Abstract
Background
The ability to predict potential for relapse to substance use following treatment could be very useful in targeting aftercare strategies. Recently, a number of investigators have focused on using neural activity measured by fMRI to predict relapse propensity. The purpose of the present study was to use fMRI to investigate prospective associations between brain reactivity to cocaine and response inhibition cues and relapse to cocaine use.
Methods
Thirty cocaine-dependent participants with clean cocaine urine drug screens (UDS) completed a baseline fMRI scan, including a cocaine-cue reactivity task and a go/no-go response inhibition task. After participating in a brief clinical trial of D-cycloserine for the facilitation of cocaine cue extinction, they returned for a one-week follow-up UDS. Associations between baseline activation to cocaine and inhibition cues and relapse to cocaine use were explored.
Results
Positive cocaine UDS was significantly associated with cocaine cue activation in the right putamen and insula, as well as bilateral occipital regions. Associations between positive cocaine UDS and activation to no-go cues were concentrated in the postcentral gyri, a region involved in response execution.
Conclusions
Although preliminary, these results suggest that brain imaging may be a useful tool for predicting risk for relapse in cocaine-dependent individuals. Further, larger-scale naturalistic studies are needed to corroborate and extend these findings.
Keywords: fMRI, cue, go no-go, urine drug screen, cocaine, relapse
1. INTRODUCTION
Cocaine dependence is a major public health concern associated with high rates of relapse (SAMHSA, 2012). There has been strong interest in prospectively predicting relapse to drug use in substance-dependent individuals. Efforts focused on self-reported drug craving (Tiffany and Carter, 1998) and behavioral disinhibition (Muller et al., 2008) have traditionally demonstrated limited utility in predicting relapse (Tiffany and Carter, 1998). As a result, in addition to continued efforts aimed at improving the assessment of self-reported craving (e.g., by improving its temporal resolution; Epstein et al., 2009; Preston et al., 2009), recent attempts at predicting relapse have focused on more objective (e.g., fMRI) predictors.
Across substances, a number of studies have demonstrated prospective associations between brain activation to fMRI tasks and subsequent substance use (Beck et al., 2012; Braus et al., 2001; Brewer et al., 2008; Clark et al., 2012; Grusser et al., 2004; Heinz et al., 2007; Janes et al., 2010; Kosten et al., 2006; Paulus et al., 2005; Zhiru et al., 2011). These studies can be divided into evaluations of drug cue-reactivity tasks, which are prominent because of the role of drug-cue exposure in relapse (Bossert et al., 2005), and evaluations of cognitive tasks. In terms of cue-reactivity tasks, a number of studies in alcohol-dependent individuals have demonstrated associations between relapse and brain activation to alcohol cues (e.g., medial prefrontal cortex: Beck et al., 2012; thalamus: Heinz et al., 2007; putamen: Braus et al., 2001; Grusser et al., 2004). While there are fewer studies investigating cue reactivity and cocaine relapse, one study found that positive urine toxicologies were associated with activation to cues in posterior cingulate, precentral, temporal, and occipital cortices in cocaine-dependent individuals (Kosten et al., 2006). A more recent investigation of cue-reactivity in nicotine-dependent individuals found that relapse was associated with activation to smoking cues in a variety of brain regions central to addiction (e.g., insula, anterior cingulate, posterior cingulate, amygdala, putamen, prefrontal cortex; Janes et al., 2010).
A number of studies have focused on associations between brain activation to cognitive tasks and stimulant relapse. Though a variety of different fMRI tasks have been employed, associations between relapse and task activation have tended to concentrate in the dorsal striatum (Brewer et al, 2008; Zhiru et al., 2011), insula (Paulus et al., 2005; Clark et al., 2012), and posterior cingulate (Clark et al., 2012; Paulus et al., 2005). Surprisingly, despite the prominent relevance of impulsivity to the development and maintenance of substance dependence (de Wit, 2009), no studies have investigated prospective associations between fMRI impulsivity tasks (e.g, go no-go) and subsequent drug use.
