol
Participants were assessed on two days separated by 5.1 ± 4.7 days in between (mean ± s.d.), in a counterbalanced order either after a night of normal sleep or a night of total sleep deprivation. Successful completion of total sleep deprivation was verified using sleep estimates obtained from actigraphy. None of the participants had a period of inactivity longer than 10 minutes, which might indicate a possible sleep period.
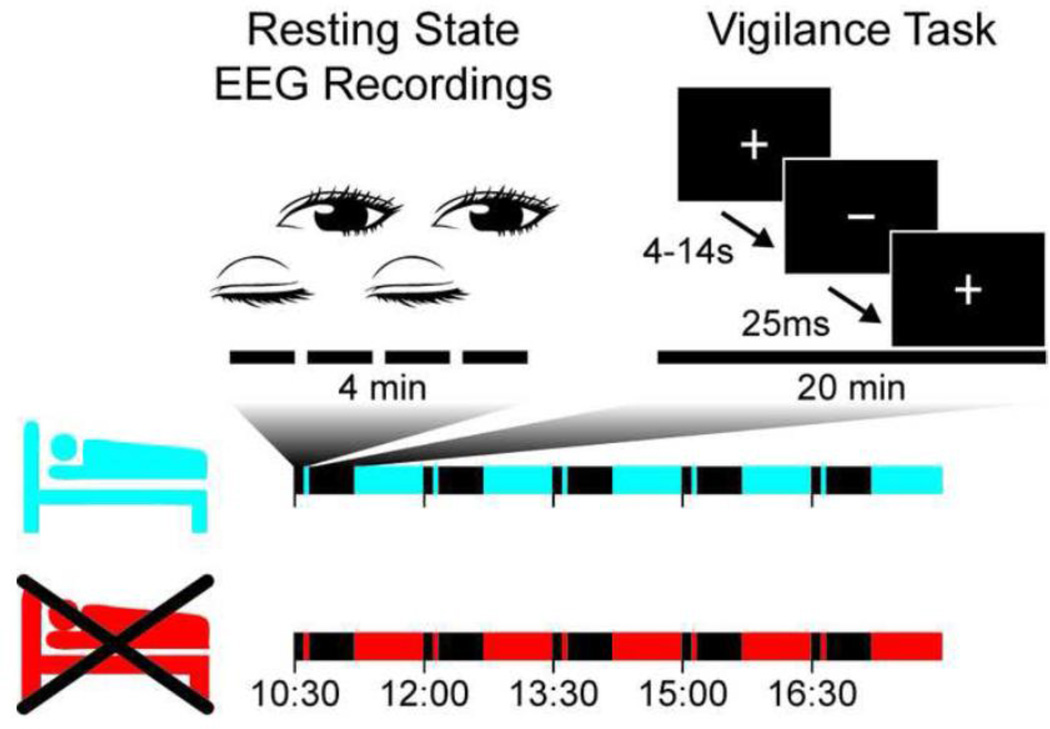
On each day, five sessions were recorded at 10:30, 12:00, 13:30, 15:00, and 16:30 (Fig. 1). A session consisted of a four-minute resting-state period: two alternations of one minute of eyes-closed and one minute of eyes-open, in a sitting position in a dimly lit room. Resting-state EEG was recorded at 1024 Hz, with a Micromed SD-LTM64 recorder (Micromed, Mogliano Veneto, Italy), using a 61-equidistant channel EEG-cap (M10, Easycap, Munich, Germany), referenced to Cz. Impedance was kept below 10 kOhm. The recording of one session of one subject was lost due to a technical malfunction.
Figure 1.
Eight participants were measured on two different days, one day after a night of normal sleep (cyan) and one day after a night of sleep deprivation (red). They performed the same protocol five times during the day. Each of the five sessions consisted of four minutes of eyes-closed and eyes-open resting state and 20 minutes of a vigilance task. During the vigilance task, participants were asked to press a button when they noticed that the plus sign turned into a minus sign.
After the resting-state EEG recording, participants performed a reaction time task with stimuli of low contrast, small size and brief duration (Romeijn and Van Someren, 2011). The task requires sustained attention to near-threshold visual stimuli for 20 minutes and was programmed in E-Prime 2 (Psychology Software Tools, Pittsburgh, PA). Participants were instructed to fixate on a small black crosshair (a plus sign, +) displayed against a gray background and to respond as fast as possible when they saw the fixation crosshair turn into a hyphen (a minus sign, −), by pressing a button with the index finger of their dominant hand (Fig. 1). The hyphen was presented for 25 milliseconds and occurred after a random interval lasting between 4 and 14 seconds (84-millisecond step resolution). The task comprised 120 stimuli per session. Non-responses and reaction times longer than 1 s were labeled as lapses.
Preprocessing
EEG data were re-referenced to the average, high-pass filtered at 1 Hz cutoff frequency (2nd-order Butterworth filter), notch-filtered at 50 Hz, and downsampled to 256 Hz, in combination with an anti-aliasing filter. Segments containing artifacts, such as muscle activity, eye blinks, and eye movements, were visually detected and manually rejected.
State-Space Formulation
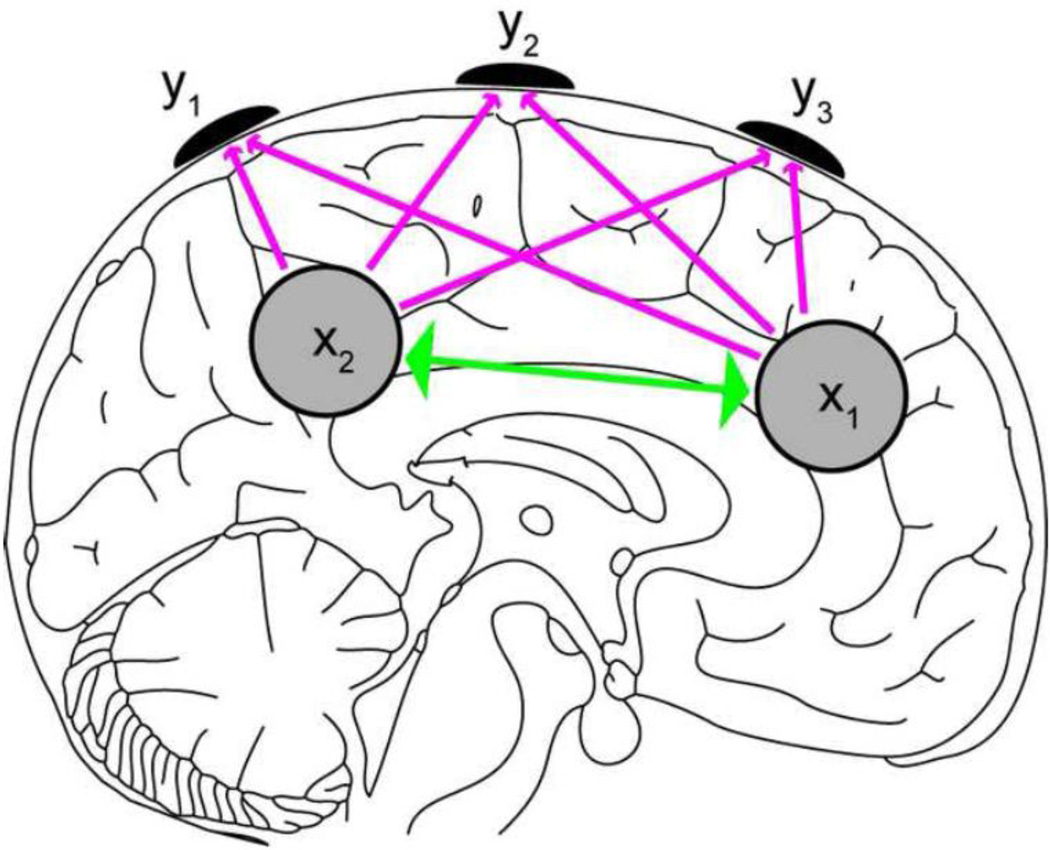
Estimation of the connectivity between sources was achieved by means of a state-space model (Cheung et al., 2010). The state-space formulation, which has been used before to improve source reconstruction (Galka et al., 2004; Yamashita et al., 2004), offers an intuitive and elegant description of the connectivity at the source level and consists of two sets of equations (Fig. 2). 1) The connectivity between the sources is represented as, xi = Azi-1 + wn, where xi is the modeled source activity at time i, A is the matrix of MVAR coefficients with dimensions M by MP (with M the number of sources and P the model order), and w ~ N(0, Q), the noise at source level. 2) The observation of source activity at the scalp electrodes is described as yi = CΛxi + vi, where C contains forward models for each cortical region of interest (ROI), Λ represents the spatial distribution of source activity within the regions of interest, and v ~ N(0, R), the noise at the sensor level. The matrix C is assumed to be known, so the ROIs should be chosen a priori based on the research question of interest and previous literature. The ROIs may also be chosen based on prior source localization of the data. If each ROI is represented by multiple dipoles (and their moments), then the parameter Λ, estimated from the data, indicates the weight of each dipole within the corresponding ROI. In this manuscript we employed a cortical patch model for each ROI by building a rank-3 patch basis set from all dipoles within the ROI following Limpiti et al. (2006). The state-space model has the following unknowns: 1) the MVAR model coefficients A for each dipole, 2) the spatial activity distribution Λ within each ROI, 3) the covariance structure of the source noise Q, 4) the error term R at the scalp level.
