Abstract
Ubiquitin mediated degradation of cyclin D1 following the G1/S transition counters its mitogen-dependent accumulation during G1 phase of the cell cycle. Although the cellular machinery responsible for this process has been identified, how this regulatory pathway interfaces to cellular stress responses, often referred to as checkpoints, remains to be established. One intensely investigated checkpoint is the cellular response to DNA damage. When DNA damage is sensed, the corresponding DNA damage checkpoint triggers the inhibition of CDK-dependent cell cycle progression, with arrest coordinated by induction of CDK inhibitors and rapid degradation of specific cyclins, such as cyclin D1. In recent work, we identified a phosphorylation- and Fbx4-dependent cyclin D1 degradation mechanism in response to genotoxic stress.18 This work revealed that loss of cyclin D1 regulation compromises the intra-Sphase response to DNA damage, promoting genomic instability and sensitization of cells to S-phase chemotherapy, highlighting a potential therapeutic strategy for cancers exhibiting cyclin D1 accumulation.
Keywords: Cyclin D1, phosphorylation, ATM, CDK4, DNA damage, intra-S-phase checkpoint, SCFFbx4-αBCrystallin, GSK3β
Introduction
Cyclin D1 expression requires Ras-dependent activation of MAP kinase pathways in various cell types.1 Because cyclin D1 is a labile protein, accumulation of threshold levels also depends on decreased ubiquitin-dependent proteolysis, mediated by phosphatidylinositol 3-kinase and AKT-dependent inactivation of GSK3β, the kinase that marks cyclin D1 for proteolytic destruction.2–4 Upon association with its catalytic partner, CDK4, the active cyclin D1/CDK4 kinase phosphorylates the retinoblastoma protein (Rb) (and related family proteins, p107 and p130). The resultant hyper-phosphorylated and inactive Rb no longer represses E2F-dependent activation of downstream targets and thus permits a gene expression program necessary for S-phase entry and progression.5–9 Cyclin D1/CDK4 also facilitates S-phase entry via stoichiometric titration of the CDK inhibitors p21Cip1 and p27Kip1, thereby facilitating maximal activation of cyclin E/CDK2.9, 10
Following the G1/S transition, cyclin D1 accumulation is antagonized by regulated proteolysis, consisting of GSK3β-dependent Thr-286 phosphorylation, nuclear export, and recognition by the SCFFbx4-αBcrystallin E3 ubiquitin ligase within the cytoplasm.4, 11–13 Recent work demonstrated that Fbx4 is inactivated through somatic mutations in primary cancers, revealing that cyclin D1 overexpression in human cancer can occur as a consequence of deregulated proteolysis.14–16 Because most cancer therapies trigger cell cycle arrest and cell death as a consequence of DNA damage and increased cyclin D1 is an early target of DNA damage-mediated proteolysis,17 disruption of the latter response could have a significant influence on cellular response and therapeutic outcome. To address this possibility, in recent work, we have evaluated the role for Thr-286 phosphorylation and SCFFbx4-αBcrystallin E3 ligase in DNA damage dependent regulation of cyclin D1.18 Here, we discuss the role of Fbx4-dependent cyclin D1 loss in maintaining genome integrity and potential therapeutic strategies for treatment of cancers overexpressing cyclin D1.
Phosphorylation-dependent cyclin D1 proteolysis following genotoxic stress
GSK3β-dependent phosphorylation on Thr-286 is essential for the increased proteolysis of cyclin D1 during S-phase and for maintenance of homeostatic levels of cyclin D1.4 Previous work suggested that this phosphorylation event may be superfluous for DNA damage mediated cyclin D1 proteolysis.17 However, we found that non-phosphorylatable D1T286A is not degraded in response to DNA damage, suggesting that phosphorylation of Thr-286 is essential for the rapid destruction of cyclin D1 following DNA damage.18 Several kinases have been implicated in the regulation of cyclin D1 Thr-286 phosphorylation during normal cell cycle progression and various stress conditions, including I kappa B (IκB) kinase alpha (IKKα) and p38SAPK.19, 20 Strikingly, manipulation of these potential kinases had no effect on cyclin D1 phosphorylation in response to DNA damage. In contrast, DNA damage was found to result in a rapid increase in GSK3β activity. Furthermore, inhibition of GSK3β via shRNA, small molecule inhibitors or dominant negative alleles all effectively inhibited cyclin D1 phosphorylation, highlighting the significance of this kinase for proper control of cyclin D1 accumulation.18 While the precise mechanisms that contribute to GSK3β activation remain to be elucidated, a framework for this regulation has been laid out as will be discussed later.