The purpose of the present study was to investigate prospective associations between brain reactivity to cocaine and response inhibition cues and subsequent relapse to cocaine use as evidenced by positive cocaine urine drug screens. Thirty participants in a larger clinical trial investigating the use of D-cycloserine (DCS) to facilitate extinction of responses elicited by cocaine cues were included in the present study based on their completion of a baseline fMRI visit and a one-week follow-up urine drug screen (UDS). The fMRI visit included both a cocaine-cue exposure task and a response inhibition (i.e., go no-go) task. We hypothesized that brain regions typically associated with exposure to cocaine (e.g., frontostriatal; Childress et al., 1999, Garavan et al., 2000) and response inhibition (e.g., frontoparietal; Swick et al., 2011) cues would prospectively predict relapse to cocaine use at the one-week follow-up visit. Furthermore, given that activations of the putamen, insula, and posterior cingulate have been frequently associated with relapse in the literature, across both substances and fMRI tasks, we hypothesized that functional activation of these regions would be associated with relapse in the present study.
2. METHODS
2.1. Participants
Thirty cocaine-dependent men and women aged 18–65 were recruited from a larger clinical trial of DCS facilitation of cocaine-cue extinction (Santa Ana et al., 2012). Participants met DSM-IV criteria for current Cocaine Dependence and were right-handed. Exclusionary criteria included medications for addiction (e.g., naltrexone, buprenorphine), major medical (e.g., diabetes, HIV) and psychiatric conditions (e.g., affective disorders, posttraumatic stress disorder), pregnancy or nursing, ferrous metal implants or pacemakers, and DSM-IV criteria for non-cocaine substance dependence (except caffeine, nicotine, marijuana, or alcohol) within the 60 days preceding the study. In order to be included in the present analysis, clinical trial participants must have completed both a baseline fMRI scan and a one-week follow-up UDS. Participants were required to provide negative alcohol breath and urine drug screens for all drugs of abuse (excluding marijuana) at their baseline fMRI visit, as well as at all subsequent study visits with the exception of the one-week follow-up visit. All study procedures were performed in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, with approval from the Medical University of South Carolina Institutional Review Board.
2.2. Procedure
Following screening, participants completed a diagnostic visit during which they completed the Substance Use Disorders module of the Structured Clinical Interview for DSM-IV (SCID; First et al., 1994) and the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Cocaine use in the three months preceding the first visit was assessed using the Timeline Follow-back method (Sobell and Sobell, 1996). Demographics were assessed using an in-house questionnaire. Once all inclusion and no exclusion criteria were met, participants were scheduled for an fMRI visit within one-week. Participants with positive breath alcohol or urine drug screens at the first MRI visit were rescheduled. On the Monday and Wednesday following their baseline fMRI scan, participants underwent two outpatient cocaine-cue extinction sessions (described fully in Santa Ana et al., 2012), separated by one day. Participants with positive breath alcohol or urine drug screens at either extinction session were excluded from further participation in extinction sessions, but were invited to return for the one-week follow-up visit. Each cocaine-cue extinction session included 4 brief alternating blocks of pre-recorded cognitive behavioral therapy skills training and in vivo handling of paraphernalia and simulated cocaine. Participants were randomly assigned to receive either 50mg of DCS or matched placebo fifteen minutes preceding the first cue exposure block, on each day of the extinction sessions. The majority of participants (n = 27) included in the present analysis completed both cue extinction sessions. Three participants completed only the first (Monday) cue extinction session; two of these participants missed the second (Wednesday) session citing personal reasons, while the third was excluded from the second session due to a positive cocaine UDS (this participant also had a positive UDS at one-week follow-up). One week following the second cue extinction session, participants returned for a one-week follow-up appointment, which included a UDS.
2.3. Cocaine cue-reactivity paradigm
The present investigation utilized a visual cocaine cue-reactivity fMRI paradigm (Prisciandaro et al., 2012). Subjects were shown pictures of cocaine and related objects (e.g., crack pipe), neutral objects (e.g., furniture), and visual control images that lack object recognition over six 120-second epochs. Each 120-second epoch contains three 30-second blocks (cocaine images, neutral objects, control images), containing five pictures displayed for 4.8 seconds each, and one 30 second rest block (i.e., cross-hair). During the final 6-seconds of each block, participants were asked to rate their craving, from zero (“none”) to four (“severe”), using a handpad. Cocaine craving scores were computed by taking participants’ average craving rating following cocaine blocks and subtracting from it their average craving rating following neutral object blocks. Image blocks were balanced with respect to luminosity (i.e., brightness). Blocks and stimuli within blocks were presented in pseudorandom order. A previous study that used the same cocaine cue paradigm as the present study in a larger sample of cocaine users (that included all participants from the present investigation) demonstrated that cocaine versus neutral images were associated with significant activation of orbitofrontal cortex, amygdala, posterior cingulate, hippocampus, dorsolateral prefrontal cortex, superior frontal gyrus, and occipital cortex (Prisciandaro et al., 2012).
