Abstract
Green tea polyphenol epigallocatechin gallate (EGCG) is a strong anti-oxidant that has previously been shown to reduce the number of plaques in HIV-infected cultured cells. Modified EGCG palmitoyl-EGCG (p-EGCG), is of interest as a topical antiviral agent for Herpes Simplex Virus (HSV-1) infections. This study evaluated the effect of p-EGCG on HSV-infected Vero cells. Results of cell viability and cell proliferation assays indicate that p-EGCG is not toxic to cultured Vero cells and show that modification of the green tea polyphenol epigallocatechin gallate (EGCG) with palmitate increases the effectiveness of EGCG as an antiviral agent. Furthermore, p-EGCG is a more potent inhibitor of Herpes Simplex Virus 1 (HSV-1) than EGCG and can be topically applied to skin, one of the primary tissues infected by HSV. Viral binding assay, plaque forming assay, PCR, real-time PCR, and fluorescence microscopy were used to demonstrate that p-EGCG concentrations of 50 µM and higher block the production of infectious HSV-1 particles. p-EGCG was found to inhibit HSV-1 adsorption to Vero cells. Thus, p-EGCG may provide a novel treatment for HSV-1 infections.
Keywords: HSV-1, Vero cells, EGCG, palmitoyl-EGCG
Introduction
Herpes (oral infection with Herpes simplex virus type 1 (HSV-1) or genital infection with HSV type 2 (HSV-2)) represents one of the most common infectious diseases in humans (Spear et al. 2006; Xu et al. 2006). In the United States (US), between 1999 and 2004, 57.7% of surveyed individuals ages 14 – 49 were seropositive for HSV-1 and 17% were seropositive for HSV-2 (Xu et al. 2006). Each year in the US, half a million people show new symptoms of HSV infections and the number of first time reported cases increases annually (Stanberry 2006). Therefore, controlling the spread of HSV is considered a significant public health issue (Stanberry et al. 2000).
Green tea polyphenols (GTPs) are a major component of extracts of green tea leaves from the Camellia sinensis plant. The most abundant GTP (that can accumulate to concentrations up to 1 mg/ml) in green tea is epigallocatechin gallate (EGCG), an antioxidant (Kanaka et al. 1989; Sharangi 2009; Sueoka et al. 2001). The US Food and Drug Administration has classified EGCG as a safe compound (Paterson and Anderson 2005) and it has been shown to have a number of beneficial effects, including antiviral activity (Ciesek et al. 2011; Hauber et al. 2009; He et al. 2011; Ho et al. 2009; Morfin and Thouvenot 2003; Sharangi 2009; Williamson et al. 2006; Yamaguchi et al. 2002). Importantly, EGCG has been shown to inhibit HSV-1 and HSV-2 infection of Vero cells (Isaacs et al. 2008). Inhibition of HSV infection by EGCG was concentration dependent and only effective prior to virus adsorption; it did not affect viral production. However, it has been reported that EGCG damaged the viral envelope of HSV-1 (Isaacs et al. 2008; Kanaka et al. 1989).
A major advantage of EGCG as a potential antiviral agent is that it is non-toxic, and can be consumed or applied topically (Paterson and Anderson 2005). However, EGCG is unstable in aqueous solution and readily oxidizes, resulting in a loss of activity (Chen et al. 2009; Chen et al. 2003). It has been proposed that fatty acid-modified polyphenols could be effective HSV antiviral agents that could be formulated in lipophilic preparations (Chen et al. 2009). EGCG lipid esters are 24-fold more effective than EGCG as inhibitors and inactivators of the influenza virus (Mori et al. 2008) and are therefore candidate HSV antiviral agents for topical application.
Here, we test the effect of palmitoyl-EGCG (p-EGCG) as compared to EGCG on HSV-1 infection of Vero cells, hypothesizing that p-EGCG will be more effective than EGCG at inhibiting HSV-1 with palmitoylation increasing the affinity of EGCG for the viral envelope (Isaacs et al. 2008; Kanaka et al. 1989).
Materials and Methods
Cells culture maintenance
Vero cells were purchased from ATCC (Manassas, VA) and were cultured until confluent in Dulbecco’s Minimal Essential Media (DMEM) with 5% Fetal Bovine Serum (FBS) and 1 µg/ml gentamicin at 37 °C and 5% CO2.
HSV-1 UL46 virus maintenance
To facilitate monitoring of the in vitro HSV-1 viral life cycle the HSV-1 UL46 virus (purchased from ATCC (Manassas, VA)) was used (Willard 2002). This virus has a green fluorescent protein (GFP) gene fused to the sequence of the C-terminus of the viral protein (UL46-GFP) UL46 under the control of the UL46 promoter that encodes the tegument protein VP11/12 (Willard 2002). Passage of virus was performed in T25 flasks and cells were allowed to reach complete cytopathic effect (CPE). The media was then collected, centrifuged to remove cellular debris, and the supernatant containing virus was stored at −80 °C.
Preparation of green tea polyphenol solutions
EGCG (>90%) was purchased from Pulimeidi Biotechnology Co., Ltd. (Hangzhou, China), and mono-palmitoyl-EGCG (p-EGCG) was a gift from Dr. Kunihiro Kaihatsu, Department of Organic Fine Chemicals, Institute of Scientific and Industrial Research, Osaka University, Osaka, Japan. EGCG dissolved in DMEM media and palmitoyl-EGCG dissolved in 100% ethanol were each used at concentrations of 12.5, 25, 50, 75, and 100 µM.
Observation of cell morphology
Cell morphology was assessed using an ACCU-Scope 3002 microscope with an attached camera by comparing treated and untreated samples. Vero cells were plated in 6-well plates, grown for 24 hours, and then different concentrations of palmitoyl-EGCG were added to the wells. After one hour, the palmitoyl-EGCG was removed by aspiration and the cells were washed with PBS. Fresh media was added to the wells, and cells were examined for morphological changes after an additional 24 hours of incubation.
Cell viability assay
Vero cells were plated in 6-well plates and after 24 hours different concentrations of p-EGCG were added to each well. After one hour, the polyphenols were aspirated and the cells were washed with PBS. DMEM media was put back into each well and cells were incubated for 24 hours. Cells were trypsinized and harvested. Then the cells were counted using a hemocytometer and trypan blue, which only stains dead cells blue. The viability was determined by the proportion of viable cells to control levels at different treatments. The result was illustrated as relative cell viability with the control as 100% viable.
Cell proliferation assay
Vero cells were plated into 96-well plates; after 24 hours, cultures of 70–80% confluent cells were treated with different concentrations of EGCG (12.5 µM, 25 µM, 50 µM, 75 µM) and palmitoyl-EGCG for 1 hour. Polyphenols were then aspirated and 100 µl of fresh DMEM was added to each well. Twenty-four hours later, cell proliferation was determined using a tetrazolium reduction-based kit (G5421, Promega Corp.). The formation of soluble formazan from [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) was measured by spectrophotometric determination of absorption at 490 nm using a plate reader (Akkarawongsa et al. 2009). The cell proliferation assay was carried out in triplicate. The relative cell proliferation was determined by the proportion of absorbance at 490 nm of treated cells to the control levels (considered as 100% proliferation) at different concentrations.
Viral titer determination using plaque assay
HSV-1 virions were treated with different concentrations of EGCG or palmitoyl-EGCG (12.5 µM, 25 µM, 50 µM, 75 µM) for 1 hour at 37 °C. No EGCG or p-EGCG was added to the control plate. Treated HSV-1 virions were then serially diluted from 10−1 to 10−7 fold prior to infection.
Vero cells were plated in 6-well plates and allowed to reach confluence. Cells in different wells were then infected with different dilutions of 100 µl HSV-1 and allowed to adsorb for 1 hour at 37 °C under 5% CO2. Viruses that had not been adsorbed were then aspirated. Plates were then overlaid with a nutrient medium-containing agar and incubated. Plaques were visualized by staining cells with crystal violet, observed, and counted within 50 hours. The plaque assay was carried out in triplicate.