SCFFbx4-αB crystallin regulates accelerated cyclin D1 degradation
The cyclin D1 E3 ubiquitin ligase is a cytoplasmic SCF complex consisting of Skp1, Cul1, F-box protein Fbx4, along with the cofactor αB crystallin.12 The pre-requisite of cyclin D1 phosphorylation for its proteolysis in response to DNA damage suggested a role for the SCFFbx4-αB crystallin ligase. The necessity for both Fbx4 and αB crystallin were assessed through use of cell lines wherein expression of either was reduced through shRNA vectors, or through use of cancer-derived cell lines known to be deficient in one of these two molecules. As would be expected if SCFFbx4-αB crystallin is a requisite regulator of cyclin D1 proteolysis, loss of either Fbx4 or αB crystallin attenuated DNA damage-mediated cyclin D1 destruction. Of significance, cyclin D1 regulation could be restored in αB crystallin-deficient cell lines following reintroduction of αB crystallin, demonstrating that the remaining components of this pathway are intact.18 It was also noted that other G1/S cyclins, including cyclin D2, cyclin E, and cyclin A, were not subject to accelerated proteolysis, highlighting the specific modulation of the cyclin D1 degradation machinery in this DNA damage response.18
These findings support a model wherein cyclin D1 degradation occurs predominantly in an Fbx4-dependent manner, contrary to the previous model of phosphorylation-independent cyclin D1 regulation by the anaphase promoting complex/cyclosome (APC/C).17 What accounts for these differences? Significantly, work describing APC/C-dependent cyclin D1 regulation was performed in MCF-7 cells,17 a cell line deficient for αB crystallin and thus, SCFFbx4-αB crystallin ligase function.12 This would imply that this cell line utilizes alternative degradation pathways to attenuate cyclin D1 protein expression. Consistent with this concept, phosphorylation-deficient cyclin D1 mutants are degraded, albeit with dramatically extended kinetics, suggesting that secondary mechanisms contribute to cyclin D1 regulation. Alternatively, our recent work has revealed that Fbx4 dimerization is regulated by GSK3β-dependent phosphorylation, triggering an increase in ligase activity as cells enter and progress through S-phase.16 Because previous work focused on cyclin D1 proteolysis during G1 phase, when GSK3β is inactive, the reduced activity of the Fbx4-dependent ligase in this phase of the cell cycle could contribute to alternative mechanisms of cyclin D1 proteolysis.
In addition to Fbx4, Fbw8/Cul7 complexes have been implicated in the regulation of cyclin D1 poly-ubiquitylation.21 Because Cul7 expression is undetectable in NIH3T3 cells,22 one of the primary experimental cell lines utilized in our recent work, it is unlikely that Fbw8/Cul7-dependent ligase contributes cyclin D1 regulation following DNA damage. The finding that Fbx4 or αB crystallin loss in tumor derived cells or via knockdown dramatically impairs cyclin D1 degradation following genotoxic stress strongly supports a model for Fbx4-dependent degradation as the primary mechanism for accelerated cyclin D1 loss.18
An unresolved question then is whether the SCFFbx4-αB crystallin ligase is itself regulated by DNA damage. GSK3β catalyzes both cyclin D1 phosphorylation, marking its degron, and Fbx4 phosphorylation, triggering its dimerization and activation.4, 16, 18 Are these events linked following DNA damage? Our data suggest that cyclin D1 phosphorylation is the critical event for degradation, as phosphorylation-deficient mutants never reach the cytoplasm and nuclear exportdeficient mutants accumulate as phospho-proteins in the nucleus.18 However, the possibility of both increased cyclin D1 phosphorylation and increased Fbx4-dependent ligase activity contributing to rapid kinetics of cyclin D1 loss is an interesting question and will be addressed in future work.
