Abstract
Problem
The specialized regulatory T cells (Treg) population, essential for maternal tolerance of the fetus, performs its suppressive actions in the critical peri-implantation phase of pregnancy. In the present work, we investigated whether trophoblast cells are able to induce Treg recruitment, differentiation, and whether these mechanisms are modified by a bacterial or viral infection.
Method of Study
Human T-regulatory cells were differentiated from naïve CD45RA+CCR7+ cells obtained from PBMCs cultured with IL-2 and TGFβ over 5 days. Induction of iTregs (CD4+Foxp3+ cells) was evaluated using low serum conditioned media (LSCM), obtained from two first trimester trophoblast cell lines, Swan-71 and HTR8. Co-culture experiments were done using transwell assays where trophoblast cells were in the absence or presence of PGN, LPS, or Poly [I:C]. Cytokine production was measured by multiplex analysis.
Results
Trophoblast cells constitutively secrete high levels of TGFβ and induced a significant increase of Foxp3 expression accompanied by a specific T-reg cytokine profile. Moreover, trophoblast cells were able to recruit iTregs in a specific-manner.
Conclusion
We demonstrate that trophoblast cells have an active role on the recruitment and differentiation of iTregs; therefore, contributing to the process of immune regulation at the placental-maternal interface.
Keywords: Regulatory T cells, early pregnancy, tolerance and pregnancy, human implantation
INTRODUCTION
Several reports have proposed that pregnancy evolves through different immunological stages with a pro-inflammatory or anti-inflammatory predominant profile, depending on the stage of gestation analyzed. Today, we can recognize three immunological phases, which coincide with the three trimesters of pregnancy. The first trimester involves the generation of a pro inflammatory environment as a prerequisite for successful implantation; the second phase of pregnancy in which the placenta and the fetus are symbiotic, is characterized by the induction of an anti-inflammatory1–5. Finally, in the last immunological phase, parturition, an inflammatory process is initiated promoting the contraction of the uterus, expulsion of the baby, and rejection of the placenta1–5.
The control of the mentioned pro/anti inflammatory states implies several regulatory and tolerogenic mechanisms at the site of fetal antigen exposure which may operate simultaneously to sustain the gestation6–8.
One emerging focus is the role of the specialized regulatory T cell (Tregs) population, essential for preventing a maternal immune response against paternal antigens released by trophoblast cells or fetal cells at the implantation site and the maternal circulation. Tregs belong to the T-lymphocyte population, and among them there are cells with distinct phenotype, cytokine secretion profile and tissue origin; all of them displaying suppressive and regulatory properties that contribute to maintaining the antigen-specific T-cell tolerance9–11. Basically, we can distinguish the natural Tregs (nTregs) derived from the thymus and constitutively expressing CD25, and the adaptive Tregs, CD4+CD25+ cells that are induced from CD25− precursors in the peripheral lymphoid organs (iTregs)11.
During early pregnancy, Tregs are further expanded in the blood, particularly with a peak phase in the second trimester and decline post-partum to the levels observed before pregnancy12,13. Several observations suggest that they have a key protective role when maternal tissue first comes into contact with embryo antigens associated with invading placental trophoblast cells.
The predominant site for the expansion of the Treg pool during pregnancy is the peripheral tissue and the draining lymph nodes of the uterus14. Tregs are stimulated through antigen-specific or non-specific pathways, thus exerting their suppressive actions is critical during the peri-implantation phase of pregnancy11. In fact, paternal antigen-specific Treg cells present at the draining lymph nodes quickly migrate to the pregnant uterus where these cells proliferate resulting in the induction of paternal antigen-specific tolerance at the early stages of pregnancy.
The mechanisms for the recruitment of Treg cells in the decidua after embryo implantation are unique and differ from those observed in the non-pregnant state. In the murine model, decidual Tregs express the chemokine receptors CCR2, CCR4, CCR5, CCR6, and the ligands CCL2, CCL3, CCL4, CCL5, CCL17, CCL20, CCL22 and CX3CL through which Tregs might selectively be recruited to the uterus from peripheral tissues11, 15. Kallikourdis et al. demonstrated that expression of CCR5 not only facilitated the selective accumulation and retention of Treg cells at the implantation site in the gravid uterus, but also represents a phenotypic marker for a murine Treg subset with highly suppressive ability16.
One key cytokine in the generation of Tregs from peripheral blood is TGFβ. TGFβ induces Foxp3 gene expression in TCR-challenged CD4+CD25− naïve T cells which suffer a transition towards a regulatory T cell phenotype with potent immunosuppressive potential17. A link between TGFβ and the induction of iTregs was also evidenced in humans18. TGFβ can induce a T cell subset with suppressive activity from naïve human peripheral blood T cells. The co-stimulatory effects of TGFβ on naïve T cells up-regulate CD25 and CTLA-4 expression and suppress the proliferation in response to alloantigens and the cytotoxic activity18,19.