Fluorescence microscopy study
Cells were grown on glass cover slips and allowed to reach confluence. They were then infected with either control HSV-1 or HSV-1 (MOI = 1) previously treated with 75 µM p-EGCG for 1 hour. Cells without treatment served as negative controls. Non-adsorbed virus was then removed by aspiration and the cells were washed with PBS followed by addition of fresh media. After a period of 8, 10 or 12 hours, cells were stained with 300 µl of 300 nM DAPI (4,6-diamidino-2-phenylindole) stain for 5 minutes at 37 °C in the dark. Cells were then fixed with a 1:1 acetone and methanol solution for 15 minutes at −20 °C. The cover slip containing cells were then glued to the slide using a drop of nail polish. Cells were then visualized under a Zeiss AxioVision fluorescence microscope with a Hamamatsu ORCA-ER digital camera.
Binding assay
The binding assay was performed at 4 °C, a temperature that allows the virus to bind to cellular receptors but not enter the cells. Thus, the only mechanism that a potential inhibitor can disrupt in this assay is virus binding to the cell. Viruses were treated with 75 µM of p-EGCG for one hour prior to performing the assay. Vero cells were plated in 6-well plates and allowed to reach confluence. The plates were then removed from the incubator and were left at room temperature for about 15 minutes. All the plates were put on ice for 15 min and incubated at 4 °C for 10 min. One hundred µl of p-EGCG treated or control viruses were used to infect the cells and plates were incubated at 4 °C for 1 hour to allow virus to bind to the cells. The unbound viruses were removed by washing wells 3 times with cold PBS. Plates were overlaid with a nutrient medium-containing agar and incubated. Plaques were visualized as described in plaque assay. The binding assay was carried out in triplicate.
DNA extraction from HSV-1 infected cells
Cells were grown on 60 mm plates and allowed to reach confluence. Cells were then infected with HSV-1 treated with EGCG, p-EGCG, or neither for 1 hour. After adsorption, cells were washed with PBS and fresh media was added. After 12 hours (Koyama and Adachi 1997; Wang et al. 2010), cells were trypsinized and DNA was extracted using the Qiagen Dneasy Blood & Tissue Kit (Qiagen Sciences, Germantown, MD, USA) following the protocol provided by the manufacturer for cultured cells. DNA concentration was then measured by using a NanoDrop™ 1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
Primer design and Polymerase chain reaction (PCR) amplification of viral genes
Three sets of primers were designed to prime different regions of the HSV-1 genome based on published sequences. Primers were designed to amplify the HSV-1 US6 (encoding glycoprotein D), HSV-1 GFP, or HSV-1 UL46 (encoding VP11/VP12) genes. The sequences, melting temperature (Tm) and size of amplicons of forward and reserve primers are list in Table 1. 100 ng of DNA extracted from HSV-1/Vero cells was added to each PCR reaction. Standard PCR amplification was performed in 25 µl reactions with an initial denaturation at 95 °C for 2 minutes followed by 30 cycles of denaturation at 95 °C for 30 seconds, annealing at 60 °C for 1 minute and extension at 72 °C for 30 seconds followed by a final extension period at 72°C for 10 minutes. Confirmation of the correct amplicon size was determined by 1% agarose gel electrophoresis and ethidium bromide staining. The densities of the amplicons were analyzed under UV light using Kodak Image Station 440CF (Perkin Elmer Life Science, Waltham, MA). Amplicons were also verified by sequencing using 3130 GENETIC ANALYZER sequencer (Applied Biosystems, Carlsbad, CA) to confirm the proper amplification.
Table 1.
The sequences, Tm and size of amplicons of the designed primers used in PCR for analysis of HSV-1/Vero cell DNA.
| Primers | Target genes |
Nucleotide sequence (5’ to 3’) | Tm (°C) |
Amplicon (nt) |
|---|---|---|---|---|
| gD1f | HSV-1 US6 | AGACGTCCGGAAACAACCCTACAA | 64.6 | 752 |
| gD1r | ACACAATTCCGCAAATGACCAGGG | 64.6 | ||
| GFPf | HSV-1 GFP | TGACCCTGAAGTTCATCTGCACCA | 64.6 | 717 |
| GFPr | AACTCCAGCAGGACCATGTGAT | 62.7 | ||
| VP11/12f | HSV-1 US46 | ACCAAGCCTTGATGCTCAACTCCA | 64.6 | 957 |
| VP11/12r | ACAACACGGTTCCCGAGAGTTTGA | 64.6 |
Real-time quantitative polymerase chain reaction of genomic HSV-1 DNA
A set of primers that amplify the HSV-1 gene US6 (encoding glycoprotein D) was designed for use in real-time polymerase chain reaction. This set includes 5’-CAACCCTACAACCTGACCATC-3’ for the forward primer and 5’-TTGTAGGAGCATTCGGTGTAC-3’ for the reverse primer to prime an approximate product size of 100 nucleotides. Real-time quantitative PCR was carried out according to the manufacturer’s protocol for SYBR Green Comparative Ct method. The samples were run on ABI StepOnePlus Real-Time PCR System (Applied Biosystems; Carlsbad, CA). The real-time PCR analysis was carried out in triplicate including negative controls (no template).
Statistical analyses
All assays were performed in triplicates and the data were analyzed using one way Analysis of Variance (ANOVA) (p<0.05) by SPSS.
Results
EGCG and p-EGCG concentrations up to 75 µM have no significant effect on Vero cell morphology
Vero cells were exposed to 0–75 µM of EGCG and palmitoyl-EGCG for only 1 hour. Cells were observed 48 hours later with phase-contrast microscopy. No significant changes in cell morphology were observed at any tested concentration of EGCG or palmitoyl-EGCG (p-EGCG) (data not shown). Thus, Vero cells appear to tolerate 1 hour exposure to EGCG and p-EGCG at concentrations up to 75 µM.
Cell viability assay shows that p-EGCG does not reduce Vero cell viability
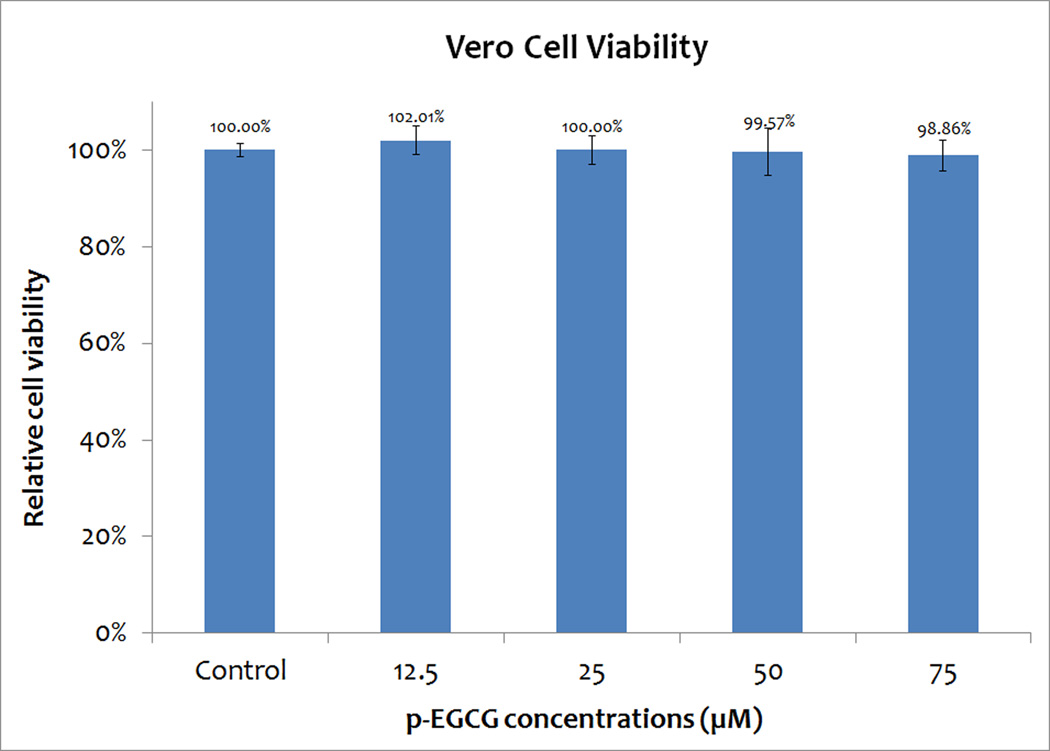
The cell viability was then determined by using trypan blue and hemocytometer direct cell count to detect the effect of p-EGCG on Vero cells. Similar numbers of cells were recovered at all concentrations of EGCG tested. The viability was determined as the percentage of viable (non-stained) cells at different treatments, compared to the control level. The results are shown in Figure 1. Figure 1 shows a minimum of 98.86% of the cells remain viable after one hour of treatment with p-EGCG at concentrations up to 75 µM. The viability of the treated cells was similar to the control. As the concentration of p-EGCG is increased, the percentage of cell death does not increase. Therefore, concentrations up to 75 µM p-EGCG can be used to treat HSV-1 and study its inhibitory effects.