Figure 2.
Description of the algorithm to estimate effective connectivity between sources x1 and x2, based on the measured signal y1, y2, y3. The state-space consists of two equations: 1) one equation describing the interaction between sources (the green arrows); 2) one equation describing the forward model, i.e. how source activity propagates from x to y (the magenta arrows).
An expectation-maximization algorithm seeks the maximum likelihood estimates of the MVAR model coefficients, the spatial activity distribution components within each ROI, and the spatial covariance matrix of the observation noise. Multiple random initial conditions are used to begin the expectation-maximization algorithm and the converged solution with maximum likelihood is selected to minimize the possibility of convergence to a local minimum. The parameters of the maximum-likelihood model are used for the computation of the connectivity at the source level.
The validity of the state-space approach has been comprehensively tested on simulations (Cheung et al., 2010, 2012) and real data (Cheung et al., 2010; Malekpour et al., 2012). This method has been shown to be less sensitive to noise than the two-step procedure, in which the inverse problem is first solved and then the MVAR model is estimated from the source time series (Babiloni et al., 2005; Hui et al., 2010). Simulations have demonstrated that the presence of unmodeled sources does not affect the estimation of the amount of directed connectivity between the ROIs (Cheung et al., 2010). Variability in the precise location of the source of interest across participants and potential misallocation were taken into account by explicitly modeling the spatial distribution of source activity within the regions of interest with the parameter Λ.
Granger Causality Calculation
GC reflects the strength of effective connectivity from one region to another by quantifying how much the signal in the seed region is able to predict the signal in the target region (Granger, 1969; Geweke, 1982). GC is defined as the log-ratio between the error variance of a reduced model, which predicts one time series based only on its own past values, and that of the full model, which in addition includes the past values of another time series. This formulation, however, requires the estimation of two models, which is computationally onerous in the case of the state-space formulation. Therefore, we calculated GC directly from the MVAR coefficients and the error covariance matrix of the full model in the frequency domain using the partitioned matrix technique of Chen et al. (2006).
Forward Model
The forward model was computed from a template T1 MRI, distributed with SPM8 (Litvak et al., 2011), which was realigned in MNI space and segmented. Based on this segmentation, a three-shell boundary element model (BEM), each consisting of 1500 vertices, was used to calculate the conduction model of the head (Oostendorp and Van Oosterom, 1991) to the electrodes. The dipoles inside the brain mesh were spaced 7mm apart.
Selection of the ROIs
The anterior and posterior cingulate cortices belong to the core of the brain network (Hagmann et al., 2008; Buckner et al., 2009; van den Heuvel and Sporns, 2011; Barrett et al., 2012), as discussed in the Introduction, and were selected as regions of interest. Studies relying on preselected ROIs always suffer from the “missing region” problem, by which it is understood that the assumption of directed connectivity between two regions might be violated by the presence of a hidden driving region (Valdés-Sosa et al., 2011). Because model complexity and computational costs would rise exponentially if we added any potential regions connected to the main hubs of the brain network (Hagmann et al., 2008; van den Heuvel and Sporns, 2011), we strived for the more modest goal of modeling effective connectivity along the cingulate cortex as accurately as possible. Because the anterior and posterior ROIs are anatomically connected by the cingulate cortex (Schmahmann and Pandya, 2006), we included an additional ROI located on the cingulate cortex between the anterior and posterior cingulate regions.
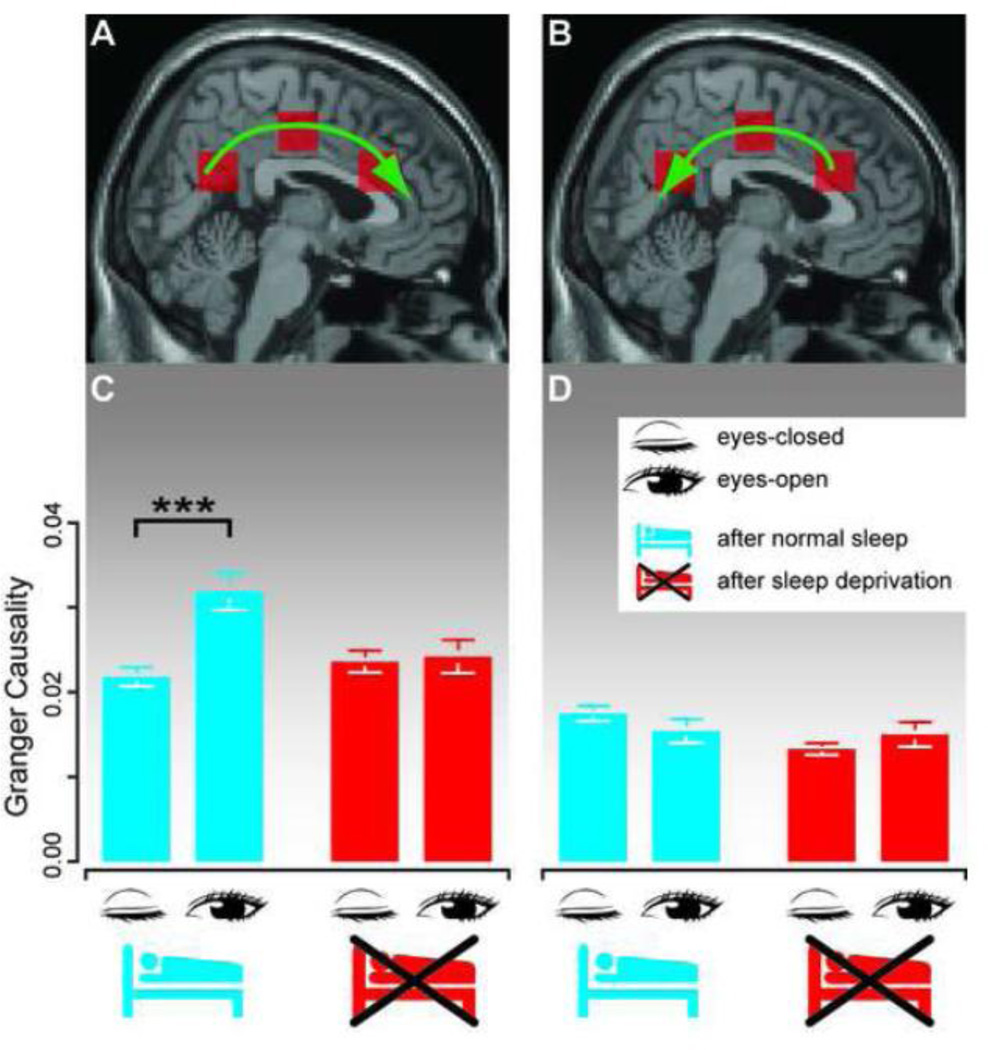
A major reason for the chosen cingulate cortex coordinates was that they comprise the most unidirectional path travelled by slow waves (Murphy et al., 2009): we hypothesized that the regions with the most prominent asynchronous backward travelling slow sleep oscillations may most clearly show a directional response to sleep deprivation (Fig. 3A-B; Anterior Cingulate MNI [min x = −3, max x = 11;min y = 17, max y = 31; min z = 29, max z = 43]; Middle Cingulate MNI [min x = −10, max x = 11;min y =−18, max y = −11; min z = 29, max z = 50]; Posterior Cingulate MNI [min x = −10, max x = 4; min y = −64, max y = −53; min z = 22, max z = 36]). In particular, in Murphy et al. (2009), slow wave activity propagates continuously along the cingulate cortex. The inclusion of the middle cingulate region allows for a more complete description of the effective connectivity along the cingulate cortex. GC was calculated for the backward and forward direction between these preselected regions along the cingulate cortex.
