Abstract
Clinical and preclinical studies provide strong evidence that nonsteroidal anti-inflammatory drugs (NSAIDs) can prevent numerous types of cancers, especially colorectal cancer. Unfortunately, the depletion of physiologically important prostaglandins due to cyclooxygenase (COX) inhibition results in potentially fatal toxicities that preclude the long-term use of NSAIDs for cancer chemoprevention. While studies have shown an involvement of COX-2 in colorectal tumorigenesis, other studies suggest that a COX-independent target may be at least partially responsible for the antineoplastic activity of NSAIDs. For example, certain NSAID derivatives have been identified that do not inhibit COX-2 but have demonstrated efficacy to suppress carcinogenesis with potential for reduced toxicity. A number of alternative targets have also been reported to account for the tumor cell growth inhibitory activity of NSAIDs, including the inhibition of cyclic guanosine monophosphate phosphodiesterases (cGMP PDEs), generation of reactive oxygen species (ROS), the suppression of the apoptosis inhibitor protein, survivin, and others. Here, we review several promising mechanisms that are being targeted to develop safer and more efficacious NSAID derivatives for colon cancer chemoprevention.
1 Introduction
Previous studies have demonstrated strong evidence that nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase-2 (COX-2) selective inhibitors have cancer chemopreventive activity against a number of cancer types, particularly colorectal cancer. For example, epidemiologic studies of the general population have shown that long-term use of NSAIDs, most notably aspirin, is associated with a significant reduction of risk from death by colorectal cancer (Chan 2002; Thun et al. 2002). Consistent with these observations, clinical studies have shown the ability of certain prescription strength NSAIDs (e.g. sulindac) to reduce the occurrence and cause the regression of precancerous adenomas in patients with familial adenomatous polyposis (Giardiello et al. 1993; Steinbach et al. 2000). A wealth of observations from preclinical studies supports these observations by showing the ability of aspirin and various non-aspirin NSAIDs to inhibit tumor formation in rodent models of chemically induced carcinogenesis.
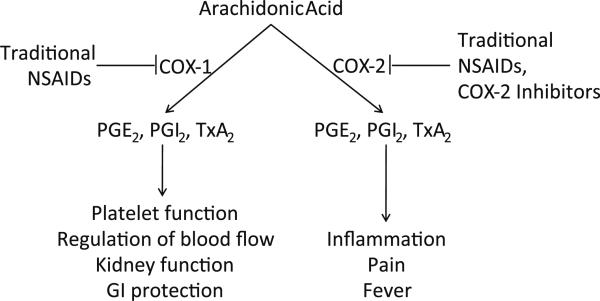
The NSAIDs, the most common of which are listed in Table 1, are a chemically diverse family of drugs that are available as either over-the-counter medications or by prescription. Long-term use is common for treating pain associated with chronic inflammatory conditions such as arthritis. The basis for the anti-inflammatory activity of NSAIDs is largely attributed to the inhibition of cyclooxygenases, which catalyze the conversion of arachidonic acid to prostaglandins (Vane and Botting 1998). At least two isoforms of the COX enzyme are expressed in humans. COX-1 is a constitutively active form of the enzyme, whereas COX-2 is an inducible form for which expression is induced during various pathophysiological conditions such as chronic inflammation (Vane et al. 1998). As shown in Fig. 1, NSAIDs generally inhibit both COX-1 and COX-2 with various degrees of selectivity, while COX-2 selective inhibitors such as celecoxib and rofecoxib have been developed to be highly selective for the inducible COX-2 isoenzyme. Unfortunately, the depletion of physiologically important prostaglandins caused by the suppression of COX-1 or COX-2 is associated with potentially fatal side effects to the gastrointestinal tract, kidneys, and cardiovascular system (Vane and Botting 1998; Vane et al. 1998). While COX-2 selective inhibitors have reduced GI and renal toxicity, their long-term use has been associated with increased risk of heart attack (Chakraborti et al. 2010; Harris 2009; Warner et al. 1999). Consequently, the widespread use of traditional NSAIDs or COX-2 selective inhibitors is precluded, especially in the high dosages administered over extended periods of time that appear to be necessary for effective chemoprevention. While aspirin appears to have unique benefits for colorectal cancer, possibly because of the irreversible nature by which it can bind COX, non-aspirin NSAIDs, especially prescription strength NSAIDs with high potency appear to act by a COX-independent mechanism.
Table 1.
Common NSAIDs and COX-2 selective inhibitors listed according to structural classification
| Carboxylic acids |
Pyrazoles | Oxicams | COX-2 selective | |||
|---|---|---|---|---|---|---|
| Salicylates | Acetic acids | Propionic acids | Fenamates | |||
| Acetylsalicylic acid salsalate diflunisal fendosal | Indomethacin acemetacin cinmetacin sulindac tolmetin zomepirac diclofenac fenclofenac isoxepac furofenac fentiazac clidanac oxepinac fenclorac lonazolac metiazinic acid clopirac amfenac benzofenac clometacine etodolac bumadizone clamidoxic acid | Ibuprofen flurbiprofen naproxen ketoprofen fenoprofen benoxaprofen indoprofen pirprofen carprofen oxaprozin pranoprofen suprofen miroprofen tioxaprofen alminoprofen cicloprofen tiaprofenic acid furaprofen butibufen fenbufen furobufen bucloxic acid protizinic acid | Mefenamic acid flufenamic acid meclofenamate niflumic acid toifenamic acid flunixin clonixin | Phenyibutazone feprazone apazone trimethazone mofebutazone kebuzone suxibuzone | Piroxicam isoxicam tenoxicam | Celecoxib rofecoxib valdecoxibe nimesulide NS 398 |
Fig. 1.