Figure 1.
Cell viability of Vero cells treated with different concentrations of p-EGCG. The numbers represent the mean of three replicates and the y-error bars represent SD.
Cell proliferation assay reveals that p-EGCG, like EGCG, is not toxic to Vero cells
To determine if Vero cells exposed to various concentrations of EGCG or p-EGCG for one hour, followed by 48 hours of EGCG/p-EGCG-free incubation, could proliferate, cellular metabolism was measured using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Although we expected Vero cells to respond to p-EGCG in a dose-dependent manner, cell proliferation experiments revealed that exposure to all concentrations of p-EGCG inhibited proliferation by ~10%, which is less than the effect on proliferation levels seen with EGCG (Figure 2). These results are consistent with the minor decrease in cell viability (p< 0.05) seen by direct counting of p-EGCG treated cells (Figure 1). Thus, Vero cells exposed to high concentrations of EGCG and p-EGCG are competent for cell proliferation, suggesting that these compounds are not toxic to Vero cells.
Figure 2.
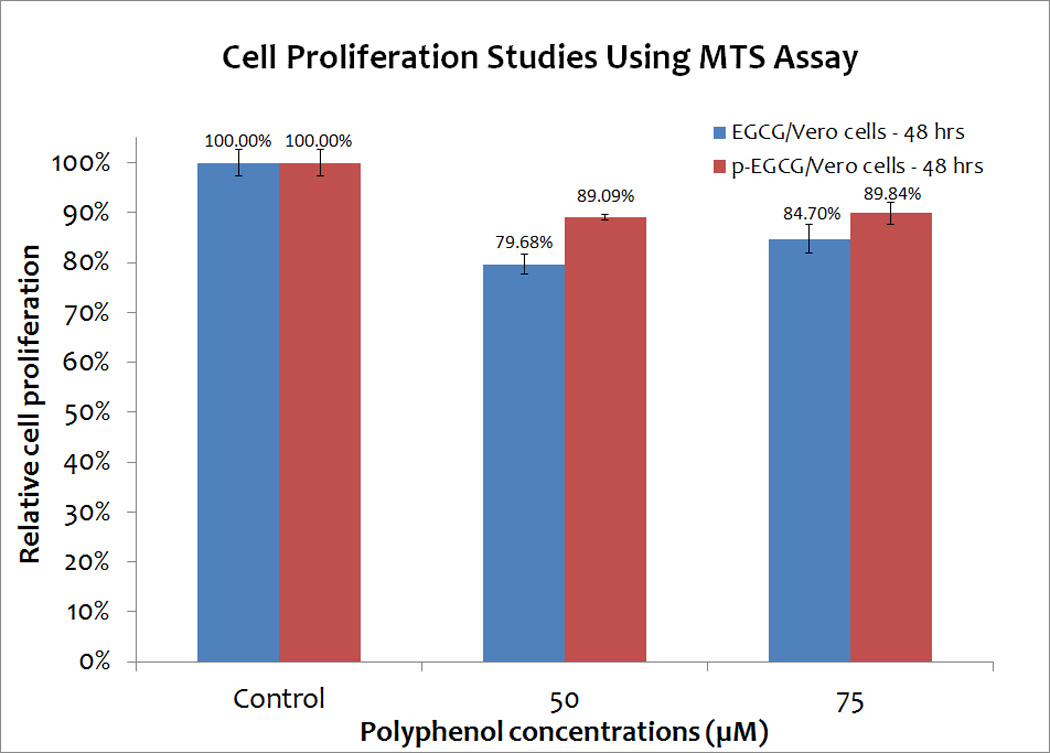
Cell proliferation studies of Vero cells treated with different concentrations (0, 50 and 75 µM) of EGCG and p-EGCG using MTS assay. The means of results of triplicate tests of EGCG treated samples (blue) and p-EGCG treated samples (red) represent the average of triplicate trials. The y-error bars represent SD.
p-EGCG reduces HSV-1 titers more effectively than EGCG
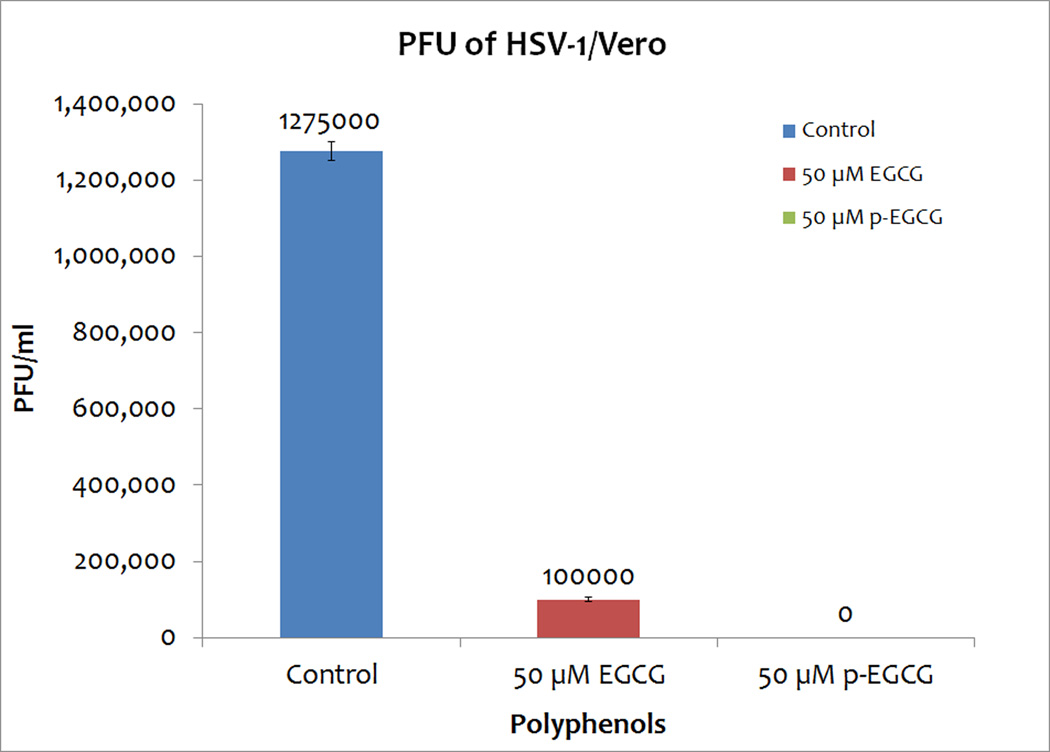
The effect of 1 hour exposure to 50 µM EGCG or palmitoyl-EGCG on HSV-1 titers was determined using a plaque assay. Treatment of a viral suspension with 50 µM EGCG decreased PFU/ml by 92.15 ± 0.15% (Figure 3). Remarkably, treatment with 50 µM p-EGCG resulted in no detectable plaques; that is, a greater than 99% reduction in plaque formation as shown in Figure 3. Thus, these results confirm our hypothesis that the palmitoylation of EGCG would increase its antiviral activity.
Figure 3.