2.4. Response inhibition (i.e., go no-go) task
The response inhibition (i.e., go no-go) task was created from an existing continuous performance task (Ogg et al., 2003). The modified task consists of 20 blocks, lasting 26.25 seconds each; 10 go no-go blocks alternate with 10 fixation blocks, in which a fixation cross is presented for the duration of the block. During go no-go blocks, participants are presented with 21 letters, one at a time, for 250 milliseconds each, followed by 1 second interstimulus intervals (i.e., black screen). They are instructed to press the button underneath their index finger on their handpad as soon as they see a letter other than “X,” but to withhold a response if they see the letter “X,” which is presented on average 20% of the time. Presentation order of letters in go no-go blocks is randomized to remove confounding effects due to the overlap of hemodynamic responses.
2.5. Image acquisition
MRI data for the present investigation were collected at baseline, prior to the application of any experimental manipulation or treatment. MRI scans were performed in a Siemens 3.0T Trio (Erlangen, Germany) MR scanner with a 12-channel head coil. Following localizer and anatomical scans, the cue reactivity scan and go no-go scans were acquired using an echo-planar gradient-echo pulse sequence (TR = 2200 ms, TE = 35 ms, flip angle = 90%). Images were acquired with approximate AC-PC alignment. Each brain volume consisted of 36 transverse slices (64 × 64 matrix, 3.0 mm thickness, no gap). Voxel size was 3.0 × 3.0 × 3.0 mm3.
2.6. Image analysis
fMRI analyses were conducted using Statistical Parametric Mapping software 8 (SPM8, The Wellcome Department of Cognitive Neurology, London). Preprocessing was conducted separately for each experimental task. All volumes within a task run were realigned to the first volume. Images were stereotactically normalized into a standard space, with a resolution of 3 × 3 × 3 mm3 voxels using a Montreal Neurological Institute (MNI) template. Data were smoothed with an isotropic 8 mm Gaussian kernel and were high-pass filtered (cue exposure paradigm cutoff period = 240s; go no-go task cut-off period = 128s). Following preprocessing, fMRI data were analyzed within a general linear model (GLM) mixed effects framework. Within-task data from individual participants was analyzed with a fixed-effects GLM, with cocaine cue exposure activity modeled as a box-car function convolved with the standard canonical hemodynamic response function and no-go responses modeled as single impulse functions specified by time of onset from the beginning of the run. Six movement parameters (3 rotation values in radian and 3 translation values in millimeter) were included as covariates to control for the influence of residual head motion. First-level analyses were conducted separately for each task. For the cue exposure paradigm, each block type (i.e., cocaine images, neutral objects, visual control images, cross-hair fixation) was represented by a separate regressor and a contrast map of cocaine pictures minus neutral objects was created for each participant. For the go no-go task, all block (task, rest) and event (correct go trial, correct no-go trial, omission error, commission error) types were represented by separate regressors. A contrast map of no-go trials (i.e., correct no-go trial, commission error) minus go trials (i.e., correct go trial, omission error) was created for each participant; the no-go minus go trials contrast was selected to represent response inhibition because it is overwhelmingly the most commonly used contrast in go no-go fMRI studies (Swick et al., 2011). Following first-level analysis, the subject-specific contrasts were entered into second-level, random-effects analyses. Second-level models were conducted separately for each task. Cocaine UDS status at one-week follow-up (0 = negative, 1 = positive) and medication status (0 = placebo, 1 = DCS) were entered as explanatory variables of the task-specific fMRI contrasts at baseline. All group-level statistical maps were thresholded using Gaussian random field theory as implemented in cluster-level thresholding in SPM8 (voxelwise threshold = p < 0.01, FWE-corrected cluster threshold = p < 0.05).
2.7. Behavioral data analysis
Participants with positive cocaine UDS versus those with negative cocaine UDS at one-week follow-up were compared on subjective craving to cocaine versus neutral cues during the cocaine cue paradigm, and number of omission and commission errors during the go no-go task, using independent samples t-tests.
