Abstract
Breast tumor kinase (Brk), also known as protein kinase-6 (PTK6), is a nonreceptor protein-tyrosine kinase that has a close functional relationship with the human epidermal growth factor receptor 2 (HER2). High levels of Brk were found in HER2-positive tumor specimens from patients with invasive ductal breast cancer; however, the underlying mechanism of the cooverexpression of Brk and HER2 remains elusive. In the current study, we explored the mechanism of HER2 and Brk co-overexpression in breast cancer cells by investigating the effect of overexpression and knockdown of HER2 on the level of Brk in breast cancer cells. We found that Brk was more stable in HER2-elevated cells than in control vector-transfected cells and was less stable in HER2 siRNA-treated cells than in control siRNA-treated cells, suggesting that HER2 regulates Brk protein stability. Further studies indicated that degradation of Brk involved a calpain-1-mediated proteolytic pathway and indicated an inverse relationship between the level of HER2 expression and calpain-1 activity. We found that HER2 inhibited calpain-1 activity through upregulating calpastatin, an endogenous calpain inhibitor. Silencing of HER2 downregulated calpastatin, and the downregulation could be rescued by overexpression of constitutively active MEK. Together, these data offer novel mechanistic insights into the functional relationship between Brk and HER2.
Keywords: HER2, Brk, Calpain, Breast Cancer, Proteolysis
1. Introduction
The discovery of human epidermal growth factor (EGF) receptor-2 (HER2) in the mid-1980s and the discovery of HER2's etiologic role in 20-25% of breast cancers have had a significant impact on understanding of breast cancer pathogenesis and on development of novel targeted agents targeting this protein for breast cancer treatment [1,2]. HER2 is an orphan receptor that has no known ligands and is activated mainly via homodimerization and heterodimerization with other members of the human EGF receptor (EGFR) family. Once activated, HER2 triggers several canonical signal transduction pathways downstream of growth factor receptors, including the Ras/Raf/MEK/Erk, PI3K/Akt, and STAT3 pathways, that collectively drive the development and progression of breast cancer. These downstream pathways, which often contain mutations in key molecules and can be cross-activated by other members of the EGFR family or other growth factor receptors, constitute a comprehensive signaling network involving a large number of signaling molecules, some of which still have not been fully studied. One such molecule is breast tumor kinase (Brk), also known as protein kinase-6 (PTK6), a nonreceptor protein tyrosine kinase reported to be highly expressed in approximately two-thirds of all breast cancers [3,4].
Brk is known to be associated with EGFR and potentiates EGF-induced proliferation of human mammary epithelial cells [5]. We recently reported that Brk can sustain activated EGFR signaling through inhibiting Cbl-mediated EGFR degradation and through transactivation of EGFR [6] and that Brk cooperates with HER2 and Src in regulating breast cancer cell survival and epithelial-to-mesenchymal transition [7]. Earlier work by others showed that Brk was present in high levels in HER2-positive breast cancer specimens [8,9] and enhanced HER2-induced activation of cell signaling, such as Ras/MAPK signaling, and activation of cyclin E/cdk2 complex [9]. In addition to interacting with EGFR and HER2, Brk can enhance EGF-stimulated HER3 phosphorylation by increasing the recruitment of phosphatidylinositol 3-kinase (PI3K) to HER3, resulting in Akt activation [10]. Furthermore, Brk regulates the process by which the HER3 ligand heregulin induces activation of ERK5 and p38 MAPK in breast cancer cells [4]. Together, these findings indicate a biological link between Brk and the members of the HER family and their downstream substrates, a link that may be clinically significant with respect to development of breast cancer and resistance of breast cancer to HER2-targeted treatment. Like HER2, Brk is an HSP-90 chaperone protein and is degraded through the ubiquitin-proteasomal pathway [11]. However, the direct mechanism by which Brk is co-expressed at high levels with HER2 and contributes to HER2-mediated oncogenic functions in breast cancer is poorly understood.
In the present study, we investigated the functional and regulatory interaction between Brk and HER2, and explored the mechanism underlying co-overexpression of HER2 and Brk in breast cancer cells. By examining the effect of overexpression and knockdown of HER2 on the level of Brk in breast cancer cells, we found that the co-overexpression of HER2 and Brk involves a mechanism through which HER2 enhances Brk stability and that the mechanism is linked to reduced activity of calpain-1 in HER2-overexpressing breast cancer cells. Calpain-1 belongs to a family of calcium-activated neutral cysteine proteases that mediate a calcium-dependent, non-lysosomal proteolytic pathway in cells [12-15]. Calpains have a wide range of cellular functions, including functions in apoptosis, cell cycle progression, and cell migration [12]. The activity of calpains is tightly regulated temporally and spatially not only by intracellular Ca2+ but also by calpastatin, an endogenous calpain inhibitor [16-19]. We found HER2 mediates inhibition of calpain-1 enzymatic activity through upregulation of calpastatin that inhibits calpain-1-mediated proteolysis of Brk, leading to co-overexpression of HER2 and Brk. Our findings provide novel mechanistic insights into the regulatory interaction between Brk and HER2 and establish a causal relationship between the levels of HER2 and Brk in breast cancer cells.
2. Materials and Methods
2.1. Reagents
Antibodies used in Western blotting for detecting total Akt and serine 473 (S473)-phosphorylated Akt, threonine 202/tyrosine 204 (T202/Y204)-phosphorylated extracellular signal-regulated kinase (Erk), total Src and Y416-phosphorylated Src, and poly(ADP-ribose) polymerase (PARP) were purchased from Cell Signaling Technology, Inc. Antibodies for detecting total Erk, Brk, calpastatin, and calpain-1 were purchased from Santa Cruz Biotechnology, Inc. Antibody for detecting HER2 was purchased from Calbiochem/EMD Chemicals, Inc.
The calpain-1 inhibitor N-acetyl-leucyl-leucyl-norleucinal (ALLN), the proteasomal inhibitor MG132, the PI3K inhibitor LY294002, the MEK inhibitor PD98059, and a fluorogenic calpain-1 substrate (substrate II, peptide sequence: Suc-Leu-Tyr-AMC) were purchased from Calbiochem/EMD Chemicals, Inc. Other chemicals were purchased from Sigma-Aldrich Corp.