PFU of HSV-1/Vero, HSV-1 treated 50 µM EGCG/Vero, HSV-1 treated 50 µM p-EGCG/Vero. The numbers represent the mean of triplicates and the y-error bars represent SD.
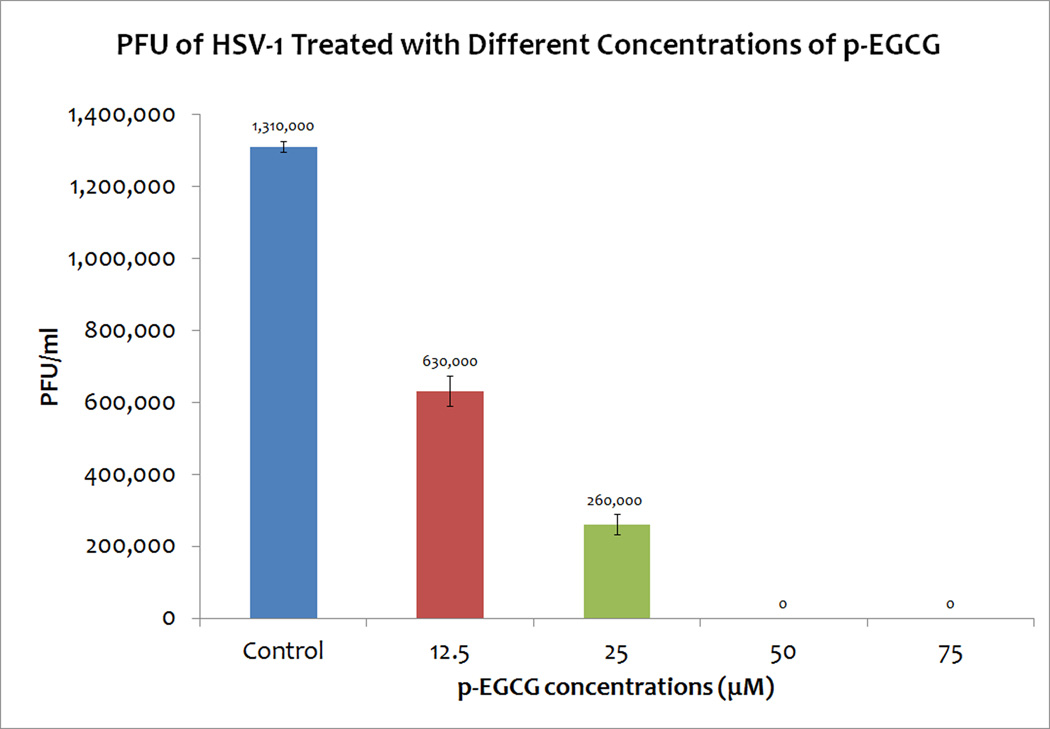
To determine what concentrations of p-EGCG effectively inhibit HSV-1, virion suspensions were treated for 1 hour at 37 °C with different concentrations of p-EGCG; treated virions were then used to infect Vero cell monolayers and the resulting titer was determined with a plaque assay. As shown in Figure 4 (repeated in triplicate), exposure of virion particles to 12.5 µM p-EGCG caused a 51.89±2.66% decrease in HSV-1 PFU; exposure to 25 µM p-EGCG caused a 80.16±1.38% decrease in HSV-1 PFU, and exposure to p-EGCG at concentrations above 50 µM resulted in a >99% decrease in titer. The plaque forming units were significantly different from the control (p< 0.05). Collectively, these experiments demonstrated that non-toxic concentrations of EGCG or p-EGCG can effectively inhibit the ability of HSV-1 to complete its lytic cycle. Since both 50 and 75 M concentrations of p-EGCG resulted in a >99% decrease in titer and were found to be non-toxic to Vero cells, we focused on the 75 µM concentration of p-EGCG in subsequent experiments.
Figure 4.
PFU of HSV-1 treated with different concentrations of p-EGCG. The numbers represent the mean of triplicate trials and the y-error bars represent SD.
Fluorescence microscopy confirms the effectiveness of EGCG and p-EGCG against HSV-1 infection of Vero cells
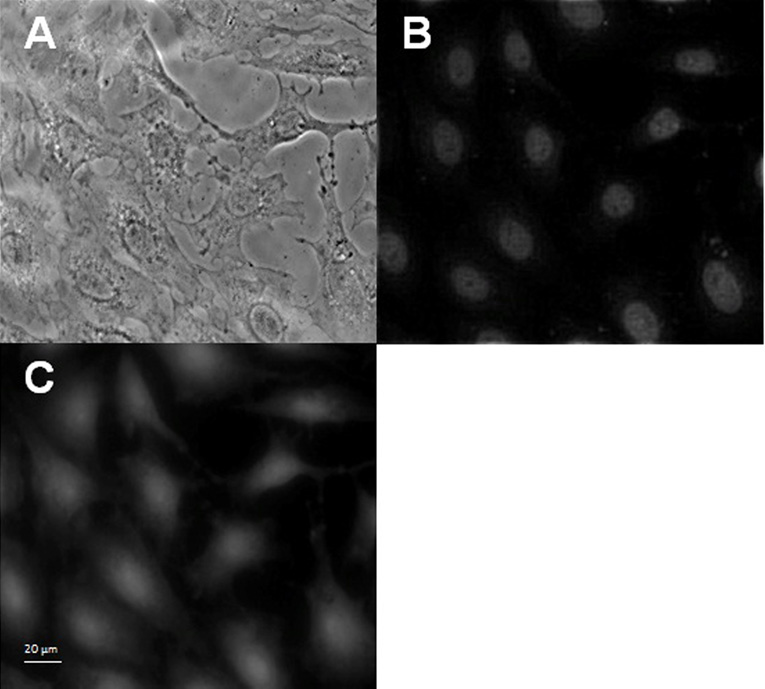
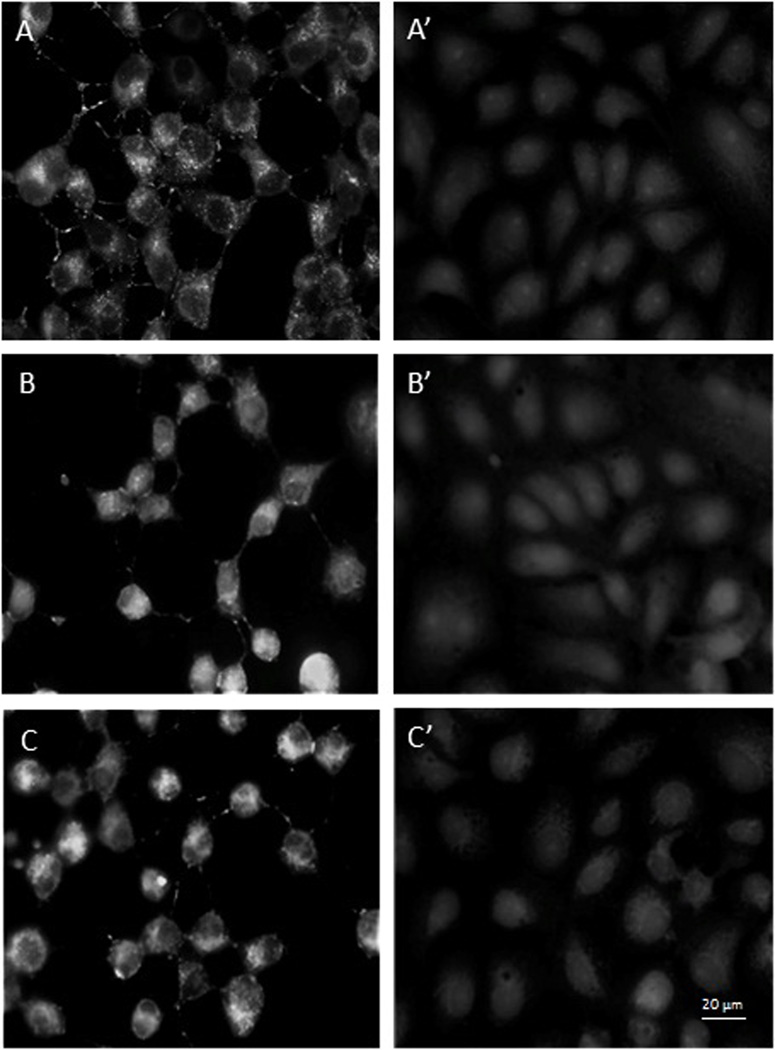
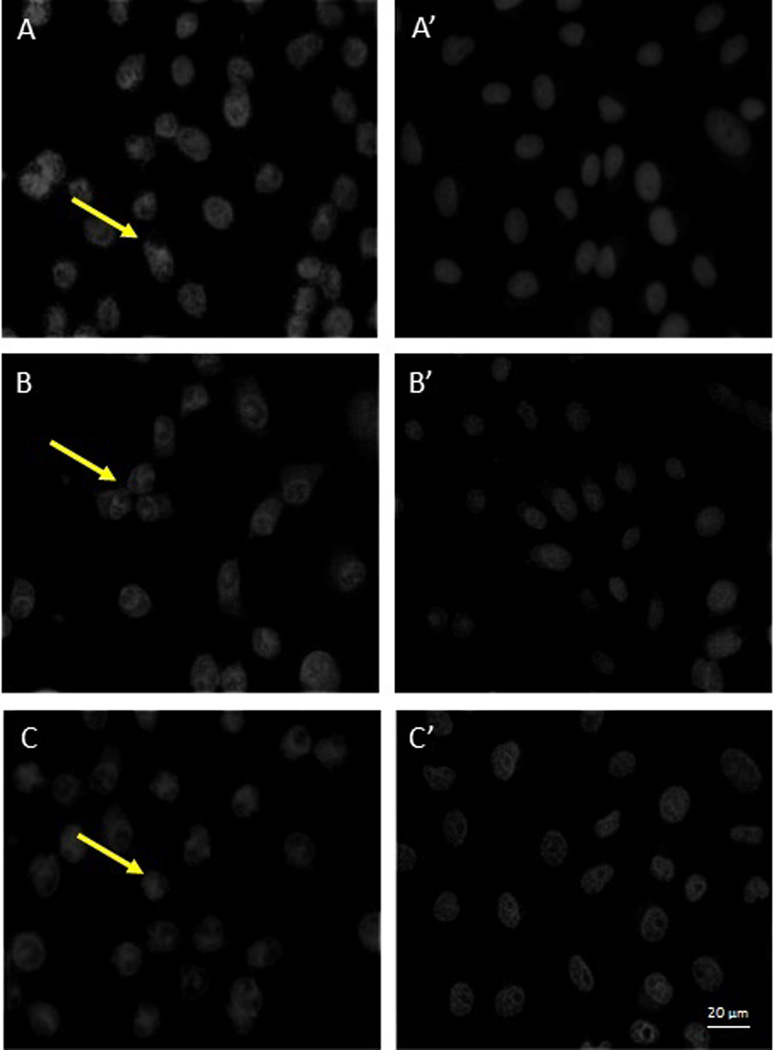
To determine if a stage of the viral replication cycle is blocked by EGCG and p–EGCG treated HSV-1, we observed the localization of tegument-GFP to see if viral particles were formed. The tegument connects the HSV-1 capsid to the host-derived viral envelope and thus represents completion of one of the last stages of the viral reproductive cycle. To this end, Vero cells infected with untreated or treated (75 µM p-EGCG for 1 hour) HSV-1 virions were examined with fluorescence microscopy at 8, 10 and 12 hours post-infection and compared to uninfected cells. The samples were stained with DAPI and observed for changes in cell nuclei and for the presence of GFP-labeled HSV-1 virions. Non-infected Vero cells showed a typical flattened “cobblestone” epithelial pattern of growth, the DAPI-stained nuclei had smooth margins