Figure 3.
Effective connectivity along the cingulate cortex is affected by eyes closure and sleep deprivation, but only in the forward direction.
(A–B) Granger Causality effective connectivity estimates were calculated between three areas in the cingulate cortex, in the forward (A) and backward (B) direction.
(C–D) Effective connectivity is higher in the forward compared to backward direction.
(C) Granger Causality during resting state EEG in the forward direction after normal sleep (blue) and sleep deprivation (red). Forward effective connectivity increases during eyes-open periods, as compared to eyes-closed, in well-rested subjects. This eyes-open increase is suppressed after sleep deprivation. Error bars represent s.e.m.
(D) Effective connectivity in the backward direction was influenced neither by eyes-closure nor by sleep deprivation. Error bars represent s.e.m.
Statistical Analysis
Linear Mixed-Effects Model Formulation
Hypothesis testing was performed using linear mixed-effects regression models (LMEM). LMEM is an extension of the linear model, which takes into account the hierarchical structure of the data and dealing with missing data (Pinheiro and Bates, 2000; Bryk and Raudenbush, 2002). A linear model can be written as y = Xβ + ε, where y is the observed values, X the design matrix, β the regression coefficients, and ε ~ N(0, σ2I) the error term. LMEM includes additional terms which capture the variance belonging to each level of the hierarchical structure (i.e. participant or session) and can be written as yi = Xiβ + Zibi + εi, where i refers to one group within a level, Zi is the design matrix for group i, bi ~ N(0, ψ) are the random-effect coefficients and ψ the covariance matrix of the random effects (Laird and Ware, 1982). The regression coefficients β cannot be calculated analytically but need to be estimated using maximum likelihood (ML). The estimated values of β and the standard error of the ML estimates are used to calculated z-values, based on the Wald test. The inclusion of covariates of interest (i.e. ‘alpha power’, see below) in this formulation is trivial. Statistical analysis was conducted using lme4 (Bates and Sarkar, 2007), a package for R 2.12 (R Development Core Team, 2010).
Linear Mixed-Effects Model Implementation
Data were structured according to their 4-level hierarchical dependency: 3 pairings of the ROIs in each direction (anterior cingulate and posterior cingulate; anterior cingulate and middle cingulate; middle cingulate and posterior cingulate), assessed in parallel in each of the 5 sessions, nested in 2 experimental days, nested in 8 participants. A first regression model including all assessments investigated whether GC in the backward direction is equally strong as GC in the opposite, forward, direction. Subsequent regression models investigated each direction separately. These latter models estimated the difference between eyes-open and eyes-closed conditions on connectivity, in the recordings obtained after normal sleep. The effect of sleep deprivation on eyes-open and eyes-closed effective connectivity was subsequently estimated using a full 2×2 model, with the factors ‘eyes open’ and ‘sleep deprivation’ (post-normal sleep and post-sleep deprivation), and their interaction. We applied log-transformation to the GC values for all analyses, so that the residuals were normally distributed; for clarity, GC values in Fig. 3 are not log-transformed.
To obtain the effects of eye-opening and sleep deprivation on integrated measures of forward and backward effective connectivity along the cingulate cortex, GC values between the three regions of interest (ACC: anterior cingulate cortex; MCC: middle cingulate cortex; PCC: posterior cingulate cortex) in the direction of interest were included as parallel observations in the linear mixed-effect models.
This procedure, however, does not reveal whether the effects of eye-opening and sleep deprivation may be restricted to a smaller part of the cingulate cortex only. Therefore, we performed post-hoc analyses separately on each of the six individual GC values (anterior-posterior, anterior-middle, and middle-posterior in each direction) to assess whether the changes in effective connectivity due to eye-opening and sleep deprivation were more pronounced in some parts of the cingulate cortex.
Alpha Power
The factors that were manipulated in our study (‘eyes open’ and ‘sleep deprivation’) are known to strongly affect resting-state EEG alpha power (8 – 12 Hz) (Corsi-Cabrera et al., 1992; Ferreira et al., 2006). Therefore, we considered it necessary to rule out the possibility that their estimated effects on effective connectivity were merely secondary to changes in alpha power. Alpha band power was calculated using Welch’s method (pwelch in Matlab). Recordings were divided in overlapping 2 s windows, multiplied by a Hamming window, and the power spectrum was averaged over the 8 – 12 Hz frequency band and over the 12 parietal electrodes with the largest alpha power across all participants. We first investigated whether changes in GC values mirrored changes in alpha power by use of the same two-factor model as described above, now estimating the effects of eye opening and sleep deprivation on log-transformed alpha power. Secondly, in order to formally test the possible confounding effect of alpha power, we included alpha power as a covariate of no interest in the full model and evaluated whether it affected the estimated effects of ‘eyes open’ and ‘sleep deprivation’ on GC.
Vigilance Prediction from Resting-State Granger Causality
In order to investigate the functional relevance of individual differences in resting-state effective connectivity along the cingulate, participants performed a brief-stimulus reaction time task (Romeijn and Van Someren, 2011). Using linear mixed-effects models, we first investigated whether sleep deprivation indeed impaired the performance on this vigilance task, quantified as the mean reaction time and the number of lapses in each session. While the distribution of reaction times did not deviate from normality, the number of lapses per session followed a Poisson distribution and was accordingly modeled in the linear mixed-effects model. Based on the observation that vulnerability to sleep deprivation varies greatly across people (Van Dongen et al., 2004, 2012; Rocklage et al., 2009), we hypothesized that sleep-deprived participants with the most marked lack of increase in effective connectivity to eye-opening would be the ones to perform worst on the brief-stimulus reaction time task. For each subject, averages over the five post-sleep deprivation sessions were calculated both for the performance measures (reaction times and lapses) and for the relative change (ratio) of GC in the forward direction during the eyes-closed relative to eyes-closed periods. Only the direction along the cingulate cortex that was significantly affected by the factors ‘eyes open’ and ‘sleep deprivation’ was included in the analysis. The individual ratios of the GC values between the eyes-open and the eyes-closed periods were correlated with their mean reaction times and the number of lapses.