2.2. Breast cancer cell lines and culture
Nonmalignant MCF10A mammary epithelial cells and the following breast cancer cell lines were originally purchased from American Type Culture Collection: BT474, HCC1954, SKBR3, MDA453, MDA361, ZR75B, T47D, MCF7, MDA157, MDA468, MDA435, and MDA231. The breast cancer cell lines SUM190, SUM149 and SUM102 were obtained from Dr. Steven Ethier's laboratory (the University of Michigan and the Karmanos Cancer Institute). The breast cancer cell lines BT20 and Hs578T were obtained from Dr. Mien-Chie Hung's laboratory (MD Anderson Cancer Center). MCF7-HER2 cells were created by lentiviral infection of MCF7 cells with a wild-type HER2 construct, as described previously [20]. Except for SUM102 cells, which were maintained in Ham's F12 medium supplemented with 5% fetal bovine serum (FBS), 5 μg/mL insulin, 1 μg/mL hydrocortisone, 2.5 μg/mL fungizone, and 5 μg/mL gentamicin, all cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS, 2 mmol/L glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin and cultured in a humidified atmosphere of 95% air and 5% CO2 at 37°C.
2.3. Western blot analysis
After desired treatments, cells were lysed in a lysis buffer containing 50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.5% NP40, 50 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride, 25 μg/mL leupeptin, and 25 μg/mL aprotinin and clarified by centrifugation (14,000g for 30 min at 4°C). The protein concentration of the cell lysates was determined using the Bradford Coomassie blue method (Pierce Chemical Corp.). Whole-cell lysates were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, transferred onto nitrocellulose by Western blotting, and probed with various primary antibodies and horseradish peroxidase-labeled secondary antibodies. The signals were visualized with an enhanced chemiluminescence detection kit (GE Healthcare).
2.4. Calpain enzymatic activity assay
Calpain enzymatic activity was measured as described in the literature [21]. Lysates were prepared in a calcium-free imidazole buffer (63.2 mmol/L imidazole-HCl, pH 7.3, 10 mmol/L 2-mercapto-ethanol, 1 mmol/L EDTA, and 10 mmol/L EGTA) plus 10 mmol/L digitonin. Equal amounts of cell lysates (500 μg of protein per sample) and fluorescently labeled calpain substrate II (25 μmol/L) were incubated in the calcium-free imidazole buffer or in a buffer containing calcium (63.2 mmol/L imidazole-HCl, pH 7.3, 10 mmol/L 2-mercapto-ethanol, and 10 mmol/L CaCl2) at 37°C for 30 min in triplicate sets. Fluorescence generated after the reaction was measured with a fluorometer (FLUOstar Omega, BMG LABTECH) at an excitation wavelength of 380 nm and an emission wavelength of 460 nm. Calpain activity was expressed as the difference in relative fluorescent units (RFU) per milligram of cell lysate protein between the lysates incubated in calcium-free and the lysates incubated in calcium-containing buffer.
2.5. RNA interference
HER2 small interfering RNA (siRNA) oligonucleotide duplexes (sense sequence #1 GGGAAACCUGGAACUCACC; complement sequence #1 GGUGAGUUCCAGGUUUCCC; sense sequence #2 GGACAUCUUCCACAAGAAC; complement sequence #2 GUUCUUGUGGAAGAUGUCC) were purchased from Ambion/Applied Biosystems. Brk siRNA oligonucleotide duplexes (sense sequence #1 AAGGUGAUUUCUCGAGACAAC; complement sequence #1 GUUGUCUCGAGAAAUCACCUU; sense sequence #2 UCUUGAGAGCUUGGCCUUAUU; complement sequence #2 UAAGGCCAAGCUCUCAAGAUU) were purchased from Dharmacon/Thermo Fisher Scientific. Transfection of siRNA oligonucleotides was performed in a six-well plate (1×105 cells/well) with Lipofectamine 2000 (Invitrogen, Inc.) using the methods recommended in the manuals provided by the manufacturer. Knockdown of targeted gene expression was examined 48 h after siRNA transfection by Western blotting with specific antibodies. β-Actin expression was used as an internal control.
HER2 lentiviral short hairpin RNA (shRNA) constructs (HER2 shRNA #1 target DNA sequence: GCCTTCGACAACCTCTATTAC; HER2 shRNA #2 target DNA sequence: TGTCAGTATCCAGGCTTTGTA; HER2 shRNA #3 target DNA sequence: GAGATCACAGGTTACCTATAC) and Brk lentiviral shRNA constructs (Brk shRNA #1 target DNA sequence: ACCTCTCCCATGACCACAATA; Brk shRNA #2 target DNA sequence: TACCTCTCCCATGACCACAAT; Brk shRNA #3 target DNA sequence: GTGCAGGAAAGGTTCACAAAT) were purchased from Sigma-Aldrich Corp. HER2 and Brk shRNA constructs were co-transfected with lentiviral packaging plasmids into HEK-293T cells. Targeted breast cancer cells were transduced with the resulting lentiviral particles for 24 h and then cultured in selecting medium with 2 μg/mL puromycin for 1 week. Knockdown of HER2 and Brk expression levels was confirmed by Western blotting with specific antibodies.