3. RESULTS
3.1. Participant characteristics
Thirty participants completed both the baseline fMRI scan and the one-week follow-up cocaine UDS. Of these participants, six had a positive cocaine UDS at one-week follow-up, suggesting cocaine use within the preceding 72 hours. Participants with positive versus negative cocaine UDS at one-week follow-up did not significantly differ on any demographic characteristics (see Table 1). Two participants (both with negative cocaine UDS at one-week follow-up) were excluded from analyses involving the cocaine cue exposure paradigm due to excessive head motion (i.e., ≥ 3 mm/degrees in any direction). Six participants (all with negative cocaine UDS at one-week follow-up) were excluded from analyses involving the go no-go task due to excessive head motion (n = 2) or invalid behavioral data (i.e., > 1/3 omission errors [n = 3], 100% commission errors [n = 1]).
Table 1.
Baseline characteristics by cocaine UDS outcome at one-week follow-up (n = 30)
| UDS − (n = 24) | UDS + (n = 6) | p | |
|---|---|---|---|
| Gender, % male | 83.3 | 83.3 | 1.00 |
| Age, M (SD) | 48.4 (8.7) | 41.2 (8.3) | 0.08 |
| Race, % African American | 83.3 | 83.3 | 1.00 |
| Marital status, % not married | 75.0 | 100.0 | 0.17 |
| Education, % ≥ high school | 87.5 | 66.7 | 0.22 |
| Employment, % unemployed | 91.7 | 83.3 | 0.54 |
| Smoking status, % smokers | 83.3 | 100.0 | 0.28 |
| Treatment randomization, % PLA | 54.2 | 50.0 | 0.86 |
| Cannabis dependence, % current | 8.3 | 0.0 | 0.46 |
| Alcohol dependence, % current | 21.0 | 0.0 | 0.22 |
Note. UDS = Urine Drug Screen. PLA = placebo
3.2. Cocaine cue-reactivity paradigm (n = 28)
Positive cocaine UDS at one-week follow-up was associated with increased cocaine-cue activation in bilateral occipital regions (i.e., intracalcarine cortex and lingual gyrus, with maximal activations in the right hemisphere), as well as right putamen and insula (Table 2; Figure 1). Negative cocaine UDS at follow-up was not associated with increased cocaine-cue activation in any brain regions. To ensure that our findings were not attributable to participants’ pre-baseline levels of cocaine use, we repeated the between-subjects model with % days of cocaine use for the 90 days preceding baseline as an additional covariate. Adding pre-baseline cocaine use to the model did not eliminate the obtained associations, but instead expanded the spatial extent of these associations (e.g., to include left subcortical regions; Supplemental Figure 11). In terms of subjective craving, participants with positive UDS at one-week follow-up (M = 0.92, SD = 0.21) did not significantly differ from those with negative UDS at follow-up (M = 0.93, SD = 0.75) in terms of subjective craving to cocaine minus neutral cues during the cocaine cue paradigm (t[26] = −0.05, p = 0.97).
Table 2.
Activation to cocaine versus neutral cues at baseline controlling for medication status
| Contrast | Cluster | Z Max | FWE P | Voxel | MNI (x, y, z) | Anatomy |
|---|---|---|---|---|---|---|
| UDS + > UDS − | 1 | 4.75 | 0.001 | 917 | 24, −67, 5 | R cerebral WM/intracalcarine cortex |
| 3.87 | 24, −52, 2 | R lingual gyrus | ||||
| 3.76 | −3, −85, −4 | R lingual gyrus (BA 18) | ||||
| 2 | 3.80 | 0.002 | 713 | 33, −4, 2 | R cerebral WM/putamen | |
| 3.74 | 27, −13, 11 | R putamen | ||||
| 3.65 | 36, −10, 8 | R insula |
Note: Analyses completed using cluster thresholding (z > 2.49 and FWE [Familywise Error] corrected cluster threshold of p < .05). Anatomy = most probable region identified using the Harvard-Oxford cortical and subcortical structural atlases. UDS +/UDS − = positive/negative for recent cocaine use according to cocaine UDS at one-week follow-up, Z Max = local maximum z-value, FWE P = familywise error-corrected cluster-level p value, MNI (x, y, z) = Montreal Neurological Institute coordinates for the local maximum, WM = white matter, BA = Brodmann Area.