Results
Resting-state activity during eyes-open and eyes-closed periods was recorded with high-density EEG, after one night of normal sleep and after one night of sleep deprivation, in counterbalanced order. Using a novel technique to estimate effective connectivity at the source level, we calculated Granger Causality (GC) in each direction between each of the anterior, middle, and posterior cingulate ROIs. The three forward GC estimates were included at the lowest level of the linear mixed-effect regression models to obtain an integrated measure of forward GC along the cingulate. The three backward GC estimates were included at the lowest level of the linear mixed-effect regression models to obtain an integrated measure of backward GC along the cingulate.
Dominant Forward Effective Connectivity
On average over the five sessions after normal sleep and the five sessions after sleep deprivation, GC was higher in the forward cingulate direction as compared to the backward direction (difference estimate 0.503 GC, standard error 0.054, z-value = 9.250, P < 0.001), in line with previous findings on effective connectivity over scalp electrodes during wakefulness (Kamiński et al., 1997; De Gennaro et al., 2004).
Increase in Forward Effective Connectivity during Eyes-Open
We investigated whether, after normal sleep, effective connectivity was different in the eyes-open period as compared to eyes-closed period. Forward GC was higher during eyes-open than during eyes-closed (difference estimate 0.381 GC, s.e. 0.100, z-value = 3.816, P < 0.001, blue bars in Fig. 3C). In the opposite, backward, direction, GC was not different between eyes-open and eyes-closed (difference estimate −0.133 GC, s.e. 0.096, z-value = −1.388, P = 0.16, blue bars in Fig. 3D; ‘eyes open’ * ‘direction’ interaction effect P = 0.001).
Lack of Increase in Effective Connectivity after Sleep Deprivation
In counterbalanced order, subjects underwent actigraphy-verified total sleep deprivation during the night preceding one of the two recording days. The increase in forward connectivity, elicited by the eyes-open condition when subjects were well-rested, no longer occurred when subjects were sleepdeprived (after sleep deprivation, difference estimate between eyes-open and eyes-closed 0.023 GC, s.e 0.104, z-value = 0.218, P = 0.83, red bars in Fig. 3C; ‘eyes open’ * ‘sleep deprivation’ interaction effect P = 0.014). As was the case after normal sleep, GC in the backward direction was not affected by the eyes-open condition after sleep deprivation (sleep deprived versus normal sleep difference estimate 0.122 GC, s.e 0.100, z-value = 1.215, P = 0.23; ‘eyes open’ * ‘sleep deprivation’ interaction effect P = 0.18, red bars in Fig. 3D).
Post-Hoc Analysis on GC between individual ROIs within the cingulate
The preceding analysis estimated the effects of eyes-opening and sleep deprivation on integrated measures forward and backward effective connectivity along the cingulate cortex. In order to evaluate whether effects of eye-opening and sleep deprivation may be restricted to a smaller part of the cingulate cortex only, we performed post-hoc analyses separately on each of the six individual GC values (anterior-posterior, anterior-middle, and middle-posterior in each direction, Table 1). After normal sleep, eye-opening increased forward GC from PCC to ACC (P = 0.001) and from PCC to MCC (P = 0.01), but not from the MCC to ACC (P = 0.37). After sleep deprivation, eye-opening did not affect forward GC between any of the individual CC ROIs (all P > 0.74). Backward GC between the individual ROIs was not affected by eye-opening or sleep deprivation (all P > 0.42). These results indicate that eye opening and sleep deprivation in particular affect effective connectivity from the posterior cingulate cortex.
Table 1.
Difference in effective connectivity between eyes-open and eyes-closed periods, measured using Granger Causality, between the three individual regions of interest. Estimates, standard errors (s.e.), z-values, and P-values are computed for the resting-state periods after normal sleep and after sleep deprivation, in the forward and in the backward direction. P-values are corrected using a Bonferroni correction; in this case they were multiplied by 3, the number of post-hoc tests. ACC: Anterior Cingulate Cortex; MCC: Middle Cingulate Cortex; PCC: Posterior Cingulate Cortex.
| Condition | Direction | From | To | Estimate | s.e. | z-value | P-value |
|---|---|---|---|---|---|---|---|
| After Normal Sleep |
Forward | PCC | ACC | 0.453 | 0.121 | 3.747 | 0.001 |
| PCC | MCC | 0.432 | 0.143 | 3.018 | 0.010 | ||
| MCC | ACC | 0.257 | 0.165 | 1.561 | 0.368 | ||
| Backward | ACC | PCC | −0.197 | 0.133 | −1.480 | 0.429 | |
| ACC | MCC | −0.138 | 0.148 | −0.933 | 1.000 | ||
| MCC | PCC | −0.064 | 0.138 | 0.461 | 1.000 | ||
| After Sleep Deprivation |
Forward | PCC | ACC | −0.174 | 0.150 | −1.163 | 0.746 |
| PCC | MCC | 0.047 | 0.128 | 0.369 | 1.000 | ||
| MCC | ACC | 0.195 | 0.186 | 1.050 | 0.892 | ||
| Backward | ACC | PCC | 0.064 | 0.134 | 0.477 | 1.000 | |
| ACC | MCC | 0.147 | 0.132 | 1.116 | 1.000 | ||
| MCC | PCC | 0.155 | 0.150 | 1.034 | 0.913 |
Alpha Power does not Affect Cingulate Connectivity
Sleep deprivation strongly attenuated eyes-closed alpha power (difference estimate −0.347 log(µV2), s.e 0.093, z-value = −3.725, P < 0.001) but not eyes-open alpha power (difference estimate 0.056 log(µV2), s.e 0.050, z-value = 1.110, P = 0.27), as reported previously (Corsi-Cabrera et al., 1992). Importantly, sleep deprivation affected alpha power and the forward GC in orthogonal ways: it affected alpha power only during the eyes-closed period and effective connectivity only during the eyes-open period. This difference rules out the possibility that the sleep deprivation-elicited suppression of the eyes-open increase in forward effective connectivity is merely secondary to alpha power modulation. To formally test absence of involvement of alpha power modulation, we verified that its inclusion as a covariate of no interest in the regression models on the modulation of effective connectivity did not change outcomes. The eyes-open increase in forward GC was confirmed to still be highly significant after normal sleep (estimate 0.649 GC, s.e. 0.157, z-value = 4.123, P < 0.001) and absent after sleep deprivation (estimate 0.095 GC, s.e. 0.119, z-value = 0.799, P = 0.43; ‘eyes open’ * ‘sleep deprivation’ interaction effect P = 0.002).
Impairment of Forward Connectivity after Sleep Deprivation Predicts Vigilance
In order to investigate the functional relevance of the sleep deprivation-induced attenuation of the increase in forward effective connectivity over the cingulate with when the eyes are open, we tested the participants on a brief-stimulus reaction time task (Romeijn and Van Someren, 2011) immediately following the resting state periods. The task requires 20 minutes of continuous sustained attention to near-threshold stimuli. We confirmed previous behavioral findings (Dinges, 1995; Philibert, 2005; Lim and Dinges, 2008) that sleep deprivation increased the average reaction time by 43.40 ms (s.e. 10.40, z-value = 4.173, P < 0.001) and the number of lapses by a factor of 2.30 (= e0.832, s.e. 0.203, z-value = 4.087, P < 0.001).
Participants with a relatively preserved forward eyes-open/eyes-closed GC ratio after sleep deprivation performed better on the subsequent vigilance task, as indicated by the shorter reaction times (−128.9 ms/GC ratio, s.e. 46.2, z-value = −2.789, P = 0.032) and lower number of lapses (−0.361 #/GC ratio, s.e. 0.148, z-value = −2.447, P = 0.014). For completeness, the same regression was estimated for the normal sleep condition. The lack of significance of the regression coefficients for either reaction time (−3.69 ms/GC ratio, s.e. 48.9, z-value = −0.075, P = 0.94) or number of lapses (−0.208 #/GC ratio, s.e. 0.226, z-value = −0.918, P = 0.36) suggests that the association is revealed only after perturbation of the system by sleep deprivation.