3. Results
3.1. HER2 overexpression upregulates Brk in breast cancer cells
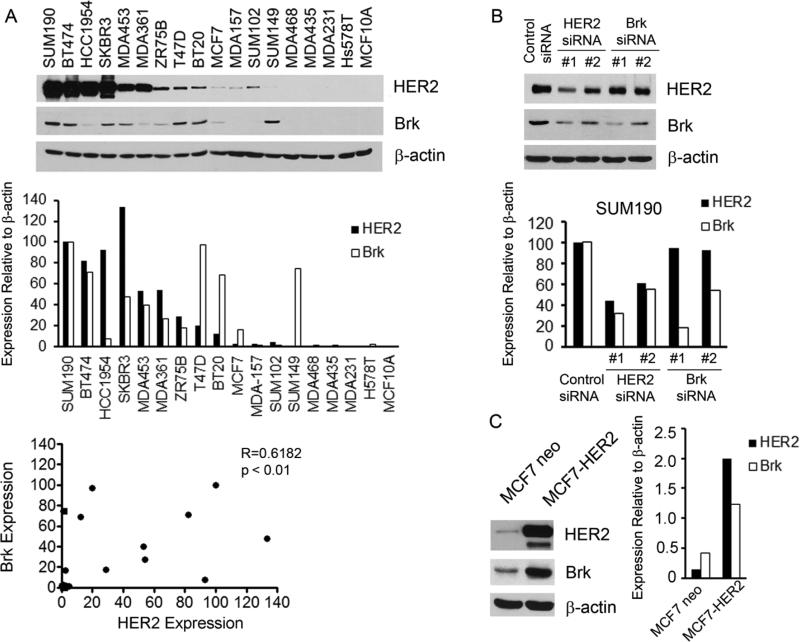
We examined the expression of Brk and HER2 in a panel of 17 breast cancer cell lines plus nonmalignant MCF10A mammary epithelial cells using Western blot analysis. Although there were exceptions, Brk was frequently co-expressed with HER2 in the breast cancer cells (Fig. 1A). Quantitative correlative analysis of the data by using the Spearman's method indicated that the correlation of the levels of HER2 and Brk was statistically significant (R = 0.6182, p < 0.01).
Fig. 1.
Correlation between the levels of Brk and HER2 in breast cancer cells. (A) A panel of 17 breast cancer cell lines and MCF10A nonmalignant mammary epithelial cells were analyzed by Western blotting with antibodies directed against Brk and HER2. β-actin served as a protein loading reference (top panel). Expression of Brk and HER2 relative to the internal control β-actin was quantified by ImageJ densitometry software and presented in a histogram (middle panel). Spearman analysis was used to analyze the correlation between Brk expression and HER2 expression in these cell lines (bottom panel). (B) SUM190 cells were transiently transfected with each of two different HER2- or Brk-specific siRNAs or control siRNA for 48 h. The cells were then lysed and analyzed by Western blotting with the indicated antibodies. Levels of Brk and HER2 relative to β-actin were quantified and presented as in A. (C) MCF7 cells stably expressing high levels of HER2 (MCF7-HER2) or a control vector (MCF7-neo) were lysed and analyzed by Western blotting with the indicated antibodies. Levels of Brk and HER2 relative to β-actin were quantified and presented as in A.
To determine whether there was a causal relationship between the levels of Brk and HER2, we examined the effect of HER2 knockdown on the level of Brk in SUM190 cells, which expressed high levels of both HER2 and Brk, and the effect of HER2 overexpression on the level of Brk in MCF7 cells, which expressed low levels of both HER2 and Brk. Knockdown of HER2 expression with two individual HER2 siRNAs was accompanied by decreases in the level of Brk in the SUM190 cells, and the decreases in Brk quantitatively correlated with the levels of HER2 knockdown by the two different HER2 siRNAs. In contrast, knockdown of Brk with two siRNAs, which resulted in slightly different levels of Brk knockdown in the cells, had no impact on the level of HER2, suggesting that the level of HER2 is subjected to the change in the level of Brk (Fig. 1B). Results from the reverse experiment in MCF7 cells with (MCF7-HER2) and without (MCF7-neo) overexpression of HER2 showed that MCF7-HER2 cells expressed a markedly higher level of Brk than did MCF7-neo cells (Fig. 1C). Together, these data indicate that the level of Brk may be regulated by HER2, but not vice versa, in breast cancer cells.
3.2. HER2 upregulates Brk through enhancing Brk protein stability
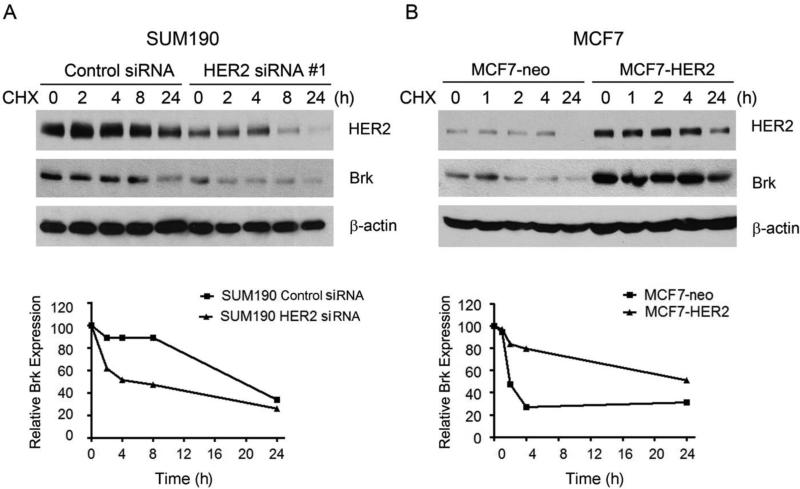
To understand how the Brk level may be regulated by HER2, we first compared the degradation of Brk in SUM190 cells with and without knockdown of HER2. Consistent with the findings in Fig. 1C, the basal level of Brk was lower in HER2 siRNA-treated SUM190 cells than in control siRNA-treated cells. Following treatment of the cells with cycloheximide (CHX), an inhibitor of protein biosynthesis, the levels of Brk declined in a time-dependent manner. In the control siRNA-treated cells, the level of Brk was stable until sometime between 8 h and 24 h after CHX treatment, similar to the stability of HER2 (Fig. 2A). In the HER2 siRNA-treated cells, the level of Brk started to decline as early as 2 h after CHX treatment (Fig. 2A). Similar results were observed in MCF7 cells with and without overexpression of HER2 (Fig. 2B). Consistent with the findings in Fig. 1C, the basal level of Brk was higher in MCF7-HER2 cells than in MCF7-neo cells. In the MCF7-neo cells, a marked decrease in Brk protein level was seen as early as 2 h after CHX treatment. In contrast, in the MCF7-HER2 cells, a marked decrease in Brk protein level was not seen within 4 hours after CHX treatment, and a substantial level of Brk remained at 24 h after CHX treatment.
Fig. 2.