Figure 1.
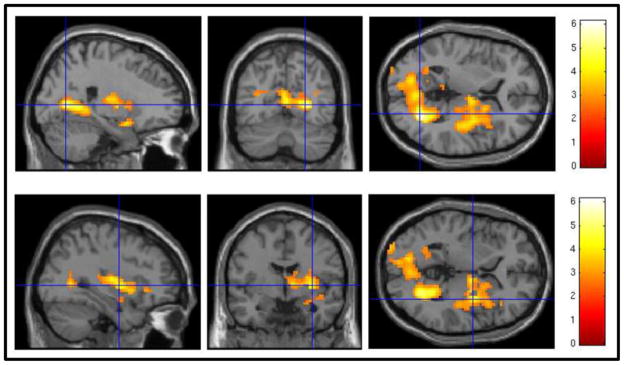
Associations between blood oxygen level dependent (BOLD) response to cocaine cues minus neutral objects and cocaine urine drug screen (UDS) status, controlling for medication status, at one-week follow-up using a voxelwise threshold of z = 2.49 and an FWE-corrected cluster threshold of p = 0.05. Positive cocaine UDS at one-week follow-up was associated with higher activation in occipital regions (top panel), as well as right putamen and insula (bottom panel).
3.3. Response inhibition (i.e., go no-go) task (n = 24)
In terms of the go no-go task, positive cocaine UDS at one-week follow-up was associated with increased activation to inhibition cues in bilateral postcentral gyri, with maximal activations in the left hemisphere (Table 3; Figure 2). Negative cocaine UDS at follow-up was not associated with increased no-go activation in any brain regions. As with the cocaine cue-reactivity paradigm analyses, we repeated the between-subjects model with % days of cocaine use for the 90 days preceding baseline as an additional covariate; once again, adding pre-baseline cocaine use to the model expanded the spatial extent of the postcentral gyrus association cluster (Supplemental Figure 22). Participants with positive UDS at one-week follow-up (omission: M = 7.50, SD = 5.61; commission: M = 10.50, SD = 5.32) did not significantly differ from those with negative UDS at follow-up (omission: M = 8.39, SD = 9.45; commission: M = 8.17, SD = 3.97) in terms of omission and commission errors during the cocaine cue paradigm (omission: t[22] = −0.22, p = 0.83; commission: t[22] = 1.15, p = 0.26).
Table 3.
Activation to no-go versus go trials at baseline controlling for medication status
| Contrast | Cluster | Z Max | FWE P | Voxel | MNI (x, y, z) | Anatomy |
|---|---|---|---|---|---|---|
| UDS + > UDS − | 1 | 3.99 | < .001 | 1216 | −42, −34, 56 | L postcentral gyrus |
| 3.95 | −30, −31, 56 | L postcentral gyrus | ||||
| 3.60 | −30, −40, 68 | L postcentral gyrus (BA 5) |
Note: Analyses completed using cluster thresholding (z > 2.52 and FWE [Familywise Error] corrected cluster threshold of p < .05). Anatomy = most probable region identified using the Harvard-Oxford cortical and subcortical structural atlases. UDS +/UDS − = positive/negative for recent cocaine use according to cocaine UDS at one-week follow-up, Z Max = local maximum z-value, FWE P = familywise error-corrected cluster-level p value, MNI (x, y, z) = Montreal Neurological Institute coordinates for the local maximum, WM = white matter, BA = Brodmann Area.
Figure 2.
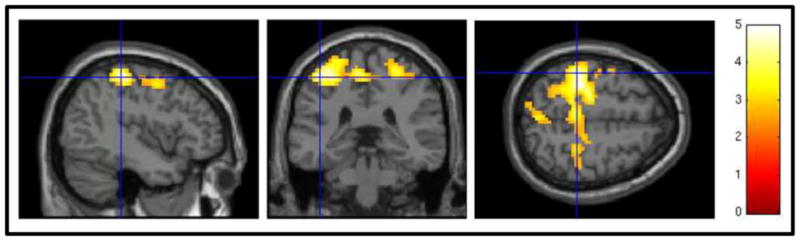
Associations between blood oxygen level dependent (BOLD) response to no-go minus go trials and cocaine urine drug screen (UDS) status, controlling for medication status, at one-week follow-up using a voxelwise threshold of z = 2.52 and an FWE-corrected cluster threshold of p = 0.05. Positive cocaine UDS at one-week follow-up was associated with higher activation in postcentral gyri.