Discussion
Main Findings
Effective connectivity along the cingulate cortex, a primary structural and functional pathway of the human brain, is strongly modulated by behavioral state and sleep deprivation. We here demonstrate that: (1) effective connectivity is higher in the forward direction, as compared to the backward direction; (2) effective connectivity increases when the eyes are opened only in well-rested subjects and only in the forward direction; (3) sleep deprivation attenuates this eyes-open enhancement of forward effective connectivity; (4) the sleep deprivation-induced attenuation of forward effective connectivity enhancement is not a confound of alpha power modulation; (5) in sleep-deprived participants, individual differences in the ratio of forward connectivity during eyes-open versus eyesclosed periods predict individual differences in vigilance performance.
Dominant Forward Effective Connectivity
The cingulate cortex has been identified as the major backbone of anatomic connectivity of the human brain (Hagmann et al., 2008; van den Heuvel et al., 2008; van den Heuvel and Sporns, 2011). This structural connectivity underlies and reflects strong functional connectivity along the cingulate cortex (Buckner et al., 2009; Honey et al., 2009), as demonstrated in resting-state fMRI studies (Greicius et al., 2003; Fransson and Marrelec, 2008). Using source-level EEG analysis, we here show for the first time that connectivity along the cingulate cortex is in fact asymmetric because Granger Causality (GC) in the forward direction was stronger than GC in the backward direction over all conditions under investigation.
The predominance of a forward effective connectivity has been consistently reported at the scalp level in several EEG studies (Kamiński and Blinowska, 1991; Kamiński et al., 1997; De Gennaro et al., 2004, 2005). Because of its central role in the brain network, the cingulate cortex might constitute one of the major underlying pathways contributing to the forward connectivity observed at the scalp level. In support of this interpretation, sleep deprivation affects both effective connectivity at the scalp level (De Gennaro et al., 2005) and, as here demonstrated, in source space along the cingulate cortex.
Increase in Forward Effective Connectivity during Eyes-Open
Even the simple experimental manipulation of asking participants to keep their eyes open versus closed strongly affects the spontaneous activity and connectivity in neuronal networks, as shown in numerous studies quantifying fMRI fluctuations (Marx et al., 2003, 2004; Yang et al., 2007; McAvoy et al., 2008; Bianciardi et al., 2009), EEG power (Berger, 1929; Barry et al., 2007; Ben-Simon et al., 2008), and the topology of functional EEG networks (Laufs et al., 2003; Stam and de Bruin, 2004; Koenis et al., 2013). Our observations refine these global findings by demonstrating a very specific effect of eye closure on effective connectivity along the cingulate cortex. Forward connectivity was selectively enhanced during the eyes-open period, while backward connectivity was not different between periods of eyes-open and eyes-closed resting state. In particular, this effect was most pronounced for the directed connectivity arising from the posterior cingulate cortex. Only after normal sleep – but not after sleep deprivation – eye opening significantly increased GC from the posterior to the anterior and middle cingulate cortex, without significantly changing GC from middle to anterior cingulate cortex. These findings suggest that forward directed connectivity leaving the posterior cingulate cortex is particularly sensitive to sleep deprivation.
At the scalp level, changes in connectivity in the anterior and posterior directions have been interpreted as reflecting the information flow along forward connections (Kamiński and Blinowska, 1991; De Gennaro et al., 2004, 2005), which subserves a bottom-up transfer of information to prefrontal association areas (Kamiński et al., 1995; De Gennaro et al., 2004). Our study identifies the cingulate cortex as the pathway where effective connectivity is increased specifically during the eyes-open condition, therefore suggesting that this condition triggers an increase in information flow in the forward direction. An alternative interpretation that is equally justified by the Granger Causality technique is that bottom-up transfer of information to more anterior association areas does not change, but rather the responsivity of these areas to incoming information (Barrett et al., 2012).
Lack of Increase in Effective Connectivity after Sleep Deprivation
Sleep deprivation strongly affects functional connectivity, either assessed during resting state (Sämann et al., 2011; De Havas et al., 2012; Koenis et al., 2013) or during task execution (Weissman et al., 2006; Acharya et al., 2010; Kar et al., 2011; van der Helm et al., 2011). We here add to these findings a specific effect of sleep deprivation on effective connectivity from the posterior cingulate to the anterior cingulate areas. The enhancement of effective connectivity elicited by opening of the eyes if subjects were well-rested was selectively suppressed after sleep deprivation. On the other hand, backward GC was not affected by sleep deprivation.
The loss of forward connectivity during eyes-open could reflect impaired communication between brain regions. As described above, decreased connectivity may indicate a degraded output of transmitting regions, poorer responsivity of the target regions, or both. Sleep deprivation has been shown to affect both the sending regions, i.e. brain areas primarily involved in processing sensory input (Corsi-Cabrera et al., 1992; Chee et al., 2008; Kong et al., 2011), and the receiving regions, i.e. areas associated with higher cognitive functioning (Cajochen et al., 1999; Thomas et al., 2000; Drummond et al., 2005; Chee et al., 2008; Lythe et al., 2012). The impairments associated with sleep deprivation observed in the present study and previous studies (Weissman et al., 2006; Chee et al., 2008, 2011; Lima et al., 2010) may result from attenuated effective connectivity, which Granger Causality can aptly quantify.
Our findings on waking connectivity after sleep deprivation complement previous research on changes in connectivity associated with sleep. A predominant backward information flow can be observed during sleep onset (De Gennaro et al., 2004) and is a defining characteristic of the traveling slow oscillations during sleep as well (Massimini et al., 2004; Riedner et al., 2007; Murphy et al., 2009). Sleep deprivation might shift the effective connectivity towards a more sleep-like state: the dominant direction of the cingulate connectivity, which is forward during well-rested wakefulness, becomes increasingly backward, the dominant direction during sleep (De Gennaro et al., 2005).
It is tempting to consider the possibility that the pronounced dominance of backward directionality during sleep might play a functional role in the maintenance of optimal information flow during wakefulness. The slow waves that are characteristic of NREM sleep preferably travel along the cingulate cortex in the backward direction (Kamiński et al., 1997; Massimini et al., 2004; Riedner et al., 2007; Murphy et al., 2009). This indicates that a preferred backward gradient exists with respect to the timing of the neuronal network-wide burst firing that underlies the slow waves. Consequently, within the network of bidirectional neuronal connections in the cingulate (Vogt et al., 1987, 1992), more frontal neurons will on average more frequently enter the burst mode just prior to when more caudal neurons enter the burst mode. Burst mode firing is highly conducive to the induction of changes in synaptic strength (Rosanova and Ulrich, 2005; Axmacher et al., 2006; Poe et al., 2010) and synaptic scaling has indeed been proposed as a primary function of slow oscillations (Tononi and Cirelli, 2006). There is a strong dependence of plasticity on the relative timing of pre- and postsynaptic activity, known as spike timing-dependent plasticity (Bi and Poo, 1998; Song and Abbott, 2001). The phenomenon has also been demonstrated in human tissue, be it with deviations from the original interpretation of a simple Hebbian rule (Testa-Silva et al., 2010; Verhoog and Mansvelder, 2011). It is thus conceivable that the dominantly unidirectional traveling of slow oscillations during slow wave sleep affects the synaptic strength of backward projections systematically differently than it affects the synaptic strength of forward projections.