Role of HER2 in Brk protein stability. (A) SUM190 cells were transfected with a HER2-specific siRNA#1 or control siRNA for 48 h. The cells were then exposed to 10 μM cycloheximide (CHX) in 0.5% FBS medium for the indicated times. The levels of HER2 and Brk were detected by Western blotting with the indicated antibodies. β-actin was used as a loading control. Levels of HER2 and Brk relative to the internal control β-actin were quantified and plotted as shown. (B) MCF7-neo and MCF7-HER2 cells were exposed to CHX (10 μM) in 0.5% FBS medium for the indicated times. The levels of HER2 and Brk were detected by Western blotting with the indicated antibodies. β-actin was used as a loading control. Levels of HER2 and Brk relative to the internal control β-actin were quantified and plotted as shown.
Together, these findings indicate that Brk is more stable in HER2-overexpressing cells than in HER2-low expressing cells and that HER2 may regulate Brk protein stability.
3.3. HER2 enhances Brk stability by inhibiting calpain-1-mediated proteolysis of Brk
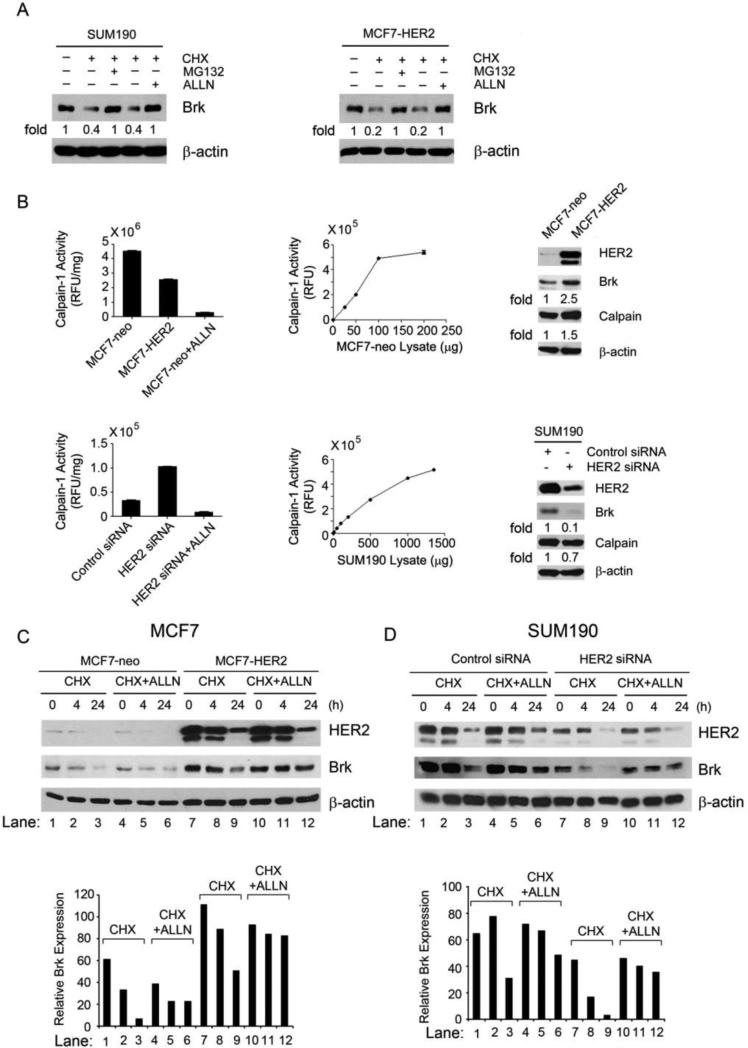
To test our hypothesis that there exists a direct link between HER2 overexpression and Brk stability and that the link may involve calpain-1, a ubiquitously expressed cysteine proteinase that plays an important role in signal transduction by catalyzing controlled proteolysis of its targeted proteins [15], we treated SUM190 and MCF7-HER2 cells with ALLN, a calpain-1 inhibitor, along with MG132, a proteasomal inhibitor. Both MG132 and ALLN inhibited degradation of Brk after CHX treatment (Fig. 3A), suggesting that Brk may be proteolytically cleaved through the calpain-1 pathway in addition to the ubiquitin-proteasomal pathway that was recently reported [11]. The enzymatic activity of calpain-1 was significantly lower in MCF7 cells after HER2 overexpression and was significantly higher in SUM190 cells after HER2 knockdown, but the level of calpain-1 was not significantly changed after HER2 knockdown (Fig. 3B). In fact, the level of calpain-1 was slightly increased in MCF7-HER2 cells compared with MCF7 cells and slightly decreased in SUM190 cells after HER2 knockdown (Fig. 3B), suggesting that there was a feedback regulation of calpain-1 expression as a result of the changes in HER2 expression in these cells. This inverse relationship between the levels of HER2 expression and calpain-1 activity suggests a HER2 level-dependent inhibition of calpain-1 enzymatic activity.
Fig. 3.
HER2-mediated inhibition of Brk proteolytic cleavage through the calpain-1 pathway. (A) SUM190 and MCF7-HER2 cells were untreated, treated with CHX (50 μg/mL) alone, or treated with CHX (50 μg/mL) plus proteasome inhibitor MG132 (5 μM) or calpain-1 inhibitor ALLN (20 μM) in 0.5% FBS medium as shown for 24 h. The cells were then lysed for Western blotting with the indicated antibodies. (B) Equal amounts (50 μg) of MCF7-neo and MCF7-HER2 cell lysates and equal amounts of control siRNA-treated and HER2 siRNA-treated SUM190 cell lysates (each in triplicate) were incubated with the fluorescently labeled calpain substrate II at 37°C for 30 min. Calpain activity was determined as described in Materials and Methods and plotted. The lysates of MCF7-neo cells and HER2 siRNA-treated SUM190 cells mixed with 25 μM ALLN were used as negative controls for calpain activity in vitro. Linear ranges of the enzymatic reaction with series of increasing amounts of MCF7-neo and SUM190 cell lysates are shown. The same lysates were also analyzed by Western blotting with the antibodies shown (right panels). (C, D) Pairs of MCF7-neo and MCF7-HER2 cells (C) and control siRNA-treated and HER2 siRNA-treated SUM190 cells (D) were untreated, treated with CHX (50 μg/mL) alone, or treated with CHX (50 μg/mL) plus ALLN (10 μM) for 4 h and 24 h, respectively. After the treatment, the cells were lysed for Western blotting with the indicated antibodies. Levels of Brk relative to the internal control β-actin were quantified and plotted as shown.