Although the presence of Tregs at the implantation site is now well characterized, the role of the placental-fetal unit on Treg recruitment, activation and differentiation is not clearly understood. Interestingly, Treg cells were found to be more abundant in implantation sites in partial and complete molar pregnancies, suggesting that the intact conceptus may not be required for the recruitment of Treg cells20, but signals coming from the placenta, and more specifically the trophoblast, are involved in Treg regulation.
In the present study we investigate the interaction between first trimester trophoblast cells and Treg cells. We demonstrate that trophoblast cells are able to induce iTreg differentiation from maternal naïve T cells from peripheral blood. Furthermore, we show that trophoblast cells are capable of recruiting Tregs and that this interaction is affected by bacterial or viral infection.
Materials and Methods
Control fertile women were defined as non-pregnant women who had two or more previous normal pregnancies without any miscarriage. The “Investigation and Ethics Committee” from the Argentinean Society of Gynecological and Reproductive Endocrinology (SAEGRE) has approved this study and all the patients provided their written consent to participate in it.
Peripheral blood mononuclear cells (PBMCs)
PBMCs from fertile women were isolated from heparinized peripheral blood by a density gradient centrifugation on Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden). Cells were extensively washed and resuspended in RPMI 1640 (Life Technologies Grand Island, NY) supplemented with 10% human AB serum, 2mM glutamine and 1% penicillin-streptomycin.
In vitro differentiation of iTregs
Naïve CD4 T cells were isolated from peripheral blood from fertile women using the Easy Sep Kit®, following manufacturer recommendations. Briefly, anti-CD45RO biotinylated Ab was added to the PBMC suspension and incubated for 15 min at room temperature, then an antibody (Ab) cocktail was added for 10 min (this cocktail contains antibodies bound in bispecific tetrameric Ab complexes, which are directed against cell surface antigens on human blood cells as CD8, CD14, CD16, CD19, CD20, CD36, CD56, CD123, TCRγδ, glycophorin A). Then magnetic nanoparticles were added for an additional 10 min and the tube was placed in the magnet for another 10 min. The suspension cells (CD45RA+CCR7+) were recovered in a new tube, while the magnetically labelled unwanted cells remained bound in the original tube by the magnetic field. Naïve CD4 T cells were cultured in pre coated plates with anti-CD3 (10 µg/ml, BD-Pharmigen, Franklin Lakes, NJ, USA) + anti-CD28 (1 µg/ml, BD-Pharmigen, Franklin Lakes, NJ, USA) and maintained with media supplemented with IL-2 (2 ng/ml, Peprotech, USA) and recombinant TGFβ (10 ng/ml, R&D System, MN, USA). Every 48 hours the media was changed and after 5 days of culture we obtained 26±4% of CD4+Foxp3+ cells. To determine the contribution of the trophoblast cells to iTreg differentiation, we performed this protocol in the presence of LSCM (ratio 1:1) obtained from Swan-71 and HTR8 cell lines. As controls, we performed the differentiation in the presence of recombinant TGFβ and LSCM from an ovarian cancer cell line. In every condition the culture media was always supplemented with IL-2 (2 ng/ml).
Trophoblast low serum conditioned media
Trophoblast cells Swan-71 (cell line derived by telomerase-mediated transformation of 7 week cytotrophoblast)21,22 and HTR823, and an ovarian cancer cell line (OVC1)24, were cultured in T25 flasks in complete DMEM 2% FCS (Gibco, Invitrogen) overnight to obtain low serum conditioned media (LSCM).
Cytokine, chemokine and growth factors quantification
LSCM (from Swan-71, HTR8, and OVC1) and supernatant recovered after five days of iTreg differentiation were quantified using the Bio-Plex® Precision Pro™ (Bio-Rad Laboratories, Inc., CA, USA). These assays use magnetic-bead-multiplex for the detection of IL-1b, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-17, G-CSF, GM-CSF, IFNγ, MCP-1, MIP-1β, RANTES, TNFα, VEGF, GROα, TGFβ1 and TGFβ2. Briefly, wells of a 96-well filter plate were loaded with either 50µl of prepared standard solution, or 50µl of cell-free supernatant and incubated with 1X antibody-coupled beads at ± 600 rpm for 30min in the dark at room temperature. Wells were then vacuum-washed three times with 100µl wash buffer. Samples were then incubated with 25µl of 1X biotinylated detection antibody at ± 600 rpm for 30 min at room temperature in the dark. After three additional washes, 50µl of 1X streptavidinphycoerythrin was added to each well and incubated for 10 min at ± 600 rpm at room temperature in the dark. After a final three washes, the beads were resuspended in 125µl of sheath buffer for measurement with the LUMINEX 200 (LUMINEX, Austin, TX, USA). Detection and analysis were performed using the Luminex 100 IS system (Upstate Biotechnology, Charlottesville, VA). Results are expressed in pg/ml ± SEM.