Along this line of reasoning, we propose that the preferential front-to-back traveling of slow oscillations along the cingulate cortex could result in a directional bias in their modulatory effect on synaptic strength. According to this framework, our findings suggest that slow waves might benefit mostly the forward connectivity pathways. The bias and its proposed underlying mechanism is amenable to testing in neuronal network models (Hill and Tononi, 2005), as well as in experimental protocols employing the selective suppression (Marshall et al., 2006; Raymann and Van Someren, 2008; Landsness et al., 2009; Van Der Werf et al., 2009) of slow waves.
Impairment of Forward Connectivity after Sleep Deprivation Predicts Vigilance
People vary greatly in their sensitivity to sleep deprivation, as measured by its effect on vigilance (Van Dongen et al., 2004, 2012; Chee et al., 2008; Chee and Tan, 2010; Mander et al., 2010; Goel et al., 2011; Lythe et al., 2012). The brain functional mechanisms underlying the trait-like individual differences remain to be elucidated. Given our finding that sleep deprivation attenuated the eyesopen response of forward effective connectivity that was seen if subjects were well-rested, we investigated whether the individual susceptibility to the effect of sleep deprivation on vigilance could be predicted by individual differences in how well the effective connectivity response to eye opening was preserved after sleep deprivation. Our findings indicate that participants that are able to maintain a relatively preserved forward effective connectivity response to eye-opening even when sleep deprived had a better vigilance, indicated by shorter reaction times and fewer lapses. Thus, after sleep deprivation, an individual’s eyes-open increase in effective connectivity represents a predictor of subsequent vigilance.
This significant correlation was observed only after sleep deprivation. It is a common observation that individual differences become relevant, and therefore measureable, only after perturbation of the system, in this case by means of sleep deprivation (Doran et al., 2001; Van Dongen et al., 2004). In fact, the same measure of effective connectivity did not predict the behavioral performance on the vigilance task after normal sleep, when the difference in the forward effective connectivity between the eyes-open period and eyes-closed period was the largest. In fact, this difference might represent a baseline shift in effective connectivity between the well-rested and sleep deprived conditions. While well rested, participants had more than enough reserve capacity to deal with the lengthy vigilance task. However, after sleep deprivation, this reserve capacity was depleted and only those participants who could maintain some degree of forward effective connectivity were able to keep a discrete level of vigilance.
Forward Granger Causality estimates the extent to which anterior regions are responsive to the information coming from posterior areas. Efficient communication between brain areas, especially along the forward axis of the cingulate cortex, is essential for task execution (Hampson et al., 2006; Agam et al., 2011) and detection of near-threshold stimuli (Sadaghiani et al., 2010). Lapsing, i.e. the failure to respond to stimuli, has been attributed to an impairment in the processing of stimuli by prefrontal regions (Chee and Tan, 2010; Chee et al., 2010; Lim et al., 2010). Furthermore, structural differences in white matter connectivity along the cingulate cortex quantified with DTI correlate with performance on error monitoring (Westlye et al., 2009; Cohen, 2011) and executive functions (Kantarci et al., 2011), suggesting a role for the cingulate cortex in the successful transmission of information between posterior and prefrontal cortical areas (Chee et al., 2008). Based on our findings, we propose that optimal performance on the vigilance task requires not only intact structural and functional connectivity of the cingulate cortex, but also unimpaired effective connectivity modulation of eyes-open as compared to eyes-closed in the forward direction.
Conclusions
The predominant direction of Granger Causality along the cingulate cortex during waking resting state is forward. During an eyes-open resting state, forward effective connectivity is significantly stronger than during an eyes-closed resting state. Sleep deprivation causes a selective attenuation of this increase in forward effective connectivity. Individual differences in the capacity to maintain the eyes-open effective connectivity increase even after sleep deprivation predicts subsequent preservation of performance on a sustained vigilance task. Forward effective connectivity thus appears to be an accurate marker of vigilance at an individual subject level. We propose that sleep, and more specifically the slow oscillations that travel over the cingulate in a predominantly backward direction, could be essential for the maintenance of optimal waking effective connectivity.
Highlights.
effective connectivity on the cingulate cortex was calculated from EEG resting state
forward effective connectivity is higher than backward connectivity
eyes-open forward connectivity is higher than during the eyes-closed period
this enhancement disappears and is no longer significant after sleep deprivation
forward connectivity after sleep deprivation correlates with the vigilance level
Acknowledgments
This work was supported by the NWO Cognition Integrated Research Project Grant 051.04.010 and NWO VICI Grant 453.07.001 to E.J.W.V.S., by the NWO VIDI Grant 016.095.359 to Y.D.v.d.W., by the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health under award R21 EB009749 to B.V.V. and B.L.P.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya UR, Chua ECP, Chua KC, Min LC, Tamura T. Analysis and automatic identification of sleep stages using higher order spectra. Int. J. Neural Syst. 2010;20:509–521. doi: 10.1142/S0129065710002589. [DOI] [PubMed] [Google Scholar]
- Agam Y, Hämäläinen MS, Lee AKC, Dyckman KA, Friedman JS, Isom M, Makris N, Manoach DS. Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proc. Natl. Acad. Sci. U.S.A. 2011;108:17556–17561. doi: 10.1073/pnas.1103475108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, Marciani MG, Baccala LA, De Vico Fallani F, Salinari S, Ursino M, Zavaglia M, Ding L, Edgar JC, Miller GA, He B, Babiloni F. Comparison of different cortical connectivity estimators for high-resolution EEG recordings. Hum. Brain Mapp. 2007;28:143–157. doi: 10.1002/hbm.20263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernández G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Res. Rev. 2006;52:170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Babiloni F, Cincotti F, Babiloni C, Carducci F, Mattia D, Astolfi L, Basilisco A, Rossini PM, Ding L, Ni Y, Cheng J, Christine K, Sweeney J, He B. Estimation of the cortical functional connectivity with the multimodal integration of high-resolution EEG and fMRI data by directed transfer function. Neuroimage. 2005;24:118–131. doi: 10.1016/j.neuroimage.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Baccala LA, Sameshima K, Baccalá LA. Partial directed coherence: a new concept in neural structure determination. Biol. Cybern. 2001;84:463–474. doi: 10.1007/PL00007990. [DOI] [PubMed] [Google Scholar]
- Barrett AB, Murphy M, Bruno M-A, Noirhomme Q, Boly M, Laureys S, Seth AK. Granger causality analysis of steady-state electroencephalographic signals during propofolinduced anaesthesia. PLoS ONE. 2012;7:e29072. doi: 10.1371/journal.pone.0029072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007;118:2765–2773. doi: 10.1016/j.clinph.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Bates D, Sarkar D. lme4: Linear mixed-effects models using S4 classes. 2007 [Google Scholar]
- Ben-Simon E, Podlipsky I, Arieli A, Zhdanov A, Hendler T. Never resting brain: simultaneous representation of two alpha related processes in humans. PLoS ONE. 2008;3:e3984. doi: 10.1371/journal.pone.0003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Über das elektrenkephalogramm des menschen. European Archives of Psychiatry and Clinical …. 1929;87:527–570. [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J. Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, Van Gelderen P, Horovitz SG, De Zwart JA, Duyn JH. Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. Neuroimage. 2009;45:160–168. doi: 10.1016/j.neuroimage.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis. 2nd ed. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res. Online. 1999;2:65–69. [PubMed] [Google Scholar]