Prostaglandins and thromboxanes produced through COX-1 have important physiological functions, whereas the prostaglandins and thromboxanes produced through COX-2 have important pathophysiological functions
The specific biochemical and cellular mechanism(s) proposed to be responsible for the cancer chemopreventive activity of the NSAIDs is controversial. While there is strong evidence that the mechanism of action involves COX-2 inhibition, there are a number of pharmacological inconsistencies that have led many investigators to conclude that the mechanism is unrelated to COX-2 inhibition (Alberts et al. 1995; Elder et al. 1997; Hanif et al. 1996; Kashfi and Rigas 2005; Piazza et al. 1997a). For example, studies have shown that NSAIDs can inhibit the growth of tumor cells that completely lack the expression COX-2 (Elder et al. 1997; Hanif et al. 1996; Grosch et al. 2001). Other studies have reported that the addition of exogenous prostaglandins cannot rescue cancer cells from the growth inhibitory activity of the NSAIDs, which would be expected if prostaglandin suppression is necessary (Kusuhara et al. 1998; Piazza et al. 1995). Furthermore, as shown in Table 2, the rank order of potency among the NSAIDs to inhibit tumor cell growth does not correlate with their potency to inhibit prostaglandin synthesis (Carter et al. 1989; de Mello et al. 1980; Erickson et al. 1999). For example, sulindac sulfide is appreciably less potent than indomethacin with regard to COX-1 or COX-2 inhibition, but is more potent in terms of its tumor cell growth inhibitory activity (Tinsley et al. 2010). In addition, certain highly specific COX-2 inhibitors such as rofecoxib fail to inhibit tumor cell growth (Soh et al. 2008). Consequently, a number of alternative targets have been suggested as potential mediators (Chan 2002; Thun et al. 2002; Shiff and Rigas 1999).
Table 2.
Potencies of select NSAIDs and COX-2 selective inhibitors for inhibition of HT-29 colon tumor cell growth, inhibition of purified ovine COX-2, and inhibition of cGMP PDE activity in HT-29 cell lysate
| IC50 (μM) | |||
|---|---|---|---|
| Growth inhibition | COX-2 inhibition | cGMP PDE inhibition | |
| Tolmetin | 313 | 0.82 | 326 |
| Meclofenamic acid | 85 | 2.9 | 80 |
| Flufenamic acid | 108 | 9.3 | 103 |
| Flurbiprofen | 550 | 5.5 | 504 |
| Celecoxib | 203 | 0.34 | 174 |
| Sulindac | 224 | >300 | 120 |
| Sulindac sulfide | 34 | 6.3 | 20 |
| Sulindac sulfone | 89 | >300 | 96 |
In this review, we consider several important developments in the study of both COX-2-dependent and COX-independent targets that can guide efforts in drug discovery and development strategies for cancer chemoprevention. We also discuss several promising novel NSAID derivatives that have been identified in recent years, which have potential safety and efficacy advantages compared to currently available NSAIDs and COX-2 inhibitors.
2 Targeting COX-2
There is strong evidence for the involvement of COX-2 in colon tumorigenesis. For example, COX-2 is overexpressed and prostaglandin levels are elevated in as many as 90 % of sporadic colon carcinomas (Eberhart et al. 1994). Furthermore, the levels of COX-2 expression correlates with colon tumor size and invasiveness (Fujita et al. 1998). The mechanism by which COX-2 may drive tumorigenesis is still unclear but likely involves multiple pathways given that prostaglandins can accelerate cellular growth, inhibit apoptosis, and induce angiogenesis; all key events in carcinogenesis (Shiff and Rigas 1999; Sawaoka et al. 1998; Tsujii et al. 1998). No matter the mechanism, studies have shown that decreasing COX-2 expression in the Apcmin mouse model of intestinal carcinogenesis reduces both the size and multiplicity of intestinal polyps (Oshima et al. 1996). Similarly, clinical studies have shown efficacy of celecoxib and rofecoxib, albeit modest, for suppressing the formation of colorectal adenomas in patients with familial adenomatous polyposis as well as the formation of sporadic adenomas (Steinbach et al. 2000; Arber et al. 2006; Baron et al. 2006; Bertagnolli et al. 2006).
While the COX-2 selective inhibitors, rofecoxib and valdecoxib, were removed from the market due to unforeseen cardiovascular toxicity, it is unclear if these side effects are directly linked with COX-2 inhibition, especially since they were less pronounced with celecoxib (Chakraborti et al. 2010). Given that the toxicity may be unrelated to COX-2 but may be compound specific, it is possible that chemical modifications to existing NSAIDs might have potential advantages. Marnett and colleagues, for example, described a series of ester and amide modifications to the carboxylic acid moiety on various NSAIDs, such as indomethacin and meclofenamic acid that increased selectivity for COX-2 (Kalgutkar et al. 2000; 2002). The rational for this approach was based on molecular modeling studies whereby a more restrictive binding domain was noted within the catalytic region of the COX-1 compared with COX-2, which permitted specificity via substitution of the carboxylic acid with bulky neutral residues. A potential concern, however, is that ester and amide linkages may not have sufficient metabolic stability whereby there is the potential to generate the parent NSAID. Studies to demonstrate in vivo antitumor efficacy and reduced toxicity compared with the parent NSAID are therefore needed for future development of this class of compounds.