We next examined the differences in Brk degradation and its sensitivity to calpain-1 inhibition with ALLN in MCF7 cells with and without HER2 overexpression and in SUM190 cells with and without HER2 knockdown. Consistent with the findings shown in Figs 1C and 2B, the basal level of Brk was higher in MCF7-HER2 cells than in MCF7-neo cells (Fig. 3C, lanes 7 and 10 versus lanes 1 and 4 and the bar graph), and the degradation of Brk after CHX treatment was slower in MCF7-HER2 cells than in MCF7-neo cells (Fig. 3C, lanes 7-9 versus lanes 1-3 and the bar graph). Brk degradation was inhibited by ALLN in both MCF7-neo and MCF7-HER2 cells (lanes 1-3 versus lanes 4-6 and lanes 7-9 versus lanes 10-12), but the inhibition was more effective in MCF7-HER2 cells (lanes 10-12 versus lanes 4-6 and the bar graph), indicating that calpain-1 activity plays a more important role in degrading Brk in HER2-overexpressing cells than in the counterpart cells. It is noteworthy that the degradation of HER2 was not affected by treatment of the cells with ALLN (Fig. 3C, lanes 10-12 versus lanes 7-9).
In SUM190 cells, the basal level of Brk was lower after HER2 knockdown (Fig. 3D, lanes 7 and 10 versus lanes 1 and 4 and the bar graph). Degradation of Brk after CHX treatment was faster in HER2 siRNA-treated cells than in control siRNA-treated cells (Fig. 3D, lanes 7-9 versus lanes 1-3 and the bar graph). Brk degradation was inhibited by ALLN in both control siRNA- and HER2 siRNA-treated cells, but the inhibition was more effective in control siRNA-treated cells (Fig. 3D, lanes 4-6 versus lanes 10-12 and the bar graph), again indicating that calpain-1 activity plays a more important role in degrading Brk in HER2-overexpressing cells than in the counterpart cells. Together, along with the in vitro data shown in Fig. 3B, these findings provide important in vivo evidence that Brk was degraded by calpain-1-mediated proteolysis and that the calpain-1-mediated Brk proteolysis was less active in cells expressing high levels of HER2 than in cells expressing low levels of HER2.
3.4. HER2 inhibits calpain-1 activity through upregulating the level of calpastatin
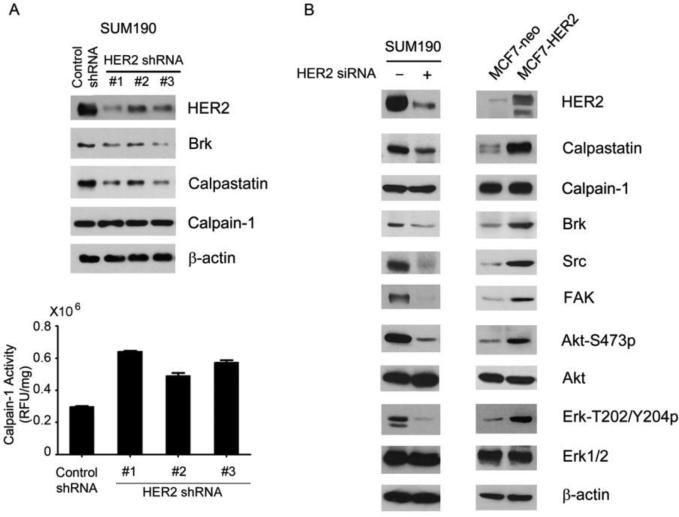
To unveil the regulatory mechanism through which HER2 inhibits calpain-1 enzymatic activity, we tested our hypothesis that HER2 can upregulate the level of calpastatin. As shown in Fig. 4A, the level of calpastatin was markedly reduced after knockdown of HER2 in SUM190 cells by each of three different HER2-specific lentiviral shRNAs. The reduced level of calpastatin was accompanied by a reduced level of Brk, unchanged level of calpain-1 protein, but reduced calpain-1 activity. To further validate these findings, we compared the levels of calpastatin and several other relevant proteins between MCF7-neo and MCF7-HER2 cells and between HER2 siRNA-treated and control siRNA-treated SUM190 cells. As shown in Fig. 4B, not only levels of Brk but also levels of two other calpain-1 substrates, Src [22] and focal adhesion kinase (FAK) [23], were increased in HER2-elevated cells and decreased in HER2-downregulated cells, whereas the level of calpain-1 was not affected by the level of HER2 in these cells. The levels of calpastatin and phosphorylated forms of the HER2 downstream substrates Akt and Erk showed changes corresponding to the levels of HER2 in the cells. Together, these results indicate that HER2 inhibits calpain-1 activity through increasing calpastatin expression, leading to upregulation of proteins such as Brk, Src, and FAK that are subjected to regulation by calpain-1-mediated proteolysis.
Fig. 4.
Regulation of calpastatin level by HER2. (A) SUM190 cells were transduced with three different HER2 lentiviral shRNAs respectively or control lentiviral shRNA for 48 h and then cultured in selecting medium with 2 μg/mL puromycin for 1 week. The cells were lysed for Western blotting with the indicated antibodies (top), and for calpain activity assay (bottom). (B) Pairs of control siRNA-treated or HER2 siRNA-treated SUM190 cells and MCF7-neo and MCF7-HER2 cells were lysed for Western blotting with the indicated antibodies.