- Chee MWL, Goh CSF, Namburi P, Parimal S, Seidl KN, Kastner S. Effects of sleep deprivation on cortical activation during directed attention in the absence and presence of visual stimuli. Neuroimage. 2011;58:595–604. doi: 10.1016/j.neuroimage.2011.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Tan JC. Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals. Neuroimage. 2010;51:835–843. doi: 10.1016/j.neuroimage.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Tan JC, Parimal S, Zagorodnov V. Sleep deprivation and its effects on object-selective attention. Neuroimage. 2010;49:1903–1910. doi: 10.1016/j.neuroimage.2009.08.067. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, Dinges DF. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J. Neurosci. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bressler SL, Ding M. Frequency decomposition of conditional Granger causality and application to multivariate neural field potential data. J. Neurosci. Methods. 2006;150:228–237. doi: 10.1016/j.jneumeth.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Cheung BLP, Nowak R, Lee HC, Van Drongelen W, Van Veen BD. Cross validation for selection of cortical interaction models from scalp EEG or MEG. IEEE Trans. Biomed. Eng. 2012;59:504–514. doi: 10.1109/TBME.2011.2174991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BLP, Riedner BA, Tononi G, Van Veen BD. Estimation of cortical connectivity from EEG using state-space models. IEEE Trans. Biomed. Eng. 2010;57:2122–2134. doi: 10.1109/TBME.2010.2050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo W-C, Lee W-W, Venkatraman V, Sheu FS, Chee MWL. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005;25:579–587. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Error-related medial frontal theta activity predicts cingulate-related structural connectivity. Neuroimage. 2011;55:1373–1383. doi: 10.1016/j.neuroimage.2010.12.072. [DOI] [PubMed] [Google Scholar]
- Corsi-Cabrera M, Ramos J, Arce C, Guevara MA, Ponce-de León M, Lorenzo I, Leon MP. Changes in the waking EEG as a consequence of sleep and sleep deprivation. Sleep. 1992;15:550–555. doi: 10.1093/sleep/15.6.550. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, Depaulis A. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 2008;6:2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Vecchio F, Ferrara M, Curcio G, Rossini PM, Babiloni C. Changes in fronto-posterior functional coupling at sleep onset in humans. J. Sleep Res. 2004;13:209–217. doi: 10.1111/j.1365-2869.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Vecchio F, Ferrara M, Curcio G, Rossini PM, Babiloni C, De Gennaro L, Maria P. Antero-posterior functional coupling at sleep onset: changes as a function of increased sleep pressure. Brain Res. Bull. 2005;65:133–140. doi: 10.1016/j.brainresbull.2004.12.004. [DOI] [PubMed] [Google Scholar]
- De Havas JA, Parimal S, Soon CS, Chee MWL. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage. 2012;59:1745–1751. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- De Vico Fallani F, Astolfi L, Cincotti F, Mattia D, Marciani MG, Salinari S, Kurths J, Gao S, Cichocki A, Colosimo A, Babiloni F. Cortical functional connectivity networks in normal and spinal cord injured patients: Evaluation by graph analysis. Hum. Brain Mapp. 2007;28:1334–1346. doi: 10.1002/hbm.20353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vico Fallani F, Nicosia V, Sinatra R, Astolfi L, Cincotti F, Mattia D, Wilke C, Doud A, Latora V, He B, Babiloni F. Defecting or not defecting: how to “read” human behavior during cooperative games by EEG measurements. PLoS ONE. 2010;5:e14187. doi: 10.1371/journal.pone.0014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Corbetta M. The dynamical balance of the brain at rest. Neuroscientist. 2011;17:107–123. doi: 10.1177/1073858409354384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa VK, McIntosh AR. Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Ding L, Worrell GA, Lagerlund TD, He B. Ictal source analysis: localization and imaging of causal interactions in humans. Neuroimage. 2007;34:575–586. doi: 10.1016/j.neuroimage.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges D. An overview of sleepiness and accidents. J. Sleep Res. 1995;4:4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch. Ital. Biol. 2001;139:253–267. [PubMed] [Google Scholar]
- Drummond SPA, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- Ferreira C, Deslandes A, Moraes H, Cagy M, Pompeu F, Basile LF, Piedade R, Ribeiro P. Electroencephalographic changes after one night of sleep deprivation. Arq. Neuropsiquiatr. 2006;64:388–393. doi: 10.1590/s0004-282x2006000300007. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Hum. Brain Mapp. 1994;2:56–78. [Google Scholar]
- Galka A, Yamashita O, Ozaki T, Biscay R, Valdés-Sosa PA. A solution to the dynamical inverse problem of EEG generation using spatiotemporal Kalman filtering. Neuroimage. 2004;23:435–453. doi: 10.1016/j.neuroimage.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Geweke J. Measurement of Linear Dependence and Feedback Between Multiple Time Series. J. Am. Stat. Assoc. 1982;77:304. [Google Scholar]
- Goel N, Banks S, Lin L, Mignot E, Dinges DF. Catechol-O-methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS ONE. 2011;6:e29283. doi: 10.1371/journal.pone.0029283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Herrero G, Atienza M, Egiazarian K, Cantero JL. Measuring directional coupling between EEG sources. Neuroimage. 2008;43:497–508. doi: 10.1016/j.neuroimage.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Granger CWJ. Investigating Causal Relations by Econometric Models and Cross-spectral Methods. Econometrica. 1969;37:424. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo S-S, Hu P, Walker MP. The unrested resting brain: sleep deprivation alters activity within the default-mode network. J. Cogn. Neurosci. 2010;22:1637–1648. doi: 10.1162/jocn.2009.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J. Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J. Neurophysiol. 2005;93:1671–1698. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui HB, Pantazis D, Bressler SL, Leahy RM. Identifying true cortical interactions in MEG using the nulling beamformer. Neuroimage. 2010;49:3161–3174. doi: 10.1016/j.neuroimage.2009.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Kamiński M, Blinowska K. A new method of the description of the information flow in the brain structures. Biol. Cybern. 1991;65:203–210. doi: 10.1007/BF00198091. [DOI] [PubMed] [Google Scholar]
- Kamiński M, Blinowska K, Szclenberger W. Topographic analysis of coherence and propagation of EEG activity during sleep and wakefulness. Electroencephalogr. Clin. Neurophysiol. 1997;102:216–227. doi: 10.1016/s0013-4694(96)95721-5. [DOI] [PubMed] [Google Scholar]
- Kamiński M, Blinowska K, Szelenberger W. Investigation of coherence structure and EEG activity propagation during sleep. Acta Neurobiol. Exp (Wars) 1995;55:213–219. doi: 10.55782/ane-1995-1078. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, Przybelski SA, Edmonson HA, Vemuri P, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Petersen RC, Jack CRJ. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77:26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar S, Routray A, Nayak BP. Functional network changes associated with sleep deprivation and fatigue during simulated driving: validation using blood biomarkers. Clin. Neurophysiol. 2011;122:966–974. doi: 10.1016/j.clinph.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Koenis MMG, Romeijn N, Piantoni G, Verweij I, Van der Werf YD, Van Someren EJW, Stam CJ. Does sleep restore the topology of functional brain networks? Hum. Brain Mapp. 2013;523:167–170. doi: 10.1002/hbm.21455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Soon CS, Chee MWL. Reduced visual processing capacity in sleep deprived persons. Neuroimage. 2011;55:629–634. doi: 10.1016/j.neuroimage.2010.12.057. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Landsness EC, Crupi D, Hulse BK, Peterson MJ, Huber R, Ansari H, Coen M, Cirelli C, Benca RM, Ghilardi MF, Tononi G. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32:1273–1284. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann. N. Y. Acad. Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 2010;136:375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Tan JC, Parimal S, Dinges DF, Chee MWL. Sleep deprivation impairs object-selective attention: a view from the ventral visual cortex. PLoS ONE. 2010;5:e9087. doi: 10.1371/journal.pone.0009087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B, Singer W, Chen NH, Neuenschwander S. Synchronization dynamics in response to plaid stimuli in monkey V1. Cereb. Cortex. 2010;20:1556–1573. doi: 10.1093/cercor/bhp218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpiti T, Van Veen BD, Wakai RT. Cortical patch basis model for spatially extended neural activity. IEEE Trans. Biomed. Eng. 2006;53:1740–1754. doi: 10.1109/TBME.2006.873743. [DOI] [PubMed] [Google Scholar]