3 COX-Independent Targets
3.1 Inhibition of cGMP PDEs
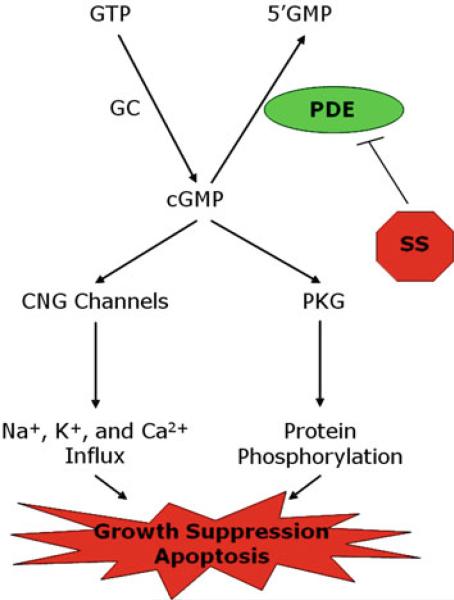
One COX-independent target of NSAIDs that has been used to guide the synthesis of more potent non-COX inhibitory derivatives includes members of the cyclic GMP phosphodiesterase (PDE) superfamily. PDE enzymes are metallophosphohydrolases that hydrolyze the 3′,5′-cyclic phosphate on second messenger cyclic nucleotides, cAMP or cGMP, to 5′-monophosphate. This modification terminates intracellular signaling following activation of cyclic nucleotide coupled receptors (Beavo 1995). The PDE superfamily comprises an estimated 100 distinct protein isoforms divided into 11 protein families, which differ from one another in selectivity for cAMP or cGMP, sensitivity to inhibitors and activators, as well as tissue, cellular, or intracellular distributions (Beavo 1995; Sonnenburg and Beavo 1994). Inhibition of PDE would result in an increase in the magnitude and/or duration of the cAMP and/or cGMP signal depending on the isozyme selectivity of the inhibitor. Increased intracellular cyclic nucleotide levels can activate specific signaling pathways, which, in the case of cGMP, can lead to activation of cGMP-dependent protein kinase (PKG), cyclic nucleotide-gated ion channels, or certain cGMP binding PDEs; all of which play important roles in regulating cellular activity (Beavo 1995; Lincoln and Cornwell 1993).
Studies have suggested that the cGMP pathway may be perturbed during colorectal tumorigenesis. For example, human colon tumors have been shown to express high levels of the cGMP-specific PDE5 isozyme (Tinsley et al. 2010). In addition, Shailubhai and colleagues demonstrated that mRNA levels for the membrane-associated guanylyl cyclase agonist, uroguanylin, are reduced in both colon adenomas and adenocarcinomas. Interestingly, oral administration of uroguanylin inhibited tumor formation in the Apcmin mouse model of intestinal tumorigenesis, which was associated with increased apoptosis rates within the tumors (Shailubhai et al. 2000). These findings are consistent with the observations that certain cGMP phosphodiesterases may be elevated in colon tumors, leading to reduced cGMP levels, which is necessary to limit tumor cell survival or suppress proliferation. Despite the evidence for a role of cGMP in colon cancer development, the relevant cGMP PDE isozymes that regulate its synthesis and downstream signaling pathways have not been well studied with regard to a role in colon tumorigenesis.
Certain NSAIDs and COX-2 inhibitors can also inhibit cGMP PDE isozymes to increase intracellular cGMP levels and activate cGMP signaling in colon cancer cell lines, as depicted in Fig. 2 (Soh et al. 2000, 2008; Silvola et al. 1982; Thompson et al. 2000; Tinsley et al. 2009). While these effects require high concentrations (Table 2) that cannot be safely achieved in vivo, a strong correlation exists between concentrations of the NSAIDS required to inhibit tumor cell growth in vitro and their ability to inhibit cGMP PDE, especially the cGMP-specific PDE isozyme, PDE5 (Tinsley et al. 2010). In addition, certain known PDE5-specific inhibitors (e.g. MY5445) and non-selective cGMP PDE inhibitors (e.g. MY5445, trequinsin) could also suppress tumor cell growth, as well as knockdown of PDE5 by siRNA or antisense (Tinsley et al. 2011; Zhu et al. 2005). However, other highly selective PDE5 inhibitors (e.g. sildenafil), commonly used for the treatment of erectile dysfunction, required high concentrations compared with those required to inhibit tumor cell growth, which suggest that additional cGMP PDE isozymes may be involved (Tinsley et al. 2011).
Fig. 2.
Mechanistic model of colon tumor cell growth inhibition mediated by inhibition of cGMP PDE and activation of cGMP signaling
Among a number of NSAIDs evaluated for cGMP PDE inhibition, sulindac sulfide was found to be the most active (Tinsley et al. 2010). Since the non-COX inhibitory sulfone form of sulindac can also inhibit cGMP PDE, albeit with less potency, it is likely that the COX inhibitory activity of this class of compounds can be uncoupled from cGMP PDE inhibitory activity (Piazza et al. 1995, 1997b). Initial efforts to synthesize sulindac derivatives that lack COX inhibitory activity, but have improved cGMP PDE inhibitory activity focused on modifications to either the sulfonyl or the carboxylic acid moieties (Thompson et al. 2000). While this approach yielded certain derivatives with high in vitro potency to inhibit colon tumor cell growth and induce apoptosis, poor oral bioavailability and metabolic instability halted further development (Thompson et al. 2000).