4. DISCUSSION
The present study examined prospective associations between brain reactivity to cocaine and response inhibition cues and subsequent relapse to cocaine use, as evidenced by positive cocaine UDS. Consistent with previous studies, brain activation to cocaine cues was associated with positive cocaine UDS at one-week follow-up. Similar to findings in alcohol (Braus et al., 2001; Grusser et al., 2004) and nicotine dependence (Janes et al., 2010), elevated activation to cocaine cues in the putamen and insula were prospectively associated with cocaine relapse; these regions have been strongly implicated in habitual drug-seeking (Kalivas and O’Brien, 2008) and the conscious urge to use drugs (Naqvi et al., 2008), respectively. The only other study that has investigated prospective associations between cocaine cue reactivity and relapse found that cocaine urine toxicologies correlated with cue activation in the posterior cingulate, as well as precentral, temporal, and occipital cortices (Kosten et al., 2006). The present study similarly demonstrated an association between occipital activation to cues and cocaine relapse, but did not find associations between relapse and posterior cingulate, precentral, or temporal region cue activations. Discrepancies between the present study and that of Kosten and colleagues may be entirely due to methodological differences. For example, whereas the present study utilized images of cocaine and paraphernalia as cues, Kosten and colleagues utilized the first 30 seconds of 3-minute videotapes of cocaine use. Furthermore, all participants in Kosten study were depressed, which could have impacted their reactivity to cocaine cues.
The present study is among the first to investigate the prospective association between brain activation to response inhibition (i.e., no-go) cues and relapse to drug use in substance dependent individuals. We found evidence for these associations primarily in the left postcentral gyrus. Interestingly, although activation of the postcentral gyrus is not typically associated with response inhibition (Swick et al., 2011), it is commonly associated with response execution (Brown et al., 2012; Menon et al., 2001). Our findings suggest that, on occasions that call for response inhibition, activation of neural response execution structures (i.e., preparing to respond when one should be withholding a response) represents a risk factor for relapse in cocaine dependent individuals.
Although brain reactivity to cocaine and response inhibition cues predicted relapse, behavioral analogues of these responses (subjective craving and omission/commission errors, respectively) did not. This dissociation between neurobiological and behavioral markers is very well documented in the literature (Tiffany and Carter, 1998), and, in the case of subjective craving, may reflect the relatively poor ecological validity of laboratory cue-induced craving paradigms (Epstein et al., 2009; Preston et al., 2009). Alternatively, given the small number of individuals with positive cocaine UDS in the present study (n = 6), the lack of significant associations between craving and omission/commission errors and relapse may have been due to a lack of power to detect such effects.
Recent research has demonstrated that, when collected using ecological momentary assessment methods, self-reported craving may indeed prospectively predict drug use and relapse (Epstein et al., 2009; Preston et al., 2009). Although these findings could be seen as a challenge to the importance of investigating biomarkers of relapse, we would argue that both approaches are complementary. First, no study has demonstrated that any single predictor accounts for all, or a majority, of the variability in relapse; it is likely that a number of variables will be necessary to adequately predict relapse. Second, identification of cognitive (e.g., subjective craving) and neurological (e.g., brain activation to response inhibition cues) predictors may each provide distinct insights into how to prevent relapse. For example, whereas understanding the role of subjective craving in drug use may aid in the refinement of cognitive-behavioral strategies for preventing relapse, identifying biomarkers of relapse may aid in the development of novel pharmacologic or neurostimulatory preventative interventions.
The above conclusions should be evaluated in light of the present study’s limitations. First, as noted above, only 6 of our 30 participants had a positive cocaine UDS at one-week follow-up. Although roughly on par with other similar studies (e.g., n = 5 in Grusser et al. 2004; n = 8 in Kosten et al. 2006), and although our overall sample size was larger than most similar studies, our results should be considered preliminary. Second, the cocaine cue exposure paradigm was always administered prior to the go no-go task. Although this fixed task presentation order could have potentially produced order-effects, this concern was mitigated by two facts: 1) task presentation order was the same between groups and should therefore not differentially impact UDS-positive and UDS-negative cocaine users and 2) our data suggested that the cocaine cue exposure paradigm did not induce substantial enduring increases in cocaine craving. Third, participants were randomized to receive either DCS or placebo, although it should be noted that medication randomization was statistically controlled in the present study, medication status did not differ between UDS-positive and UDS-negative participants, and DCS did not significantly impact clinical outcomes (Santa Ana et al., 2012). However, future research should consider naturalistic designs in which substance dependent individuals are scanned and subsequently followed over time without intervention. A larger scale study designed specifically to examine neural predictors of relapse would represent a substantial contribution to the field. Identification of neural predictors of relapse may allow researchers and practitioners to delineate a priori which of their participants/patients are most likely to relapse to drug use. Such at-risk individuals could subsequently be selected for relatively more intense intervention and follow-up.