- Litvak V, Mattout J, Kiebel S, Phillips C, Henson R, Kilner J, Barnes G, Oostenveld R, Daunizeau J, Flandin G, Penny W, Friston K. EEG and MEG data analysis in SPM8. Comput. Intell. Neurosci. 2011;2011:852961. doi: 10.1155/2011/852961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lythe KE, Williams SCR, Anderson C, Libri V, Mehta MA. Frontal and parietal activity after sleep deprivation is dependent on task difficulty and can be predicted by the fMRI response after normal sleep. Behav. Brain Res. 2012;233:62–70. doi: 10.1016/j.bbr.2012.04.050. [DOI] [PubMed] [Google Scholar]
- Malekpour S, Li Z, Cheung BLP, Castillo EM, Papanicolaou AC, Kramer LA, Fletcher JM, Van Veen BD. Interhemispheric effective and functional cortical connectivity signatures of spina bifida are consistent with callosal anomaly. Brain connectivity. 2012;2:142–154. doi: 10.1089/brain.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Reid KJ, Baron KG, Tjoa T, Parrish TB, Paller KA, Gitelman DR, Zee PC. EEG measures index neural and cognitive recovery from sleep deprivation. J. Neurosci. 2010;30:2686–2693. doi: 10.1523/JNEUROSCI.4010-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Marx E, Deutschländer A, Stephan T, Dieterich M, Wiesmann M, Brandt T. Eyes open and eyes closed as rest conditions: impact on brain activation patterns. Neuroimage. 2004;21:1818–1824. doi: 10.1016/j.neuroimage.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Marx E, Stephan T, Nolte A, Deutschländer A, Seelos KC, Dieterich M, Brandt T. Eye closure in darkness animates sensory systems. Neuroimage. 2003;19:924–934. doi: 10.1016/s1053-8119(03)00150-2. [DOI] [PubMed] [Google Scholar]
- Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J. Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy M, Larson-Prior L, Nolan TS, Vaishnavi SN, Raichle ME, d’Avossa G. Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. J. Neurophysiol. 2008;100:922–931. doi: 10.1152/jn.90426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin. Neurophysiol. 2006;117:1885–1901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp T, Van Oosterom A. The potential distribution generated by surface electrodes in inhomogeneous volume conductors of arbitrary shape. IEEE Trans. Biomed. Eng. 1991;38:409–417. doi: 10.1109/10.81559. [DOI] [PubMed] [Google Scholar]
- Philibert I. Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Sleep. 2005;28:1392–1402. doi: 10.1093/sleep/28.11.1392. [DOI] [PubMed] [Google Scholar]
- Piantoni G, Poil S-S, Linkenkaer-Hansen K, Verweij IM, Ramautar JR, Van Someren EJW, Van Der Werf YD. Individual differences in white matter diffusion affect sleep oscillations. J. Neurosci. 2013;33:227–233. doi: 10.1523/JNEUROSCI.2030-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed Effects Models in S and S-Plus. New York, NY: Springer; 2000. [Google Scholar]
- Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog. Brain Res. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Auditory processing across the sleep-wake cycle: simultaneous EEG and fMRI monitoring in humans. Neuron. 2000;28:991–999. doi: 10.1016/s0896-6273(00)00169-0. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Austria: Vienna; 2010. [Google Scholar]
- Raymann RJEM, Van Someren EJW. Diminished capability to recognize the optimal temperature for sleep initiation may contribute to poor sleep in elderly people. Sleep. 2008;31:1301–1309. [PMC free article] [PubMed] [Google Scholar]
- Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, Tononi G. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–1657. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklage M, Williams V, Pacheco J, Schnyer DM. White matter differences predict cognitive vulnerability to sleep deprivation. Sleep. 2009;32:1100–1103. doi: 10.1093/sleep/32.8.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeijn N, Van Someren EJW. Correlated fluctuations of daytime skin temperature and vigilance. J. Biol. Rhythms. 2011;26:68–77. doi: 10.1177/0748730410391894. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J. Neurosci. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Scheeringa R, Lehongre K, Morillon B, Giraud A-L, Kleinschmidt A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J. Neurosci. 2010;30:10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sämann PG, Wehrle R, Hoehn D, Spoormaker VI, Peters H, Tully C, Holsboer F, Czisch M. Development of the brain’s default mode network from wakefulness to slow wave sleep. Cereb. Cortex. 2011;21:2082–2093. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW. Network modelling methods for FMRI. Neuroimage. 2011;54:875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000;48:555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Song S, Abbott LF. Cortical development and remapping through spike timing-dependent plasticity. Neuron. 2001;32:339–350. doi: 10.1016/s0896-6273(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Stam CJ, De Bruin EA. Scale-free dynamics of global functional connectivity in the human brain. Hum. Brain Mapp. 2004;22:97–109. doi: 10.1002/hbm.20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supp GG, Schlögl A, Trujillo-Barreto N, Müller MM, Gruber T. Directed cortical information flow during human object recognition: analyzing induced EEG gamma-band responses in brain’s source space. PLoS ONE. 2007;2:e684. doi: 10.1371/journal.pone.0000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa-Silva G, Verhoog MB, Goriounova NA, Loebel A, Hjorth J, Baayen JC, De Kock CPJ, Mansvelder HD. Human synapses show a wide temporal window for spike-timingdependent plasticity. Front. Synaptic Neurosci. 2010;2:12. doi: 10.3389/fnsyn.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J. Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang RL, Telang F, Boronikolas V, Jayne MC, Wang G-J, Fowler JS, Volkow ND. Impairment of attentional networks after 1 night of sleep deprivation. Cereb. Cortex. 2009;19:233–240. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med. Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Valdés-Sosa PA, Roebroeck A, Daunizeau J, Friston K. Effective connectivity: influence, causality and biophysical modeling. Neuroimage. 2011;58:339–361. doi: 10.1016/j.neuroimage.2011.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MP, Mandl R, Luigjes J, Hulshoff Pol H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J. Neurosci. 2008;28:10844–10851. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J. Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Helm E, Gujar N, Nishida M, Walker MP. Sleep-dependent facilitation of episodic memory details. PLoS ONE. 2011;6:e27421. doi: 10.1371/journal.pone.0027421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De Rijke W, Van Someren EJW. Sleep benefits subsequent hippocampal functioning. Nat. Neurosci. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- Van Dongen HPA, Bender AM, Dinges DF. Systematic individual differences in sleep homeostatic and circadian rhythm contributions to neurobehavioral impairment during sleep deprivation. Accid. Anal. Prev. 2012;(45 Suppl):11–16. doi: 10.1016/j.aap.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoog MB, Mansvelder HD. Presynaptic ionotropic receptors controlling and modulating the rules for spike timing-dependent plasticity. Neural Plast. 2011;2011:870763. doi: 10.1155/2011/870763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb. Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J. Comp. Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Bjørnerud A, Due-Tønnessen P, Fjell AM. Error-related negativity is mediated by fractional anisotropy in the posterior cingulate gyrus--a study combining diffusion tensor imaging and electrophysiology in healthy adults. Cereb. Cortex. 2009;19:293–304. doi: 10.1093/cercor/bhn084. [DOI] [PubMed] [Google Scholar]
- Yamashita O, Galka A, Ozaki T, Biscay R, Valdés-Sosa PA. Recursive penalized least squares solution for dynamical inverse problems of EEG generation. Hum. Brain Mapp. 2004;21:221–235. doi: 10.1002/hbm.20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Long X-Y, Yang Y, Yan H, Zhu C-Z, Zhou X-P, Zang Y-F, Gong Q-Y. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]