In light of information gained from molecular modeling docking sulindac sulfide into the COX active site, our laboratory has focused on chemically modifying the carboxylic acid moiety of sulindac sulfide and substituting with a positive-charged amide to interfere with COX binding (Piazza et al. 2009). This approach yielded an interesting series of compounds that retained ability to inhibit cGMP PDE and had improved anticancer activity both in vitro and in vivo compared with sulindac (Piazza et al. 2009). These derivatives serve to validate cGMP PDE as a target to optimize for anticancer efficacy, while reducing toxicity. Although problems with poor absorption following oral administration necessitated high dosages limited the development of this class of compounds for colorectal cancer chemoprevention, alternative derivatives or formulations with improved oral bioavailability may have advantages.
3.2 Generation of Reactive Oxygen Species
Reactive oxygen species (ROS) such as the superoxide anion or nitric oxide have long been recognized as by-products of mitochondrial oxygen metabolism that can be toxic to cells (Genestra 2007). Furthermore, ROS generated by certain organ-elles such as peroxisomes, especially in cellular types such as macrophages, mediate important physiological processes including macromolecular anabolism and pathogen destruction (Genestra 2007; Klaunig and Kamendulis 2004). However, recent evidence suggests that these highly reactive molecules can serve as signaling molecules within cells (redox signaling), interacting with numerous canonical signaling pathways to mediate a variety of cellular functions including proliferation, apoptosis, and differentiation (Frein et al. 2005).
Aberrant redox signaling has been documented in cancer, although more work is necessary to determine whether ROS are a driving force in tumorigenesis. For example, abnormally high levels of ROS have been observed in multiple types of cancer (Toyokuni et al. 1995). Furthermore, chronic inflammation, which can promote tumorigenesis, results in elevated levels of ROS. High ROS levels have been reported in tumors and are associated with genomic instability, proliferation, angiogenesis, and aberrant apoptosis, although the effect may be dependent on intracellular levels (Frein et al. 2005; Toyokuni et al. 1995; Halliwell 2007).
Two promising new classes of NSAID derivatives, the nitric oxide NSAIDs (NO-NSAIDs) and the phospho-NSAIDs, demonstrate chemopreventive efficacy through a mechanism that appears to involve the elevation of intracellular ROS levels and activation of proapoptotic redox signaling pathways as described by Rigas and colleagues. The NO-NSAIDs were designed with an NO-releasing moiety attached to a parent compound such as aspirin, sulindac, or naproxen through a chemical linker. The intended design was to increase NO levels in the gut where it could serve a protective function to increase vascular blood supply to reduce the GI toxicity of the parent NSAID (Rigas and Kashfi 2004). Initial studies with the NO-NSAIDs showed enhanced colon cancer chemopreventive activity when compared to the parent compound both in vitro and in vivo (Williams et al. 2004; Yeh et al. 2004). In order to identify the importance of the NO moiety for the enhanced chemopreventive activity, additional modifications were made in which the NO was replaced with a diethylphosphate. Interestingly, these phospho-NSAID derivatives demonstrated similar chemopreventive activity as the NO-NSAIDs, suggesting that release of the NO was not solely responsible for the enhanced activity of these new classes of compounds, but rather a chemical modification to the NSAIDs (Huang et al. 2010; Mackenzie et al. 2010).
Whether the parent NSAID was linked to NO or to the diethylphosphate substituent, the new derivatives caused substantial increases in ROS in tumor cells in vitro and showed advantages in efficacy in animal models of colon cancer (Rigas and Kashfi 2004; Kashfi and Rigas 2007; Rigas and Kozoni 2008; Rigas and Williams 2008; Sun et al. 2011; Xie et al. 2011). While further studies are necessary to develop this class of derivatives, their ability to induce apoptosis of colon tumor cells through activation of redox signaling makes them a promising class of agents for colorectal cancer chemoprevention.
3.3 Downregulation of Survivin
Survivin is an apoptosis inhibitor protein that prevents activation of caspases that are important mediators of apoptosis (Altieri 2003a, b; Ambrosini et al. 1997). Not normally found to be expressed in adult tissues, survivin is overexpressed in the vast majority of human cancers and increased levels of this protein strongly correlate with increased tumor stage and a poor prognosis (Altieri 2003a; Ambrosini et al. 1997; Zaffaroni et al. 2005). Furthermore, survivin expression appears to be strongly connected to p53 status and sensitivity to chemotherapy (Hoffman et al. 2002; Li 2005).
A number of NSAIDs and COX-2 selective inhibitors have been shown to reduce survivin expression and/or activity in cancer cells, but the best studied of these is celecoxib (Tinsley et al. 2010, 2011; Konduri et al. 2009; Pyrko et al. 2006). Interestingly, the celecoxib derivative 2,5-dimethyl-celecoxib (DMC) lacks COX-2 inhibitory activity but retains the anticancer activity of the parent compound and is actually more potent than celecoxib for suppressing survivin (Pyrko et al. 2006). Even more promising, DMC has shown in vivo chemopreventive efficacy in various models of human cancer (Pyrko et al. 2006; Kardosh et al. 2005).
3.4 Other COX-Independent Targets
Additional targets that are known to play a role in colorectal cancer tumorigenesis have also been implicated as targets of NSAIDs. However, the association between these targets and the anticancer activity of the NSAIDs is weaker and has not been established for non-COX-inhibitory derivatives, but nonetheless represent potential targets for future efforts in drug discovery.