with no evident granules; green fluorescent granules (viral particles) were absent as shown in Figure 5.1. In contrast, Vero cells 8 to 12 hours post-infection with untreated HSV-1 showed significant morphological changes typical of lytic viral infection (Figure 5.2). A significant level of GFP expression was observed, and there were numerous fluorescent clusters of viral particles. GFP positive granules could be observed through 12 hours post-infection and looked very similar at 8 or 10 hours post infection. The cells were rounded, and the nuclei showed significant granulation and loss of margins. The extent of nuclear granulation and margin loss continued to increase through 12 hours post infection (Figure 5.3). The morphology of cells infected with virions treated with 75 µM p-EGCG closely resembled non-infected cells at all time points. The cells maintained their flattened cobblestone appearance, and the nuclei remained intact. Relatively few GFP-positive granules could be seen. Together, these observations indicate that treatment of HSV-1 virus particle with 75 µM p-EGCG prevented completion of the lytic cycle in Vero cells.
Figure 5.
Fluorescence microscopic observations of control Vero cells and p-EGCG treated HSV-1 infection of Vero Cells. 5.1. Control Vero cells monolayer (400×). (A) Phase contrast; (B) DAPI stain; (C) GFP. 5.2. GFP expression and 5.3. DAPI stain at 8–12 hours post-infection for HSV-1 (+/− p-EGCG) of Vero cells at (A) 8 hours no p-EGCG (A’) 8 hours + p-EGCG; (B) 10 hours no p-EGCG; (B’) 10 hours + p-EGCG; (C) 12 hours no p-EGCG; (C’) 12 hours + p-EGCG. The arrows show the granulation and loss of margins in the HSV-1 infected cells.
Binding assay
To determine if p-EGCG is able to inhibit the binding of HSV-1 to Vero cells, a binding assay was carried out to allow the virus to bind to cellular receptors but not to enter the cells. Attached viral particles can later enter the cells after incubation at higher temperature, and they can complete the lytic cycle. The results (repeated in triplicate) indicated that p-EGCG is able to inhibit >99% of binding. This suggests that p-EGCG is able to block viral glycoprotein(s) and efficiently inhibit the binding of HSV-1 to host receptors (Figure 6).
Figure 6.
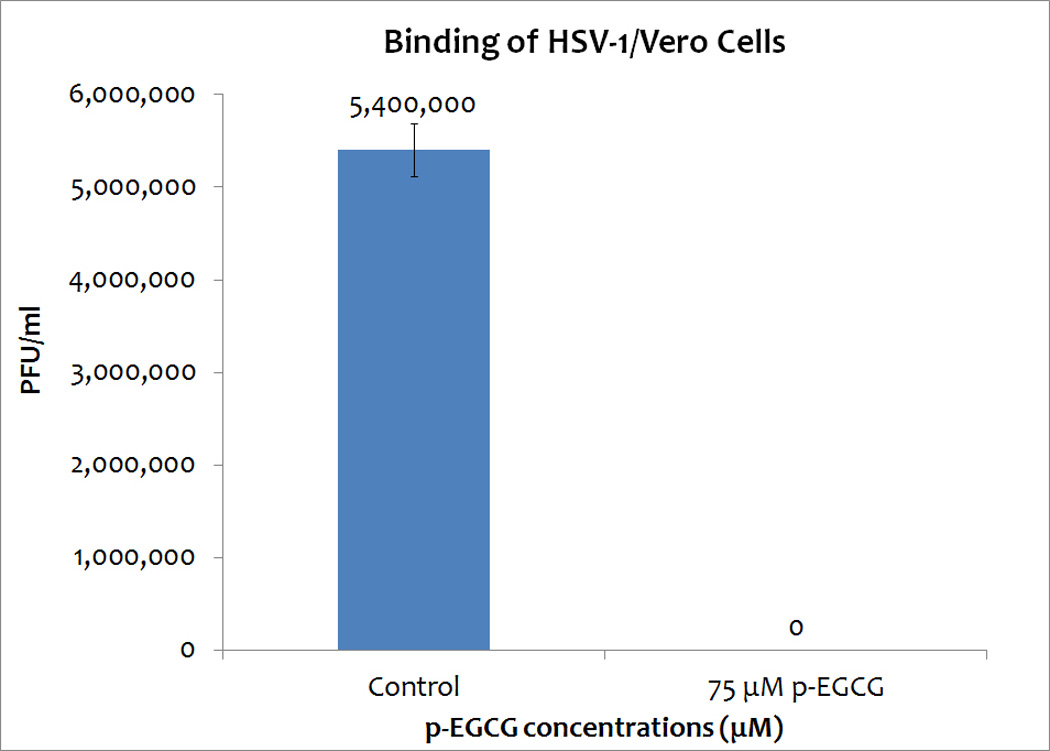
Binding assay of HSV-1, with or without 75 µM p-EGCG, with Vero cells. The numbers represent the mean of three trials and the y-error bars represent SD.