Degradation of cyclin D1 is critical for intra-S-phase checkpoint integrity
The rapid GSK3β- and Fbx4-dependent destruction of cyclin D1 suggests that the canonical cyclin D1 proteolysis pathway is regulated by DNA damage signalling effectors. Significantly, bona fide DSB induction is required to initiate this process. Replication stress associated with single strand DNA (ssDNA) accumulation and subsequent ATM and Rad3-related (ATR) kinase activation has been suggested to induce cyclin D1 phosphorylation and degradation during S-phase.23 In contrast to this work, we found that DSB-inducing treatments including HU, γIR, and camptothecin (CPT) induced rapid cyclin D1 phosphorylation and degradation downstream of Ataxia Telangiectasia-mutated (ATM) activation, whereas activation of ATR via aphidicolin treatment was not sufficient to trigger cyclin D1 loss.18 Further analysis of the ATM versus ATR pathways contributing to accelerated cyclin D1 proteolysis revealed that ATM inhibition or ablation (ATM-null murine embryonic fibroblast cell lines) attenuates cyclin D1 phosphorylation and degradation; however, conditional deletion of ATR (ATRflox/-MEFs) or shRNA-mediated knockdown had no effect on the cyclin D1 degradation pathway following DNA damage.18
While ATM signalling is required for genotoxic stress-induced cyclin D1 phosphorylation, the precise molecular underpinnings that connect ATM and cyclin D1 remain to be established. Several different models of ATM-dependent cyclin D1 phosphorylation can be considered. First, activation of ATM and its downstream effectors, such as Chk2, could directly activate GSK3β.24 Consistent with this notion, an increase in GSK3β kinase activity following DNA damage was noted.18 Second, ATM-dependent regulation of GSK3β interacting proteins could bring the kinase in proximity to cyclin D1 in the nucleus, increasing the efficiency of phosphorylation. Finally, ATM-dependent modification of a cyclin D1-interacting protein could induce a conformational change, making cyclin D1 a better GSK3β substrate. Our initial work to parse out this ATM-dependent signalling pathway reveal that Chk2, an ATM-effector kinase implicated in cell cycle arrest,25 only modestly stabilizes cyclin D1 at early time points following DNA damage,18 suggesting that ATM itself or alternative downstream effector(s) regulate this DNA damage response. Consequently, further characterizing the connection between ATM and cyclin D1 regulation is an exciting question and a current area of investigation.
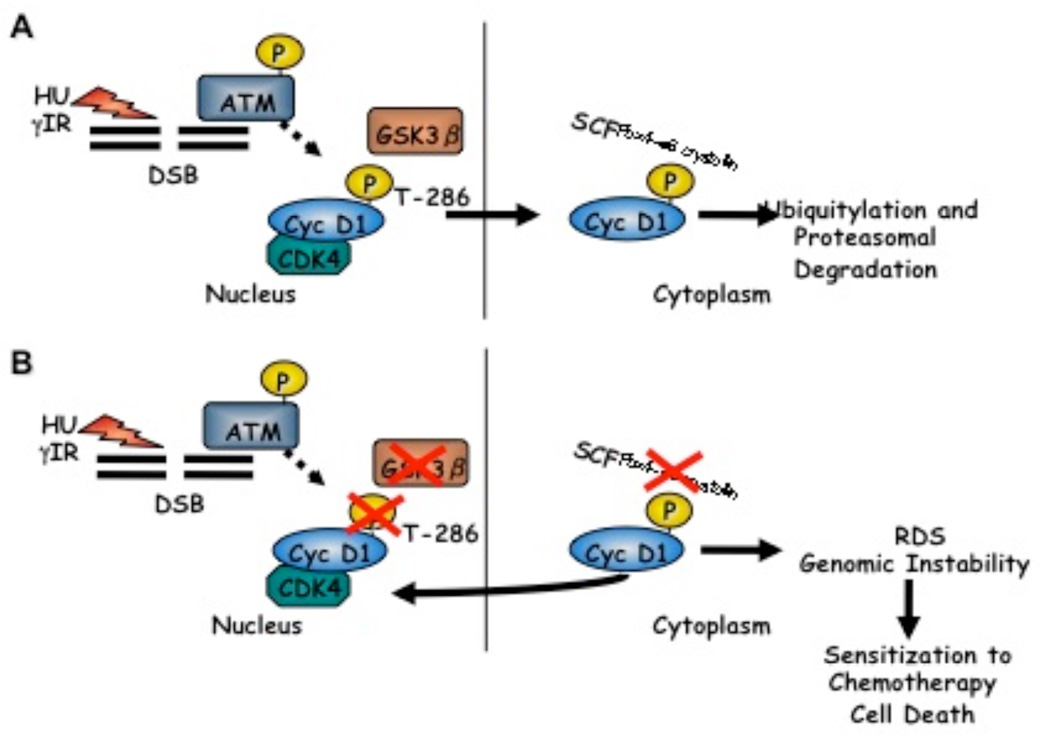