One such target is peroxisomal proliferator-activated receptor γ (PPARγ). PPARγ is overexpressed in colon cancer cells and can inhibit growth and promote differentiation (DuBois et al. 1998; Mueller et al. 1998). Numerous studies have shown that certain NSAIDs can directly bind to and activate PPARγ and that this activity is associated with inhibition of neoplastic growth (Lehmann et al. 1997). However, the activation of PPARγ is not a characteristic shared by all antineo-plastic NSAIDs (Lehmann et al. 1997; Lefebvre et al. 1998; Saez et al. 1998).
NFκB is another potential COX-independent antineoplastic target of the NSAIDs. NFκB is a transcription factor that commonly promotes cellular growth and inhibits apoptosis (Ljungdahl et al. 1997). Increased NFκB activity has been observed in multiple types of cancer and can be attributed to a number of alterations including overexpression of NFκB or decreased expression of IκB, a regulatory protein that sequesters NFκB in the cytosol in order to prevent its transcriptional activity (Rayet and Gelinas 1999). Some NSAIDs, particularly the salicylates, have been found to inhibit NFκB-mediated transcription, presumably by preventing degradation of IκB (Schwenger et al. 1998; Yin et al. 1998). However, these effects are most pronounced among the least active NSAIDs in terms of in vitro colon cancer prevention (Yin et al. 1998).
One of the most novel mechanisms proposed for the anticancer activity of the NSAIDs involves induction of the NSAID-activated gene (NAG-1) as reported by Baek and colleagues. NAG-1 is a member of the transforming growth factor β (TGF-β) superfamily that has both proapoptotic and antiproliferative properties, although the exact mechanism responsible for these effects has not been well defined (Baek et al. 2001a, b). A number of different NSAIDs including indomethacin, aspirin, and ibuprofen have been shown to increase expression of NAG-1 in colon cancer cells and this effect appears to be independent of COX expression (Baek et al. 2001a, 2002). However, there is little association between the anticancer activity of an NSAID and its potency for activating NAG-1 expression, as NAG-1 induction occurs at significantly lower concentrations than those necessary for induction of apoptosis or inhibition of growth (Baek et al. 2002). Furthermore, the effects of the NSAIDs on NAG-1 and the effects of NAG-1 expression have yet to be observed in vivo.
Although not a direct target, microRNA may be another important factor that contributes to the sensitivity of tumor cells to NSAIDs. microRNAs are a set of naturally occurring small RNA molecules that are capable of regulating approximately 30 % of human genes through direct binding with the cognate target genes and are involved in many essential biological processes such as cell growth, differentiation, apoptosis, and tumorigenesis(Carmell et al. 2002; Esquela-Kerscher and Slack 2006), which highlights its clinical applications in tumor diagnosis, prognosis, and therapy (Nakajima et al. 2006; Xi et al. 2006). Our studies, for example, have shown that a panel of microRNAs (miR-10b, -17, -19, -21, and -9) are suppressed in human colon tumor cells treated with sulindac sulfide. Interestingly, these microRNAs are known to be elevated during cancer metastasis and invasion (Huang et al. 2009; Ma et al. 2010a, b, 2007; Song et al. 2010; Yu et al. 2010; Zhu et al. 2008) and may mediate the ability of sulindac sulfide to inhibit tumor cell invasion.
Another complicating aspect of determining the mechanisms of the actions of NSAIDs in the prevention of neoplasia is the effects of various types of NSAIDs on inflammation in general. Inflammation in the setting of long standing, continuing damage, inflammation, and repair that occurs in ulcerative colitis and the associated development of colon cancers generates not only increased ROS, but also increased cellular death and proliferation. These associated inflammatory changes may lead to molecular changes in epithelial cells that result in the inhibition of enzymes that repair DNA to molecular changes in signaling pathways associated with the initiation of neoplasia as a result of genetic mutations (Baek et al. 2001b, 2002). Thus, some changes related to the chemopreventive actions of NSAIDs may be related specifically to their anti-inflammatory activity involving COX-inhibition, while others (e.g. tumor cell growth inhibition and apoptosis induction) are more related to their ability to suppress tumorigenesis. Whether the two activities can be fully uncoupled remains to be determined and awaits the development of new derivatives that lack COX inhibitory activity.
4 Conclusions
NSAIDs represent a chemically diverse group of drugs that have multiple biological effects, some of which are related to their anticancer activities, while others are responsible for toxicity. Experimental studies over the past 20 years have provided strong evidence that the mechanism responsible for their chemopreventive activity may not require COX inhibition. As such, it should be feasible to develop improved drugs with greater antitumor efficacy and reduced toxicity. Given that the anticancer activity of the NSAIDs is undoubtedly complex, a number of reports have suggested many alternative targets as summarized in Table 3. Traditional NSAIDs and COX-2 selective inhibitors most likely inhibit tumorigenesis through a combination of COX-dependent and COX-independent mechanisms. However, the development of NSAID derivatives that lack COX inhibitory activity have shown promise for improved potency and selectivity to inhibit tumor cell growth. As such, the elucidation of COX-independent mechanisms of the NSAIDs is an important area of research that offers the promise to design a highly specific new class of chemopreventive agents. The greatest challenges will be to identify which molecular target(s) is most important for colon tumorigenesis and which chemical scaffold can yield suitable lead compounds for preclinical development with optimal pharmaceutical, efficacy, and toxicity properties.
Table 3.