PCR amplification of HSV-1 genes in EGCG or p-EGCG treated Vero cells suggests that the replication of the viral genes encoding glycoprotein D, GFP and VP11/12 is reduced
To determine if EGCG and p-EGCG treatment resulted in the production of fewer viral genomes, PCR was used to measure the relative levels of viral DNA using three different HSV-1 genes. PCR products from each sample were purified and sequenced to confirm the expected genes coding for glycoprotein D, GFP, or VP11/12. As shown in Figure 7, treatment of viral particles with 75 µM EGCG or p-EGCG resulted in decreased viral replication relative to untreated virions. The detectable levels of viral genes encoding glycoprotein D, GFP and VP11/12 12 hours post infection were lower (Figure 7), suggesting there is a significant reduction in viral genome replication in EGCG and p-EGCG treated cultures.
Figure 7.
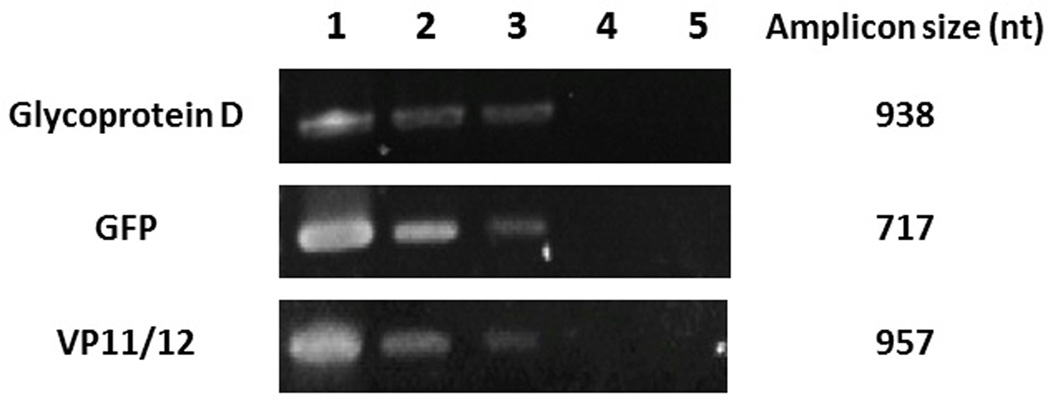
Gel electrophoresis of PCR products. Top: PCR of HSV-1 glycoprotein D gene US6. 100 ng of DNA extracted from HSV-1/Vero cells was added to each PCR reaction. Middle: PCR of HSV-1 GFP gene UL46. Bottom: PCR of HSV-1 VP11/12 tegument gene UL46. Lane 1: HSV-1/Vero; Lane 2: 75 µM EGCG-HSV-1/Vero; Lane 3: 75 µM palmitoyl-EGCG-HSV-1/Vero; Lane 4: Vero cells only; Lane 5: Negative control.
Real-time PCR confirms that EGCG and p-EGCG treatments block the replication of the viral genes encoding glycoprotein D, GFP and VP11/12
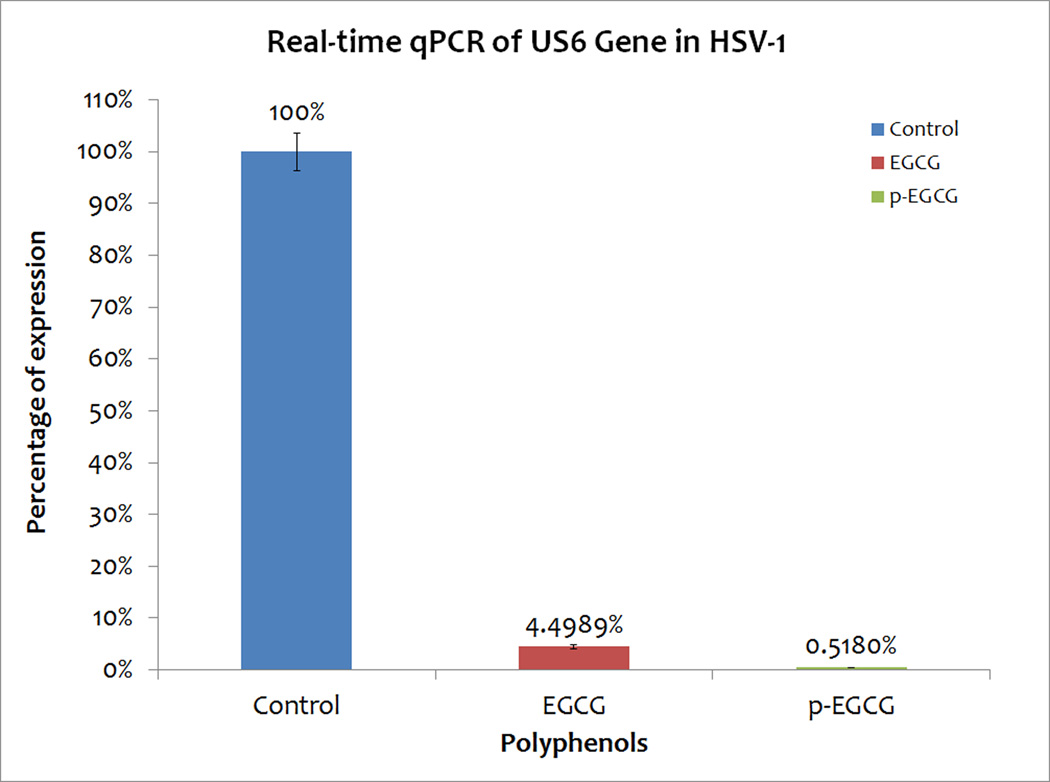
Since PCR suggested a reduction in the levels of viral genomic DNA, quantitative real-time PCR of the viral US6 gene encoding glycoprotein D was therefore used to quantify the relative block of viral DNA replication 12 hours after infection with EGCG or p-EGCG treated virions. The DNA from control HSV-1/Vero cells, EGCG treated, and p-EGCG treated HSV-1/ Vero cells was isolated at 12 hours post-infection and US6 gene levels were measured with real time PCR. Treatment with EGCG caused a 95% reduction in the concentration of HSV-1’s US6 gene relative to concentration in Vero cells infected with untreated virions as shown in Figure 8 (repeated in triplicate) whereas p-EGCG had a much greater effect with a 99.5 % reduction in concentration of HSV-1’s US6 gene (Figure 8). Thus, the concentration of HSV-1 DNA in treated cells was significantly reduced in the presence of EGCG or p-EGCG (p< 0.05).
Figure 8.
Percentage relative to control of concentration of US6 gene in EGCG-HSV-1/Vero and p-EGCG-HSV-1/Vero cells relative to HSV-1/ Vero cells with no EGCG or p-EGCG based on RT-PCR. Percentage calculated based on the relative expression with comparative Ct (n=3). Y-error bars indicate SD.