Consistent with accelerated cyclin D1 proteolysis downstream of ATM activation18 and previous work establishing that nuclear accumulation of cyclin D1 could perturb normal S-phase progression,26 expression of phosphorylation-deficient cyclin D1 mutants or inactivation of Fbx4 promotes a radio-resistant DNA synthesis (RDS) phenotype associated with Cdt1 stabilization and maintenance of MCM proteins on chromatin.18 These recent findings support a model wherein DSB-dependent ATM activation triggers a rapid cell cycle arrest via Fbx4-dependent degradation of cyclin D1 (Figure 1A). In contrast, tumors wherein Fbx4 mediated control of cyclin D1 is lost are subject to DNA replication in the face of DNA damage, an event that will potentiate loss of genomic integrity. While such genomic instability can drive genetic alterations that could provide a growth advantage to proliferating cells, severe DNA damage can invoke cell death through apoptosis or mitotic failure. Significantly, our recent work demonstrated that nuclear accumulation of cyclin D1 sensitizes cells to the S-phase-specific chemotherapeutic agent CPT.18 Ultimately, these findings imply that loss of Fbx4 control compromises the cellular checkpoint response to DNA-damaging chemotherapy, thereby driving substantial genomic instability and cell death (Figure 1B).
Figure 1. Cyclin D1 regulation following genotoxic stress is required to maintain genome integrity.
A) Genotoxic stress induces GSK3β-dependent cyclin D1 Thr-286 phosphorylation downstream of ATM activation. Phosphorylated cyclin D1 is exported from the nucleus, and polyubiquitylated by the SCF-Fbx4 E3 ligase, triggering proteasomal degradation. B) Mutations impairing cyclin D1 Thr-286 phosphorylation, nuclear export, and Fbx4-dependent ligase function result in nuclear accumulation of cyclin D1 and loss of intra-S-phase checkpoint integrity, ultimately sensitizing cells to treatment with S-phase DNA damaging chemotherapy.
Considering these findings, could cyclin D1 overexpression or inactivating mutations in Fbx4 serve as biomarkers for cancer therapy? Recent identification of Fbx4 mutations rendering 9 impaired ligase activity and cyclin D1 overexpression in primary esophageal tumors highlights the importance for such development.16 Additional work assessing sensitivity of tumor cells exhibiting deregulated cyclin D1 proteolysis to DNA damaging chemotherapy including CPT, cisplatin, and other DSB-inducing agents in vitro and in animal models is required to validate the therapeutic value of such treatments.
Concluding Remarks
Fbx4-dependent cyclin D1 degradation following genotoxic stress is a critical process to maintain cellular integrity. Our recent experiments demonstrate that loss of cyclin D1 post-translational control compromises the intra-S-phase checkpoint response to DNA damage. Significantly, loss of Fbx4-dependent cyclin D1 regulation sensitizes cells to DSB-inducing chemotherapy in vitro, providing insight into a potential avenue for treatment of tumors exhibiting stabilized cyclin D1. Future studies in vitro and in vivo evaluating the cellular response to chemotherapy in the presence or absence of cyclin D1 accumulation are necessary to determine whether such treatment regimens will be effective in human cancer.
Acknowledgements
This work was supported by NIH CA93237: JAD is a Leukemia Lymphoma Society Scholar.