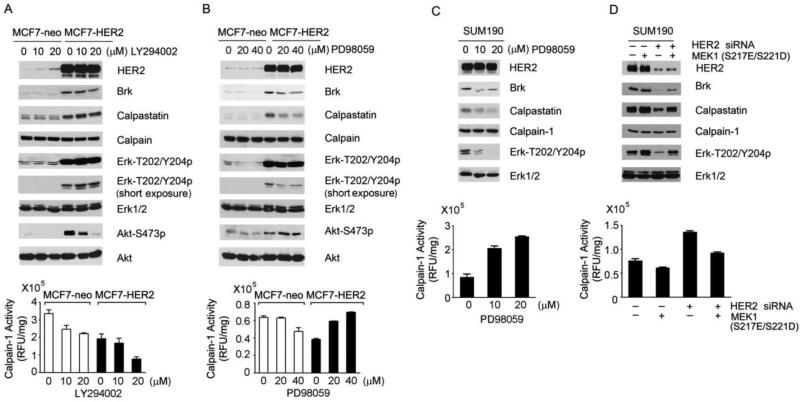
To identify the molecular players downstream of HER2 through which HER2 upregulates the level of calpastatin, we assessed the impact of inhibition of the PI3K/Akt pathway with LY294002 and inhibition of the MEK/Erk pathway with PD98059 on the levels of calpastatin and calpain-1 and the levels of calpain-1 activity. Treatment of MCF7-neo and MCF7-HER2 cells with LY294002 or PD98059 specifically inhibited the respective pathways, as shown by inhibition of Akt phosphorylation but not Erk phosphorylation by LY294002 and by inhibition of Erk phosphorylation but not Akt phosphorylation by PD98059 (Fig. 5A and B). Inhibition of the MEK/Erk pathway, but not inhibition of the PI3K/Akt pathway, decreased the levels of calpastatin and Brk protein in MCF7-HER2 cells (Fig. 5A and B). Consistent with this finding, PD98059 treatment elevated calpain-1 enzymatic activity in MCF7-HER2 cells in a dose-dependent manner but did not have such an effect in MCF7-neo cells. In fact, the enzymatic activity of calpain-1 seemed inhibited by PD98059 at high doses in MCF7-neo cells for unknown reasons (Fig. 5B).
Fig. 5.
Upregulation of calpastatin by HER2 in a MEK/Erk-dependent manner. (A and B). MCF7-neo and MCF7-HER2 cells were treated with the indicated doses of LY294002 (A) or PD98059 (B) in 0.5% FBS DMEM for 24 h. Cell lysates were prepared and analyzed by Western blotting with the indicated antibodies and by calpain activity assay (bottom). (C) SUM190 cells were treated with PD98059 in 0.5% FBS DMEM for 24 h. Cell lysates were prepared and analyzed by Western blotting with the indicated antibodies and by calpain activity assay (bottom). (D) SUM190 cells were transfected with control siRNA or HER2 siRNA for 24 h and then transfected with constitutively active MEK1 or control vector as shown for an additional 24 h. Cells were lysed for Western blotting with the indicated antibodies.
We further confirmed these observations in SUM190 cells. PD98059 treatment of SUM190 cells strongly inhibited Erk phosphorylation, leading to downregulation of Brk and calpastatin levels and upregulation of calpain-1 activity (Fig. 5C). To provide further causal evidence supporting a role of the MEK/Erk pathway in mediating HER2-induced upregulation of calpastatin, we performed a rescue experiment in which a constitutively active MEK1 mutant (S217E/S221D) was overexpressed in SUM190 cells (Fig. 5D). Co-transfection of the SUM190 cells with the constitutively active MEK1 construct counteracted the effects of HER2 knockdown by HER2 siRNA, including downregulation of Brk and calpastatin levels, inhibition of Erk phosphorylation, and increase in calpain-1 activity (Fig. 5D).
Together, these data support a model in which HER2 upregulates calpastatin level via activating the MEK/Erk pathway and calpastatin in turn inactivates calpain-1 enzyme activity, leading to an increase in the Brk protein level.
4. Discussion
In the work described in this paper, we investigated the mechanism underlying the previously reported co-overexpression of HER2 and Brk in breast cancer [8,9,24]. We recently reported that Brk cooperates functionally with HER2 and Src in regulating breast cancer cell survival and epithelial-to-mesenchymal transition [7]. In the current study, we identified a novel mechanism by which HER2 regulates Brk stability. We found that Brk is a substrate of calpain-1. We also found evidence that proteolysis of Brk by calpain-1 was inversely correlated with the level of HER2 expression in breast cancer cells and that the low enzymatic activity of calpain-1 in HER2-overexpressing cells was caused by upregulation by HER2 of the level of the endogenous calpain inhibitor calpastatin. The inverse correlation between the level of HER2 and calpain-1 proteolytic activity resulted in upregulation of Brk in HER2-overexpressing breast cancer cells.
Brk was reported to be highly expressed in two-thirds of all breast cancers, whereas HER2 is overexpressed in only 20-25% of all breast cancers [1]. Therefore, most likely there exist other mechanisms besides the one we have identified here that contribute to the high level of Brk expression in breast cancer. For example, in our study, we found that SUM149 cells expressed no detectable HER2 and T47D cells expressed a very low level of HER2 but the level of Brk in these cells was as high as the level of Brk in HER2-overexpressing cell lines, such as SUM190 and BT474. Apparently, the mechanisms underlying the high level of Brk expression in these cells are unrelated to HER2; these mechanisms may be due to gene amplification or overexpression of Brk or alteration in the degradation pathways of Brk—for example, the proteasomal pathway [11]. We also found that not all HER2-overexpressing breast cancer cells expressed a high level of Brk; for example, HCC1954 cells had a high level of HER2 but a low level of Brk. However, in our panel of 17 breast cancer cell lines, our data indicated a statistically significant correlation between high levels of HER2 and high levels of Brk, and our mechanistic studies demonstrated that HER2 regulated Brk degradation via upregulation of calpastatin that inhibited calpain-1-mediated proteolysis of Brk. Cell lines that do not fit the pattern of correlation of high HER2 levels with high Brk levels, such as HCC1954 cells, may have defects in signaling pathways or regulatory mechanisms leading to failure of HER2-mediated Erk signaling to upregulate calpastatin or failure of calpastatin to inhibit calpain-1-mediated proteolysis.