Summary of drugs and COX-independent targets that have been studied for the chemopreventive activity of the NSAIDs and COX-2 selective inhibitors
| Target | Active NSAIDs | Reference |
|---|---|---|
| cGMP PDE | Sulindac and metabolites | Tinsley et al. (2009, 2010, 2011), Soh et al. (2000, 2008), Silvola et al. (1982), Thompson et al. (2000) |
| Celecoxib | ||
| Indomethacin | ||
| Meclofenamic acid | ||
| Naproxen | ||
| Tolfenamic acid | ||
| Diclofenac | ||
| NSAID derivatives | ||
| ROS | NO-NSAIDs | Huang et al. (2010), Mackenzie et al. (2010), Kashfi and Rigas (2007) |
| Phospho-NSAIDs | ||
| Survivin | Sulindac and metabolites | Tinsley et al. (2009, 2010, 2011), Konduri et al. (2009), Pyrko et al. (2006) |
| Tolfenamic acid | ||
| Celecoxib and derivatives | ||
| PPARγ | Indomethacin | Lehmann et al. (1997) |
| Fenoprofen | ||
| Ibuprofen | ||
| Flufenamic acid | ||
| NFκB | Sodium salicylate | Schwenger et al. (1998), Yin et al. (1998) |
| Aspirin | ||
| NAG-1 | Indomethacin and derivatives | Baek et al. (2001b, 2002) |
| Piroxicam | ||
| Diclofenac | ||
| Aspirin | ||
| Sulindac | ||
| microRNA | Sulindac | Unpublished |
Acknowledgments
Funding provided by NIH grants R01 CA131378 and R01 CA148817 and a UAB Breast Cancer SPORE grant.
Contributor Information
Heather N. Tinsley, Department of Biology, Chemistry, and Mathematics, University of Montevallo, Montevallo, AL, USA
William E. Grizzle, Department of Pathology, The University of Alabama at Birmingham, Birmingham, AL, USA
Ashraf Abadi, Faculty of Pharmacy and Biotechnology, German University of Cairo, Cairo, Egypt.
Adam Keeton, Drug Discovery Research Center Mitchell Cancer Institute, University of South Alabama, 1660 Springhill Avenue, Suite 3029, Mobile, AL 36604, USA.
Bing Zhu, Drug Discovery Research Center Mitchell Cancer Institute, University of South Alabama, 1660 Springhill Avenue, Suite 3029, Mobile, AL 36604, USA.
Yaguang Xi, Drug Discovery Research Center Mitchell Cancer Institute, University of South Alabama, 1660 Springhill Avenue, Suite 3029, Mobile, AL 36604, USA.
Gary A. Piazza, Drug Discovery Research Center Mitchell Cancer Institute, University of South Alabama, 1660 Springhill Avenue, Suite 3029, Mobile, AL 36604, USA gpiazza@usouthal.edu
References
- Alberts DS, et al. Do NSAIDs exert their colon cancer chemoprevention activities through the inhibition of mucosal prostaglandin synthetase? J Cell Biochem Suppl. 1995;22:18–23. doi: 10.1002/jcb.240590804. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin in apoptosis control and cell cycle regulation in cancer. Prog Cell Cycle Res. 2003a;5:447–452. [PubMed] [Google Scholar]
- Altieri DC. Survivin and apoptosis control. Adv Cancer Res. 2003b;88:31–52. doi: 10.1016/s0065-230x(03)88303-3. [DOI] [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Arber N, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Horowitz JM, Eling TE. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3. J Biol Chem. 2001a;276(36):33384–33392. doi: 10.1074/jbc.M101814200. [DOI] [PubMed] [Google Scholar]
- Baek SJ, et al. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001b;59(4):901–908. [PubMed] [Google Scholar]
- Baek SJ, et al. Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): inhibition of cyclooxygenase and induction of NSAID-activated gene. J Pharmacol Exp Ther. 2002;301(3):1126–1131. doi: 10.1124/jpet.301.3.1126. [DOI] [PubMed] [Google Scholar]
- Baron JA, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75(4):725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Bertagnolli MM, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- Carmell MA, et al. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002;16(21):2733–2742. doi: 10.1101/gad.1026102. [DOI] [PubMed] [Google Scholar]
- Carter CA, Ip MM, Ip C. A comparison of the effects of the prostaglandin synthesis inhibitors indomethacin and carprofen on 7,12-dimethylbenz[a]anthracene-induced mammary tumorigenesis in rats fed different amounts of essential fatty acid. Carcinogenesis. 1989;10(8):1369–1374. doi: 10.1093/carcin/10.8.1369. [DOI] [PubMed] [Google Scholar]
- Chakraborti AK, et al. Progress in COX-2 inhibitors: a journey so far. Curr Med Chem. 2010;17(15):1563–1593. doi: 10.2174/092986710790979980. [DOI] [PubMed] [Google Scholar]
- Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002;3(3):166–174. doi: 10.1016/s1470-2045(02)00680-0. [DOI] [PubMed] [Google Scholar]
- de Mello MC, Bayer BM, Beaven MA. Evidence that prostaglandins do not have a role in the cytostatic action of anti-inflammatory drugs. Biochem Pharmacol. 1980;29(3):311–318. doi: 10.1016/0006-2952(80)90506-7. [DOI] [PubMed] [Google Scholar]
- DuBois RN, et al. The nuclear eicosanoid receptor, PPARgamma, is aberrantly expressed in colonic cancers. Carcinogenesis. 1998;19(1):49–53. doi: 10.1093/carcin/19.1.49. [DOI] [PubMed] [Google Scholar]
- Eberhart CE, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Elder DJ, et al. Induction of apoptotic cell death in human colorectal carcinoma cell lines by a cyclooxygenase-2 (COX-2)-selective nonsteroidal anti-inflammatory drug: independence from COX-2 protein expression. Clin Cancer Res. 1997;3(10):1679–1683. [PubMed] [Google Scholar]
- Erickson BA, et al. The effect of selective cyclooxygenase inhibitors on intestinal epithelial cell mitogenesis. J Surg Res. 1999;81(1):101–107. doi: 10.1006/jsre.1998.5511. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs–microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Frein D, et al. Redox regulation: a new challenge for pharmacology. Biochem Pharmacol. 2005;70(6):811–823. doi: 10.1016/j.bcp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Fujita T, et al. Size- and invasion-dependent increase in cyclooxygenase 2 levels in human colorectal carcinomas. Cancer Res. 1998;58(21):4823–4826. [PubMed] [Google Scholar]
- Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19(9):1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Grosch S, et al. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. Faseb J. 2001;15(14):2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401(1):1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- Hanif R, et al. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52(2):237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- Harris RE. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology. 2009;17(2):55–67. doi: 10.1007/s10787-009-8049-8. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, et al. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277(5):3247–3257. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- Huang GL, et al. Clinical significance of miR-21 expression in breast cancer: SYBR-Green I-based real-time RT-PCR study of invasive ductal carcinoma. Oncol Rep. 2009;21(3):673–679. [PubMed] [Google Scholar]
- Huang L, et al. Phospho-sulindac (OXT-922) inhibits the growth of human colon cancer cell lines: a redox/polyamine-dependent effect. Carcinogenesis. 2010;31(11):1982–1990. doi: 10.1093/carcin/bgq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalgutkar AS, et al. Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J Med Chem. 2000;43(15):2860–2870. doi: 10.1021/jm000004e. [DOI] [PubMed] [Google Scholar]
- Kalgutkar AS, et al. Amide derivatives of meclofenamic acid as selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett. 2002;12(4):521–524. doi: 10.1016/s0960-894x(01)00792-2. [DOI] [PubMed] [Google Scholar]
- Kardosh A, et al. Multitarget inhibition of drug-resistant multiple myeloma cell lines by dimethyl-celecoxib (DMC), a non-COX-2 inhibitory analog of celecoxib. Blood. 2005;106(13):4330–4338. doi: 10.1182/blood-2005-07-2819. [DOI] [PubMed] [Google Scholar]
- Kashfi K, Rigas B. Is COX-2 a ‘collateral’ target in cancer prevention? Biochem Soc Trans. 2005;33(Pt 4):724–727. doi: 10.1042/BST0330724. [DOI] [PubMed] [Google Scholar]
- Kashfi K, Rigas B. The mechanism of action of nitric oxide-donating aspirin. Biochem Biophys Res Commun. 2007;358(4):1096–1101. doi: 10.1016/j.bbrc.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Konduri S, et al. Tolfenamic acid enhances pancreatic cancer cell and tumor response to radiation therapy by inhibiting survivin protein expression. Mol Cancer Ther. 2009;8(3):533–542. doi: 10.1158/1535-7163.MCT-08-0405. [DOI] [PubMed] [Google Scholar]
- Kusuhara H, et al. Induction of apoptotic DNA fragmentation by nonsteroidal anti-inflammatory drugs in cultured rat gastric mucosal cells. Eur J Pharmacol. 1998;360(2–3):273–280. doi: 10.1016/s0014-2999(98)00679-7. [DOI] [PubMed] [Google Scholar]
- Lefebvre AM, et al. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6 J-APCMin/+ mice. Nat Med. 1998;4(9):1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, et al. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272(6):3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- Li F. Role of survivin and its splice variants in tumorigenesis. Br J Cancer. 2005;92(2):212–216. doi: 10.1038/sj.bjc.6602340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. Faseb J. 1993;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Ljungdahl S, Shoshan MC, Linder S. Inhibition of the growth of 12 V-ras-transformed rat fibroblasts by acetylsalicylic acid correlates with inhibition of NF-kappa B. Anticancer Drugs. 1997;8(1):62–66. doi: 10.1097/00001813-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- Ma L, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010a;28(4):341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, et al. MiR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010b;12(3):247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie GG, et al. Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology. 2010;139(4):1320–1332. doi: 10.1053/j.gastro.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller E, et al. Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell. 1998;1(3):465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- Nakajima G, et al. Non-coding microRNAs hsa-let-7 g and hsa-miR-181b are associated with chemoresponse to S-1 in colon cancer. Cancer Genomics Proteomics. 2006;3(5):317–324. [PMC free article] [PubMed] [Google Scholar]
- Oshima M, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Piazza GA, et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995;55(14):3110–3116. [PubMed] [Google Scholar]
- Piazza GA, et al. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997a;57(12):2452–2459. [PubMed] [Google Scholar]
- Piazza GA, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997b;57(14):2909–2915. [PubMed] [Google Scholar]
- Piazza GA, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila Pa) 2009;2(6):572–580. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrko P, et al. Downregulation of survivin expression and concomitant induction of apoptosis by celecoxib and its non-cyclooxygenase-2-inhibitory analog, dimethyl-celecoxib (DMC), in tumor cells in vitro and in vivo. Mol Cancer. 2006;5:19. doi: 10.1186/1476-4598-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18(49):6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- Rigas B, Kashfi K. Nitric-oxide-donating NSAIDs as agents for cancer prevention. Trends Mol Med. 2004;10(7):324–330. doi: 10.1016/j.molmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Rigas B, Kozoni V. The novel phenylester anticancer compounds: Study of a derivative of aspirin (phoshoaspirin). Int J Oncol. 2008;32(1):97–100. [PubMed] [Google Scholar]