References
- 1.Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 2.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase 3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 3.Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 4.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatakeyama M, Brill JA, Fink GR, Weinberg RA. Collaboration of G1 cyclins in the functional inactivation of the retinoblastoma protein. Genes Dev. 1994;8:1759–1771. doi: 10.1101/gad.8.15.1759. [DOI] [PubMed] [Google Scholar]
- 6.Harbour JW, Luo RX, Dei Santi A, Postigo AA, Dean DC. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 7.Calbo J, Parreno M, Sotillo E, Yong T, Mazo A, Garriga J, et al. G1 cyclin/cyclin-dependent kinase-coordinated phosphorylation of endogenous pocket proteins differentially regulates their interactions with E2F4 and E2F1 and gene expression. J Biol Chem. 2002;277:50263–50274. doi: 10.1074/jbc.M209181200. [DOI] [PubMed] [Google Scholar]
- 8.Leng X, Noble M, Adams PD, Qin J, Harper JW. Reversal of growth suppression by p107 via direct phosphorylation by cyclin D1/cyclin-dependent kinase 4. Mol Cell Biol. 2002;22:2242–2254. doi: 10.1128/MCB.22.7.2242-2254.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 10.Cheng M, Sexl V, Sherr CJ, Roussel MF. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1) Proc Natl Acad Sci U S A. 1998;95:1091–1096. doi: 10.1073/pnas.95.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alt JR, Cleveland JL, Hannink M, Diehl JA. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 2000;14:3102–3114. doi: 10.1101/gad.854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin DI, Barbash O, Kumar KG, Weber JD, Harper JW, Klein-Szanto AJ, et al. Phosphorylation-dependent ubiquitination of cyclin D1 by the SCF(FBX4-alphaB crystallin) complex. Mol Cell. 2006;24:355–366. doi: 10.1016/j.molcel.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diehl JA, Zindy F, Sherr CJ. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 14.Benzeno S, Lu F, Guo M, Barbash O, Zhang F, Herman JG, et al. Identification of mutations that disrupt phosphorylation-dependent nuclear export of cyclin D1. Oncogene. 2006;25:6291–6303. doi: 10.1038/sj.onc.1209644. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Bueno G, Rodriguez-Perales S, Sanchez-Estevez C, Hardisson D, Sarrio D, Prat J, et al. Cyclin D1 gene (CCND1) mutations in endometrial cancer. Oncogene. 2003;22:6115–6118. doi: 10.1038/sj.onc.1206868. [DOI] [PubMed] [Google Scholar]
- 16.Barbash O, Zamfirova P, Lin DI, Chen X, Yang K, Nakagawa H, et al. Mutations in Fbx4 inhibit dimerization of the SCF(Fbx4) ligase and contribute to cyclin D1 overexpression in human cancer. Cancer Cell. 2008;14:68–78. doi: 10.1016/j.ccr.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agami R, Bernards R. Distinct initiation and maintenance mechanisms cooperate to induce G1 cell cycle arrest in response to DNA damage. Cell. 2000;102:55–66. doi: 10.1016/s0092-8674(00)00010-6. [DOI] [PubMed] [Google Scholar]
- 18.Pontano LL, Aggarwal P, Barbash O, Brown EJ, Bassing CH, Diehl JA. Genotoxic stress-induced cyclin D1 phosphorylation and proteolysis are required for genomic stability. Mol Cell Biol. 2008;28:7245–7258. doi: 10.1128/MCB.01085-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwak YT, Li R, Becerra CR, Tripathy D, Frenkel EP, Verma UN. IkappaB kinase alpha regulates subcellular distribution and turnover of cyclin D1 by phosphorylation. J Biol Chem. 2005;280:33945–33952. doi: 10.1074/jbc.M506206200. [DOI] [PubMed] [Google Scholar]
- 20.Casanovas O, Miro F, Estanyol JM, Itarte E, Agell N, Bachs O. Osmotic stress regulates the stability of cyclin D1 in a p38SAPK2-dependent manner. J Biol Chem. 2000;275:35091–35097. doi: 10.1074/jbc.M006324200. [DOI] [PubMed] [Google Scholar]
- 21.Okabe H, Lee SH, Phuchareon J, Albertson DG, McCormick F, Tetsu O. A critical role for FBXW8 and MAPK in cyclin D1 degradation and cancer cell proliferation. PLoS ONE. 2006;1:e128. doi: 10.1371/journal.pone.0000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasper JS, Kuwabara H, Arai T, Ali SH, DeCaprio JA. Simian virus 40 large T antigen's association with the CUL7 SCF complex contributes to cellular transformation. J Virol. 2005;79:11685–11692. doi: 10.1128/JVI.79.18.11685-11692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hitomi M, Yang K, Stacey AW, Stacey DW. Phosphorylation of cyclin D1 regulated by ATM or ATR controls cell cycle progression. Mol Cell Biol. 2008;28:5478–5493. doi: 10.1128/MCB.02047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JY, Yu SJ, Park YG, Kim J, Sohn J. Glycogen synthase kinase 3beta phosphorylates p21WAF1/CIP1 for proteasomal degradation after UV irradiation. Mol Cell Biol. 2007;27:3187–3198. doi: 10.1128/MCB.01461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal P, Lessie MD, Lin DI, Pontano L, Gladden AB, Nuskey B, et al. Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev. 2007;21:2908–2922. doi: 10.1101/gad.1586007. [DOI] [PMC free article] [PubMed] [Google Scholar]