Our data showed clearly that knockdown of HER2 downregulated calpastatin whereas overexpression of HER2 upregulated calpastatin in the SUM190 and MCF7 breast cancer cell lines. To the best of our knowledge, based on searching the PubMed database, ours is the first report showing a causal relationship between the levels of HER2 and calpastatin in breast cancer cells. A question remaining, however, is how HER2-mediated activation of Erk leads to upregulation of calpastatin. Understanding of how calpastatin expression is regulated in human cells remains elusive in general. Four promoters were reported to direct calpastatin expression [25]. Structural analysis of the calpastatin promoter sequences revealed binding motifs for several transcription factors, including SP1, AP-1, GATA-1, SRY, and NF-κB, in the calpastatin promoter region [26]. Further study is needed to identify which transcription factors are involved in regulating the expression of calpastatin after activation of the MEK/Erk pathway by HER2. Another question that was not directly addressed in our study is the potential role of calpain-2 in the cell models we examined. It was previously reported that MEK/Erk-mediated cell signaling may lead to phosphorylation of calpain-2 and subsequent activation of the enzyme [27]. In our study, however, we found that overexpression of HER2 elevated the level of activated MEK/Erk and led to inhibition of total calpain enzymatic activity, whereas knockdown of HER2 enhanced total calpain enzymatic activity (Fig. 3B); therefore, the likelihood that calpain-2 contributes to the total calpain activity is minimal if not zero. Furthermore, we found that treatment of HER2-overexpressing breast cancer cells with the MEK inhibitor PD98059 resulted in an increase rather than a decrease in calpain activity (Fig. 5B), again suggesting that calpain-2 is unlikely to play a significant role in the total calpain enzymatic activity that we detected.
The calpain-calpastatin system regulates a variety of cellular functions involving many protein substrates inside cells. Therefore, it is important to stress that the mechanism by which HER2 upregulates calpastatin that we identified in our study may not be limited in its impact to Brk; rather, this mechanism may be a general mechanism by which HER2 exerts its oncogenic functions in breast cancer by regulating an array of important oncogenic partners to mediate the functions of HER2. Indeed, our data showed that HER2-mediated calpastatin upregulation correlated not only with upregulation of Brk but also with upregulation of other important proteins, such as Src and FAK (Fig. 4B).
Calpastatin, which inhibits calpain, has been implicated in lymphovascular invasion in breast cancer. It was reported that both calpastatin mRNA and protein levels were significantly directly associated with the occurrence of lymphovascular invasion of breast cancer [28]. Paradoxically, it was reported that a high level of calpain-1 may confer resistance to the anti-HER2 antibody trastuzumab through cleavage of HER2 [29], and a study using patient specimens supports a direct association between the level of calpain-1 expression and relapse-free survival in breast cancer patients treated with trastuzumab [30]. These data, which seemingly contradict our findings, may be due to a feedback regulatory mechanisms inside the cells: HER2 and calpain-1 may regulate each other, such that while overexpression of HER2 inhibits calpain-1 activity via upregulation of calpastatin, calpain-1 may overcome the effect of HER2 by cleaving the intracellular cytoplasmic domain of HER2 [29].
Lastly, it should also be mentioned that although our data support a model wherein HER2 elevates Brk through upregulation of calpastatin and inhibition of calpain-1 activity via the MEK/Erk pathway, our data do not exclude the possibility that HER2 upregulates Brk via HER2-kinase-activity-independent mechanisms, such as via HER2-Brk direct interactions, since Brk was found to be co-immunoprecipitated with HER2 and vice versa when both HER2 and Brk were experimentally overexpressed [9]. It should also be mentioned that our current work focused on the mechanism of Brk posttranslational regulation by HER2-mediated cell signaling; our work does not exclude the possibility that overexpression of HER2 or HER2-mediated cell signaling may also regulate the level of Brk at a gene transcriptional and/or protein translational level.
5. Conclusions
Our dada support a novel paradigm wherein HER2 upregulates the level of calpastatin in a MEK/Erk-dependent manner and calpastatin in turn inhibits the enzymatic activity of calpain-1, leading to a rise in Brk protein level due to inhibition of calpain-1-mediated proteolytic cleavage of Brk. Our findings provide novel mechanistic insights into the co-overexpression of HER2 and Brk in breast cancer.
Highlights.
HER2 overexpression upregulates Brk in breast cancer cells
HER2 upregulates Brk through enhancing Brk protein stability
HER2 enhances Brk stability by inhibiting calpain-1-mediated proteolysis of Brk
HER2 inhibits calpain-1 activity through upregulating the level of calpastatin
Acknowledgments
Financial Support: US Department of Defense Congressionally Directed Medical Research Programs Awards W81XWH-06-1-0544 and W81XWH-07-1-0526 (to Z.F.), The Breast Cancer Research Foundation (to Z.F.), NIH grant 5R01CA129036 (to Z.F.), and Cancer Center Support Grant CA016672 from the National Cancer Institute. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Abbreviations
- ALLN
N-acetyl-leucyl-leucyl-norleucinal
- Brk
breast tumor kinase
- CHX
cycloheximide
- DMEM
Dulbecco's modified Eagle's medium
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- Erk
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- FBS
fetal bovine serum
- HER2
human epidermal growth factor receptor 2
- OD
optical density
- PARP
poly(ADP-ribose) polymerase
- PI3K
phosphatidylinositol 3-kinase
- PTK6
protein kinase-6
- SDS
sodium dodecyl sulfate
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship contributions