Discussion
Although infection with HSV-1 or -2 is typically asymptomatic, viral activation results in epithelial lesions that are painful, recurrent, ulcerative, and infectious. HSV-1 causes cold sores and lesions of the mouth and lip, as well as herpes keratitis, a leading cause of corneal blindness in the United States. HSV-1 is also emerging as an inducer of genital herpes in developing countries (Xu et al. 2006), although HSV-2 is the primary cause of most cases of genital herpes. In rare cases, HSV-2 can also cause encephalitis (Stanberry et al. 2000; Xu et al. 2006). Frequent HSV outbreaks can have major psychological and social impacts on infected individuals. Further, HSV lesions provide a facile route for HIV infection during sexual activity (Van de Perre et al. 2008; Wald and Link 2002). HSV-1 and -2 are enveloped, double stranded DNA viruses with ~152 Kb genomes and have capsids of ~200 nm in diameter (Garner 2003). The virion consists of three major structures, an outer portion called the envelope, which includes 11 glycoproteins, a tegument layer composed of 15 proteins, and an icosahedral capsid enclosing the viral DNA (Foster et al. 1998; Garner 2003; Willard 2002). The tegument proteins connect the capsid to the viral envelope.
HSV infection is initiated by envelope glycoprotein C binding to cell surface proteoglycan heparin sulfate on epithelial cells and glycoprotein D binding to one of three entry receptors; this results in strong virion attachment to the host cell (Akhtar and Shukla 2009; Carfi et al. 2001; Shukla and Spear 2001; Spear et al. 2006). Glycoproteins B, D, H and L form a complex that results in fusion of the viral envelope with the host cell membrane, allowing the virus to enter the cell. The viral capsid is transported to the nucleus where the viral DNA content is released. Persistent lifetime infection is maintained by the virus infecting and becoming latent in the cell bodies of neurons. Activation (e.g., by other viral infections, stress or injury) and axonal transport can lead to reinfection of epithelial cells and a recurrence of lesions.
Although many efforts have been made, there is currently no effective vaccine against HSV infection, which successfully evades the immune system during latent infections (Belshe et al. 2012; Bernstein and Stanberry 1999; Toka et al. 2004). Nucleotide analogs targeting viral replication represent the primary treatment for infection, but they do not provide a cure (Brady and Bernstein 2004; Fatahzadeh and Schwartz 2007; Morfin and Thouvenot 2003). Moreover, the virus can develop resistance due to mutations in the DNA polymerase gene (Brady and Bernstein 2004; Frobert et al. 2008; Lebel and Boivin 2006; Piret and Boivin 2011). Therefore, new medications are required to block infection and to prevent viral shedding by an infected individual (Morfin and Thouvenot 2003). EGCG has been reported to lack marked cytotoxic effects on non-cancerous cells (Babich et al. 2005; Weisburg et al. 2004). Data presented here indicate that both EGCG and p-EGCG have similar cytotoxicity towards African green monkey kidney-derived Vero cells at concentrations up to 75 µM. Pilot studies with 100 µM of EGCG and p-EGCG have shown no increase in toxicity. Cellular metabolism retains 90% of its normal activity, suggesting that EGCG and p-EGCG are not toxic at the concentrations used in this study. EGCG is unstable in aqueous solution and readily oxidizes, resulting in a loss of activity (Chen et al. 2009; Chen et al. 2003). Therefore, aqueous topical solutions must be prepared fresh, making it unsuitable for the preparation of medications. Several experimental approaches used here demonstrated that palmitoyl-EGCG, and to a lesser extent EGCG, had a powerful inhibitory effect on the HSV-1 lytic cycle, consistent with a previous report for the effects of EGCG (Isaacs et al. 2008). Treatment of virions for 1 hour with 50 µM EGCG and p-EGCG and removed after 1 hour absorption caused a 92% and >99% respectively, reduction in infectivity as measured by plaque formation. Unlike the effect of p-EGCG on Vero cell metabolism, the effect on HSV-1 was dose-dependent (Figure 8). Moreover, p-EGCG treatment caused a >99% reduction in the amount of HSV-1 genome DNA synthesized 12 hours after infection, whereas EGCG caused a 95% reduction.
In this study, the EGCG or p-EGCG was removed after one hour of viral adsorption, but still resulted in a significant inhibition of viral production by Vero cells. One potential mechanism for this inhibitory effect of EGCG and p-EGCG on the HSV-1 lytic cycle is by blocking virion entry into cells, which was observed for the effect of EGCG on HIV and influenza virus infection (Song et al. 2005; Williamson et al. 2006). Alternatively (or in addition), the virion particles could be damaged, as has been observed previously for the effect of EGCG on HSV (Isaacs et al. 2008). While these may be the dominant mechanism(s), some HSV-1 DNA persisted after infection of Vero cells with EGCG or p-EGCG treated virions serving as effective PCR templates for HSV-1 genes, suggesting some viral particles enter the cells and the genomes are replicated although no detectable plaques are formed. Therefore, p-EGCG may also affect intracellular genome trafficking, and/or virion assembly to form viable virus particles. Further work will be required to test these possibilities and further clarify the mechanisms of action. Collectively, the results from this study demonstrate that lipophilic p-EGCG is a potent inhibitor of HSV-1 infection of epithelial cells in vitro at non-toxic doses. The fatty acid derivative is stable to air exposure, vaginal pH (Isaacs et al. 2008; Lambert et al. 2006; Sang et al. 2005; Zhu et al. 1997), and is a more effective antiviral agent against HSV-1 than EGCG. Palmitoyl-EGCG therefore offers a potentially useful approach to prevent infection by topical application on epithelial tissues. A case study has been reported recently that the topical application of lipophilic EGCG could be a potential effective treatment for HSV (Zhao et al. 2012). Use of p-EGCG could have significant public health benefits, and warrants further study in appropriate models.
Conclusion
EGCG is a strong anti-oxidant that has previously been shown to reduce the number of HIV plaques in tissue culture (Williamson et al. 2006). Modification of the green tea polyphenol epigallocatechin gallate (EGCG) with palmitate increases the effectiveness of EGCG as an antiviral agent. Palmitoyl-EGCG (p-EGCG) is of interest since it can be topically applied to skin, one of the primary tissues infected by Herpes Simplex Virus. Here, we show that p-EGCG is a more potent inhibitor of Herpes Simplex Virus 1 (HSV-1) than EGCG and that p-EGCG is not toxic to Vero cells in culture by cell viability and cell proliferation assays. Significantly, p-EGCG was found to inhibit HSV-1 adsorption to Vero cells. Thus, p-EGCG may provide a novel treatment for HSV-1 infections.
Highlights.
We show that palmitoyl-EGCG (p-EGCG) is a potent inhibitor of Herpes Simplex Virus type 1.
Viral titers show no plaque formation in p-EGCG treated cells.
Real-time PCR shows p-EGCG has a 99.5 % reduction of HSV-1 glycoprotein D US6 gene.
Fluorescence microscopy demonstrates that p-EGCG block the production of HSV-1.
Acknowledgements
We thank Dr. Kunihiro Kaihatsu for generously providing p-EGCG. AO was supported by Novartis Graduate Scholarship. SDA and LHL were supported by Montclair State University Faculty Scholarship Program. SRM and JH were supported by NIH Grant GM084860 awarded to SRM. TC was supported by SHU Biological Sciences Department Annual Research Fund, William and Doreen Wong Foundation, and the California State University Northridge IRIS visiting scholar program. The authors thank Jonathan Yarborough for microscopy that was not shown in the manuscript and Math Cuajungco for critical reading of the manuscript.
List of abbreviations
- HSV
Herpes simplex virus
- HSV-1
Herpes simplex virus type 1
- HSV-2
Herpes simplex virus type 2
- EGCG
epigallocatechin gallate
- p-EGCG
palmitoyl-EGCG
- GFP
green fluorescent protein
- DAPI
4,6-diamidino-2-phenylindole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
No competing interests.
Authors’ contributions
TC, SDA, LHL and SDH designed this study. TC, LHL, AO, SDA and DD drafted the manuscript. SDH and PC synthesized and modified EGCG and p-EGCG. TC, SDA, LHL supervised AO and carried out experiments. SRM supervised and worked with JRH on fluorescence microscopy and image analysis. TC and AO performed data analyses.