- Rigas B, Williams JL. NO-donating NSAIDs and cancer: an overview with a note on whether NO is required for their action. Nitric Oxide. 2008;19(2):199–204. doi: 10.1016/j.niox.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez E, et al. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med. 1998;4(9):1058–1061. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- Sawaoka H, et al. Effects of NSAIDs on proliferation of gastric cancer cells in vitro: possible implication of cyclooxygenase-2 in cancer development. J Clin Gastroenterol. 1998;27(Suppl 1):S47–S52. doi: 10.1097/00004836-199800001-00009. [DOI] [PubMed] [Google Scholar]
- Schwenger P, et al. Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IkappaB alpha phosphorylation and degradation. Mol Cell Biol. 1998;18(1):78–84. doi: 10.1128/mcb.18.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailubhai K, et al. Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP. Cancer Res. 2000;60(18):5151–5157. [PubMed] [Google Scholar]
- Shiff SJ, Rigas B. The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal antiinflammatory drugs (NSAIDs). J Exp Med. 1999;190(4):445–450. doi: 10.1084/jem.190.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvola J, et al. Effects of nonsteroidal anti-inflammatory drugs on rat gastric mucosal phosphodiesterase activity. Agents Actions. 1982;12(4):516–520. doi: 10.1007/BF01965936. [DOI] [PubMed] [Google Scholar]
- Soh JW, et al. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin Cancer Res. 2000;6(10):4136–4141. [PubMed] [Google Scholar]
- Soh JW, et al. Celecoxib-induced growth inhibition in SW480 colon cancer cells is associated with activation of protein kinase G. Mol Carcinog. 2008;47(7):519–525. doi: 10.1002/mc.20409. [DOI] [PubMed] [Google Scholar]
- Song B, et al. MicroRNA-21 regulates breast cancer invasion partly by targeting tissue inhibitor of metalloproteinase 3 expression. J Exp Clin Cancer Res. 2010;29:29. doi: 10.1186/1756-9966-29-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg WK, Beavo JA. Cyclic GMP and regulation of cyclic nucleotide hydrolysis. Adv Pharmacol. 1994;26:87–114. doi: 10.1016/s1054-3589(08)60052-6. [DOI] [PubMed] [Google Scholar]
- Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- Sun Y, et al. Oxidative stress mediates through apoptosis the anticancer effect of phospho-NSAIDs: implications for the role of oxidative stress in the action of anticancer agents. J Pharmacol Exp Ther. 2011;338(3):775–783. doi: 10.1124/jpet.111.183533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WJ, et al. Exisulind induction of apoptosis involves guanosine 3’,5’-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60(13):3338–3342. [PubMed] [Google Scholar]
- Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94(4):252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- Tinsley HN, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8(12):3331–3340. doi: 10.1158/1535-7163.MCT-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley HN, et al. Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res (Phila) 2010;3(10):1303–1313. doi: 10.1158/1940-6207.CAPR-10-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley HN, et al. Inhibition of PDE5 by Sulindac Sulfide Selectively Induces Apoptosis and Attenuates Oncogenic Wnt/{beta}-Catenin-Mediated Transcription in Human Breast Tumor Cells. Cancer Prev Res (Phila) 2011;4(8):1275–1284. doi: 10.1158/1940-6207.CAPR-11-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokuni S, et al. Persistent oxidative stress in cancer. FEBS Lett. 1995;358(1):1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- Tsujii M, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93(5):705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- Vane JR, Botting RM. Mechanism of action of antiinflammatory drugs. Int J Tissue React. 1998;20(1):3–15. [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Warner TD, et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclooxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96(13):7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL, et al. NO-donating aspirin inhibits intestinal carcinogenesis in Min (APC(Min/+)) mice. Biochem Biophys Res Commun. 2004;313(3):784–788. doi: 10.1016/j.bbrc.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Xi Y, et al. Prognostic values of microRNAs in colorectal cancer. Biomark Insights. 2006;2:113–121. [PMC free article] [PubMed] [Google Scholar]
- Xie G, et al. Phospho-ibuprofen (MDC-917) is a novel agent against colon cancer: efficacy, metabolism, and pharmacokinetics in mouse models. J Pharmacol Exp Ther. 2011;337(3):876–886. doi: 10.1124/jpet.111.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh RK, et al. NO-donating nonsteroidal antiinflammatory drugs (NSAIDs) inhibit colon cancer cell growth more potently than traditional NSAIDs: a general pharmacological property? Biochem Pharmacol. 2004;67(12):2197–2205. doi: 10.1016/j.bcp.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396(6706):77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- Yu Z, et al. MicroRNA 17/20 inhibits cellular invasion and tumor metastasis in breast cancer by heterotypic signaling. Proc Natl Acad Sci U S A. 2010;107(18):8231–8236. doi: 10.1073/pnas.1002080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffaroni N, Pennati M, Daidone MG. Survivin as a target for new anticancer interventions. J Cell Mol Med. 2005;9(2):360–372. doi: 10.1111/j.1582-4934.2005.tb00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, et al. Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. J Cell Biochem. 2005;94(2):336–350. doi: 10.1002/jcb.20286. [DOI] [PubMed] [Google Scholar]
- Zhu S, et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18(3):350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]