In conclusion, the present study demonstrated that baseline brain activation to cocaine and response inhibition cues were prospectively associated with relapse to cocaine use in a group of thirty cocaine-dependent individuals. Although preliminary, these results suggest that brain imaging may be a useful tool for predicting which cocaine-dependent individuals are most at risk for relapse to cocaine use and therefore require intensive intervention and follow-up.
Supplementary Material
Acknowledgments
Role of Funding Source: Funding for this study was provided by NIH grant R01 DA023188 (Brady); the NIH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. Dr. Prisciandaro was supported by NIDA F32 DA032250.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors: Dr. Brady designed the study and wrote the protocol. Dr. McRae-Clark coordinated the study implementation. Dr. Myrick and Mr. Henderson designed the imaging protocol. Dr. Prisciandaro conducted the analyses and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest: No conflict declared.
Clinical Trials Registration:D-Cycloserine Facilitation of Cocaine-cue Extinction, ClinicalTrials.gov Identifier: NCT00759473 (http://clinicaltrials.gov/ct2/show/NCT00759473).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Mann K, Heinz A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry. 2012;69:842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during Stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MRG, Lebel RM, Dolcos F, Wilman AH, Silverstone PH, Pazderka H, Fujiwara E, Wild TC, Carroll AM, Hodlevskyy O, Zedkova L, Zwaigenbaum L, Thompson AH, Greenshaw AJ, Dursun SM. Effects of emotional context on impulse control. Neuroimage. 2012;63:434–446. doi: 10.1016/j.neuroimage.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Beatty GK, Anderson RE, Kodituwakku P, Phillips JP, Lane TDR, Kiehl KA, Calhoun VD. Reduced fMRI activity predicts relapse in patients recovering from stimulant dependence. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22184. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic-diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition. New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation ofthe striatum and medial prefrontal cortex is associated with subsequent relapse inabstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Heinz A, Wrase J, Kahnt T, Beck A, Bromand Z, Grusser SM, Kienast T, Smolka MN, Flor H, Mann K. Brain activation elicited by affectively positive stimuli is associated with a lower risk of relapse in detoxified alcoholic subjects. Alcohol Clin Exp Res. 2007;31:1138–1147. doi: 10.1111/j.1530-0277.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Fredrick BB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien CO. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacol Rev. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacol. 2006;31:644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a go/nogo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller SE, Weijers HG, Boning J, Wiesbeck GA. Personality traits predict treatment outcome in alcohol-dependent patients. Neuropsychobiology. 2008;57:159–164. doi: 10.1159/000147469. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2008;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg RJ, Zou P, Allen DN, Hutchins SB, Dutkiewicz RM, Mulhem RK. Neural correlates of a clinical continuous performance test. Magn Reson Imag. 2008;26:504–512. doi: 10.1016/j.mri.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–8. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DA. Cocaine craving and use during daily life. Psychopharmacology. 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT. Brain activation to cocaine cues and motivation/treatment status. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00446.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Ana EJ, Prisciandaro JJ, Saladin ME, McRae-Clark AL, Shaftman SR, Brady KT. Effect of D-cycloserine and cue exposure therapy on physiological and subjective craving in cocaine dependence. 2012 doi: 10.1111/ajad.12191. Unpublished Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavas J, Weiller E, Hergueta T, Baker R, Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiat. 1998;59:22–33. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-44, HHS Publication No (SMA) Rockville, MD: 2012. pp. 12–4713. [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback User’s Guide: A Calendar Method for Assessing Alcohol and Drug Use. Addiction Research Foundation; Toronto: 1996. [Google Scholar]
- Swick D, Ashley V, Turken AU. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Zhiru J, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