MA and ZF designed the experiments, generated the figures, and wrote the manuscript. MA, YL, and SQ carried out the experiments. MA, YL, SQ, and ZF analyzed the data. All authors approved the final version of the manuscript.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Barker KT, Jackson LE, Crompton MR. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15:799–805. doi: 10.1038/sj.onc.1201241. [DOI] [PubMed] [Google Scholar]
- 4.Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 2007;67:4199–4209. doi: 10.1158/0008-5472.CAN-06-3409. [DOI] [PubMed] [Google Scholar]
- 5.Kamalati T, Jolin HE, Mitchell PJ, Barker KT, Jackson LE, Dean CJ, Page MJ, Gusterson BA, Crompton MR. Brk, a breast tumor-derived non-receptor protein-tyrosine kinase, sensitizes mammary epithelial cells to epidermal growth factor. J. Biol. Chem. 1996;271:30956–30963. doi: 10.1074/jbc.271.48.30956. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Lu Y, Liang K, Hsu JM, Albarracin C, Mills GB, Hung MC, Fan Z. Brk/PTK6 sustains activated EGFR signaling through inhibiting EGFR degradation and transactivating EGFR. Oncogene. 2012;31:4372–4383. doi: 10.1038/onc.2011.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ai M, Liang K, Lu Y, Qiu S, Fan Z. Brk/PTK6 cooperates with HER2 and Src in regulating breast cancer cell survival and epithelial-to-mesenchymal transition. Cancer Biol. Ther. 2013;14 doi: 10.4161/cbt.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Born M, Quintanilla-Fend L, Braselmann H, Reich U, Richter M, Hutzler P, Aubele M. Simultaneous over-expression of the Her2/neu and PTK6 tyrosine kinases in archival invasive ductal breast carcinomas. J. Pathol. 2005;205:592–596. doi: 10.1002/path.1720. [DOI] [PubMed] [Google Scholar]
- 9.Xiang B, Chatti K, Qiu H, Lakshmi B, Krasnitz A, Hicks J, Yu M, Miller WT, Muthuswamy SK. Brk is coamplified with ErbB2 to promote proliferation in breast cancer. Proc. Natl. Acad. Sci. U S A. 2008;105:12463–12468. doi: 10.1073/pnas.0805009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamalati T, Jolin HE, Fry MJ, Crompton MR. Expression of the BRK tyrosine kinase in mammary epithelial cells enhances the coupling of EGF signalling to PI 3-kinase and Akt, via erbB3 phosphorylation. Oncogene. 2000;19:5471–5476. doi: 10.1038/sj.onc.1203931. [DOI] [PubMed] [Google Scholar]
- 11.Kang SA, Cho HS, Yoon JB, Chung IK, Lee ST. Hsp90 rescues PTK6 from proteasomal degradation in breast cancer cells. Biochem. J. 2012;447:313–320. doi: 10.1042/BJ20120803. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki H, Kawashima S. Regulation of the calpain-calpastatin system by membranes (review) Mol. Membr. Biol. 1996;13:217–224. doi: 10.3109/09687689609160599. [DOI] [PubMed] [Google Scholar]
- 13.Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- 14.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 15.Campbell RL, Davies PL. Structure-function relationships in calpains. Biochem. J. 2012;447:335–351. doi: 10.1042/BJ20120921. [DOI] [PubMed] [Google Scholar]
- 16.Murachi T. Intracellular regulatory system involving calpain and calpastatin. Biochem. Int. 1989;18:263–294. [PubMed] [Google Scholar]
- 17.Maki M, Bagci H, Hamaguchi K, Ueda M, Murachi T, Hatanaka M. Inhibition of calpain by a synthetic oligopeptide corresponding to an exon of the human calpastatin gene. J. Biol. Chem. 1989;264:18866–18869. [PubMed] [Google Scholar]
- 18.Murachi T, Takano E, Maki M, Adachi Y, Hatanaka M. Cloning and expression of the genes for calpains and calpastatins. Biochem. Soc. Symp. 1989;55:29–44. [PubMed] [Google Scholar]
- 19.Asada K, Ishino Y, Shimada M, Shimojo T, Endo M, Kimizuka F, Kato I, Maki M, Hatanaka M, Murachi T. cDNA cloning of human calpastatin: sequence homology among human, pig, and rabbit calpastatins. J. Enzyme Inhib. 1989;3:49–56. doi: 10.3109/14756368909030363. [DOI] [PubMed] [Google Scholar]
- 20.Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol. Cancer Ther. 2003;2:1113–1120. [PubMed] [Google Scholar]
- 21.Edelstein CL. Calpain activity in rat renal proximal tubules. An in vitro assay. Methods Mol. Biol. 2000;144:233–238. doi: 10.1385/1-59259-050-0:233. [DOI] [PubMed] [Google Scholar]
- 22.Oda A, Druker BJ, Ariyoshi H, Smith M, Salzman EW. pp60src is an endogenous substrate for calpain in human blood platelets. J. Biol. Chem. 1993;268:12603–12608. [PubMed] [Google Scholar]
- 23.Cooray P, Yuan Y, Schoenwaelder SM, Mitchell CA, Salem HH, Jackson SP. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 1996;318(Pt 1):41–47. doi: 10.1042/bj3180041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aubele M, Auer G, Walch AK, Munro A, Atkinson MJ, Braselmann H, Fornander T, Bartlett JM. PTK (protein tyrosine kinase)-6 and HER2 and 4, but not HER1 and 3 predict long-term survival in breast carcinomas. Br. J. Cancer. 2007;96:801–807. doi: 10.1038/sj.bjc.6603613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raynaud P, Jayat-Vignoles C, Laforet MP, Leveziel H, Amarger V. Four promoters direct expression of the calpastatin gene. Arch. Biochem. Biophys. 2005;437:69–77. doi: 10.1016/j.abb.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Melloni E, Averna M, Stifanese R, De TR, Defranchi E, Salamino F, Pontremoli S. Association of calpastatin with inactive calpain: a novel mechanism to control the activation of the protease? J. Biol. Chem. 2006;281:24945–24954. doi: 10.1074/jbc.M601449200. [DOI] [PubMed] [Google Scholar]
- 27.Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, Blair HC, Wells A. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol. Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storr SJ, Mohammed RA, Woolston CM, Green AR, Parr T, Spiteri I, Caldas C, Ball GR, Ellis IO, Martin SG. Calpastatin is associated with lymphovascular invasion in breast cancer. Breast. 2011;20:413–418. doi: 10.1016/j.breast.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni S, Reddy KB, Esteva FJ, Moore HC, Budd GT, Tubbs RR. Calpain regulates sensitivity to trastuzumab and survival in HER2-positive breast cancer. Oncogene. 2010;29:1339–1350. doi: 10.1038/onc.2009.422. [DOI] [PubMed] [Google Scholar]
- 30.Storr SJ, Woolston CM, Barros FF, Green AR, Shehata M, Chan SY, Ellis IO, Martin SG. Calpain-1 expression is associated with relapse-free survival in breast cancer patients treated with trastuzumab following adjuvant chemotherapy. Int. J. Cancer. 2011;129:1773–1780. doi: 10.1002/ijc.25832. [DOI] [PubMed] [Google Scholar]