References
- Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276(24):7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkarawongsa R, Pocaro NE, et al. Multiple peptides homologous to herpes simplex virus type 1 glycoprotein B inhibit viral infection. Antimicrob Agents Chemother. 2009;53(3):987–996. doi: 10.1128/AAC.00793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babich H, Krupka ME, et al. Differential in vitro cytotoxicity of (−)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol In Vitro. 2005;19(2):231–242. doi: 10.1016/j.tiv.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Belshe RB, Leone PA, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366(1):34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DI, Stanberry LR. Herpes simplex virus vaccines. Vaccine. 1999;17(13–14):1681–1689. doi: 10.1016/s0264-410x(98)00434-4. [DOI] [PubMed] [Google Scholar]
- Brady RC, Bernstein DI. Treatment of herpes simplex virus infections. Antiviral Res. 2004;61(2):73–81. doi: 10.1016/j.antiviral.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Carfi A, Willis SH, et al. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell. 2001;8(1):169–179. doi: 10.1016/s1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- Chen P, Dickinson D, et al. Lipid-soluble green tea polyphenols:stabilized for effective formulation. In: McKinley H, Jamieson M, editors. Handbook of Green Tea & Health Research. Lancaster: Nova Science Publishers: 500; 2009. [Google Scholar]
- Chen P, Tan Y, et al. A novel long-chain acyl-derivative of epigallocatechin-3-O-gallate prepared and purified from green tea polyphenols. J Zhejiang Univ Sci. 2003;4(6):714–718. doi: 10.1631/jzus.2003.0714. [DOI] [PubMed] [Google Scholar]
- Ciesek S, von Hahn T, et al. The green tea polyphenol epigallocatechin-3-gallate (EGCG) inhibits hepatitis C virus (HCV) entry. Hepatology. 2011 doi: 10.1002/hep.24610. [DOI] [PubMed] [Google Scholar]
- Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57(5):737–763. doi: 10.1016/j.jaad.2007.06.027. quiz 64-6. [DOI] [PubMed] [Google Scholar]
- Foster TP, Rybachuk GV, et al. Expression of the enhanced green fluorescent protein by herpes simplex virus type 1 (HSV-1) as an in vitro or in vivo marker for virus entry and replication. J Virol Methods. 1998;75(2):151–160. doi: 10.1016/s0166-0934(98)00107-4. [DOI] [PubMed] [Google Scholar]
- Frobert E, Cortay JC, et al. Genotypic detection of acyclovir-resistant HSV-1: characterization of 67 ACV-sensitive and 14 ACV-resistant viruses. Antiviral Res. 2008;79(1):28–36. doi: 10.1016/j.antiviral.2008.01.153. [DOI] [PubMed] [Google Scholar]
- Garner JA. Herpes simplex virion entry into and intracellular transport within mammalian cells. Adv Drug Deliv Rev. 2003;55(11):1497–1513. doi: 10.1016/j.addr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Hauber I, Hohenberg H, et al. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proc Natl Acad Sci U S A. 2009;106(22):9033–9038. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Li LX, et al. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication - inducible cell line. World J Gastroenterol. 2011;17(11):1507–1514. doi: 10.3748/wjg.v17.i11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HY, Cheng ML, et al. Antiviral effect of epigallocatechin gallate on enterovirus 71. J Agric Food Chem. 2009;57(14):6140–6147. doi: 10.1021/jf901128u. [DOI] [PubMed] [Google Scholar]
- Isaacs CE, Wen GY, et al. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob Agents Chemother. 2008;52(3):962–970. doi: 10.1128/AAC.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaka S, Kim M, et al. Antibacterial substances in Japanese green tea extract against streptococcus mutans, a cariogenic bacterium. Agricultural and Biological Chemistry. 1989;53:2307–2311. [Google Scholar]
- Koyama AH, Adachi A. Induction of apoptosis by herpes simplex virus type 1. J Gen Virol. 1997;78(Pt 11):2909–2912. doi: 10.1099/0022-1317-78-11-2909. [DOI] [PubMed] [Google Scholar]
- Lambert JD, Kim DH, et al. Transdermal delivery of (−)-epigallocatechin-3-gallate, a green tea polyphenol, in mice. J Pharm Pharmacol. 2006;58(5):599–604. doi: 10.1211/jpp.58.5.0004. [DOI] [PubMed] [Google Scholar]
- Lebel A, Boivin G. Pathogenicity and response to topical antiviral therapy in a murine model of acyclovir-sensitive and acyclovir-resistant herpes simplex viruses isolated from the same patient. J Clin Virol. 2006;37(1):34–37. doi: 10.1016/j.jcv.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Morfin F, Thouvenot D. Herpes simplex virus resistance to antiviral drugs. J Clin Virol. 2003;26(1):29–37. doi: 10.1016/s1386-6532(02)00263-9. [DOI] [PubMed] [Google Scholar]
- Mori S, Miyake S, et al. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: effect of alkyl chain length. Bioorg Med Chem Lett. 2008;18(14):4249–4252. doi: 10.1016/j.bmcl.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Paterson I, Anderson EA. Chemistry. The renaissance of natural products as drug candidates. Science. 2005;310(5747):451–453. doi: 10.1126/science.1116364. [DOI] [PubMed] [Google Scholar]
- Piret J, Boivin G. Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother. 2011;55(2):459–472. doi: 10.1128/AAC.00615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang S, Lee MJ, et al. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J Agric Food Chem. 2005;53(24):9478–9484. doi: 10.1021/jf0519055. [DOI] [PubMed] [Google Scholar]
- Sharangi AB. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.) – A review. Food Research International. 2009;42(5–6):529–535. [Google Scholar]
- Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108(4):503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Lee KH, et al. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005;68(2):66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Spear PG, Manoj S, et al. Different receptors binding to distinct interfaces on herpes simplex virus gD can trigger events leading to cell fusion and viral entry. Virology. 2006;344(1):17–24. doi: 10.1016/j.virol.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Stanberry LR. Understanding Herpes, 2nd Revised. Jackson: University Press of Mississippi; 2006. [Google Scholar]
- Stanberry LR, Cunningham AL, et al. Prospects for control of herpes simplex virus disease through immunization. Clin Infect Dis. 2000;30(3):549–566. doi: 10.1086/313687. [DOI] [PubMed] [Google Scholar]
- Sueoka N, Suganuma M, et al. A new function of green tea: prevention of lifestyle-related diseases. Ann N Y Acad Sci. 2001;928:274–280. doi: 10.1111/j.1749-6632.2001.tb05656.x. [DOI] [PubMed] [Google Scholar]
- Toka FN, Suvas S, et al. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J Virol. 2004;78(23):13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Perre P, Segondy M, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis. 2008;8(8):490–497. doi: 10.1016/S1473-3099(08)70181-6. [DOI] [PubMed] [Google Scholar]
- Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185(1):45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu L, et al. Egress of HSV-1 capsid requires the interaction of VP26 and a cellular tetraspanin membrane protein. Virol J. 2010;7:156. doi: 10.1186/1743-422X-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg JH, Weissman DB, et al. In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity. Basic Clin Pharmacol Toxicol. 2004;95(4):191–200. doi: 10.1111/j.1742-7843.2004.pto_950407.x. [DOI] [PubMed] [Google Scholar]
- Willard M. Rapid directional translocations in virus replication. J Virol. 2002;76(10):5220–5232. doi: 10.1128/JVI.76.10.5220-5232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson MP, McCormick TG, et al. Epigallocatechin gallate, the main polyphenol in green tea, binds to the T-cell receptor, CD4: Potential for HIV-1 therapy. J Allergy Clin Immunol. 2006;118(6):1369–1374. doi: 10.1016/j.jaci.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Honda M, et al. Inhibitory effects of (−)-epigallocatechin gallate on the life cycle of human immunodeficiency virus type 1 (HIV-1) Antiviral Res. 2002;53(1):19–34. doi: 10.1016/s0166-3542(01)00189-9. [DOI] [PubMed] [Google Scholar]
- Zhao M, Jiang J, et al. A proprietary topical preparation containing EGCG-stearate and glycerin with inhibitory effects on herpes simplex virus: case study. Inflamm Allergy Drug Targets. 2012 doi: 10.2174/187152812803251033. [DOI] [PubMed] [Google Scholar]
- Zhu QY, Zhang A, et al. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997;45(12):4624–4628. [Google Scholar]