Flow-cytometry analysis
Intracellular staining for Foxp3 detection was performed with a commercial kit (eBioscience, USA) following the manufacturer recommendations. Briefly, 1 × 106 cells were surface stained with an anti-CD4 Ab for 30 minutes at room temperature, then washed with PBS and resuspended in the Fixation/Permeabilization working solution. After an hour of incubation at 4°C in the dark, cells were washed with the permeabilization buffer and then the anti-human Foxp3 Ab or the isotype control were added to the pellet in the presence of permeabilization buffer. After washing, cells were resuspended in an appropriate volume of flow cytometry staining buffer and analyzed on the cytometer.
Ten thousand events were acquired in a FACS Aria II cytometer® and results were analyzed using the WinMDI software®. Negative control samples were incubated in parallel with an irrelevant, isotype-matched Ab. Results for positive cells are expressed as a percentage of the respective population and the quadrant was set using the irrelevant isotype specific Ab.
Cell growth quantification
Cellular proliferation was measured with an IncuCyte™ Long-term live cell imaging system (Essen Instruments, USA). This system acquires phase contrast images of living cells in microplates, by a packaged automated microscope which takes auto-focused images at user-defined times. Once the digital images are collected, the image processing software calculates image metrics and the percentage of confluence.
Suppression Assays
Maternal iTregs were differentiated from naïve T cells in the presence of IL-2 + TGFβ as described above. After five days of treatment, cells were recovered and resuspended in complete RPMI-1640 and 30 × 103 cells/well or 60 × 103 cells/well were respectively supplemented with 70 × 103 cells/well or 40 × 103 cells/well syngenic PBMCs to test their suppression ability (the “Responder cells”). These responder cells were co-cultured with 1 × 105 cells/well mismatched allogenic healthy donor-PBMCs (the “Stimulator cells”). These last ones were previously treated with mitomycin C (0.5 ng/ml, Sigma–Aldrich, St. Louis, MO) for 30 minutes at 37°C, to inhibit DNA synthesis by cross-linking DNA at guanine and adenine residues, disrupting base pairing, hence these cells only have the ability to stimulate the proliferation of the responder cells. As a positive control of proliferation we performed cultures with 1 × 105 cells/well responder cells and 1 × 105 cells/well mismatched allogeneic healthy donor-PBMCs as stimulator cells. The mixture of responder and stimulator cells was incubated in a U-shape microtitre plate, in the presence of 10% human AB serum. After five days, cells were pulsed with 1 µCi/well of methyl-[3H]-thymidine [3H]TdR (NEN, Boston, MA) during the last 18 h of cell culture, and then harvested on glass fiber filters using a Packard Filtermate cell harvester (Packard Instruments, LaGrange, IL). Incorporated radioactivity was measured in a liquid scintillation β-counter (Packard Instruments). Tests were conducted in triplicate and results were expressed as the % of proliferation compared to the positive control of proliferation (100%) and also they were expressed as the % of suppression obtained as 100% – % of proliferation with the addition of iTregs.
Migration assays
We performed two sets of migration assays. In the first set of experiments we evaluated the migration of the Foxp3+ cells using the different SFCM as stimuli. The naïve T cells, after 5 days of differentiation with IL-2 + TGFβ, were seeded in 8µm-inserts (4 × 104 cells/insert) (BD Falcon cell culture inserts) which then were set in a 24-plate-well containing the SFCM from Swan-71, H8, and OVC1. After 48 hours, the cells were recovered from the lower compartment and the frequency of CD4+Foxp3+ cells were quantified by FACS analysis. As a positive control, we used human serum. The results are expressed as the folds of increase with respect to the positive control.
In the second set of migration assays, we evaluated the migration of the iTregs towards the Swan-71 cells. The trophoblast cells were cultured in a 24-well flat bottom polystyrene plate in complete DMEM 10% FCS. When the cells reached a 70% of confluence (2 × 105 cells/well) different stimuli representing a bacterial or viral infection were added: LPS (10 µg/ml isolated from Escherichia coli (0111:B4)), PGN (10 µg/ml), or Poly[I:C] (10 µg/ml) all from (Sigma–Aldrich, St Louis, MO, USA). Then 8µm inserts were added containing the differentiated iTregs previously labeled with the red fluorescent linker dye PKH26 (Sigma–Aldrich, St Louis, MO, USA) following manufacturer recommendations. After 48 h of culture, cells from the lower compartment were recovered and analyzed by FACS, as described above. For these experiments we consider the spontaneous migration as the % of PKH26+Foxp3+ cells recovered from the lower compartment without attraction stimuli.
Statistical analysis
The significance of the results was analyzed by the Student´s t test for non-parametric samples, using the GraphPad Prism4 software (GraphPad, San Diego, CA). A value of p<0.05 was considered significant.
RESULTS
Trophoblast cells contribute to the differentiation of iTregs from maternal naïve T cells with a specific chemokine and cytokine production profile
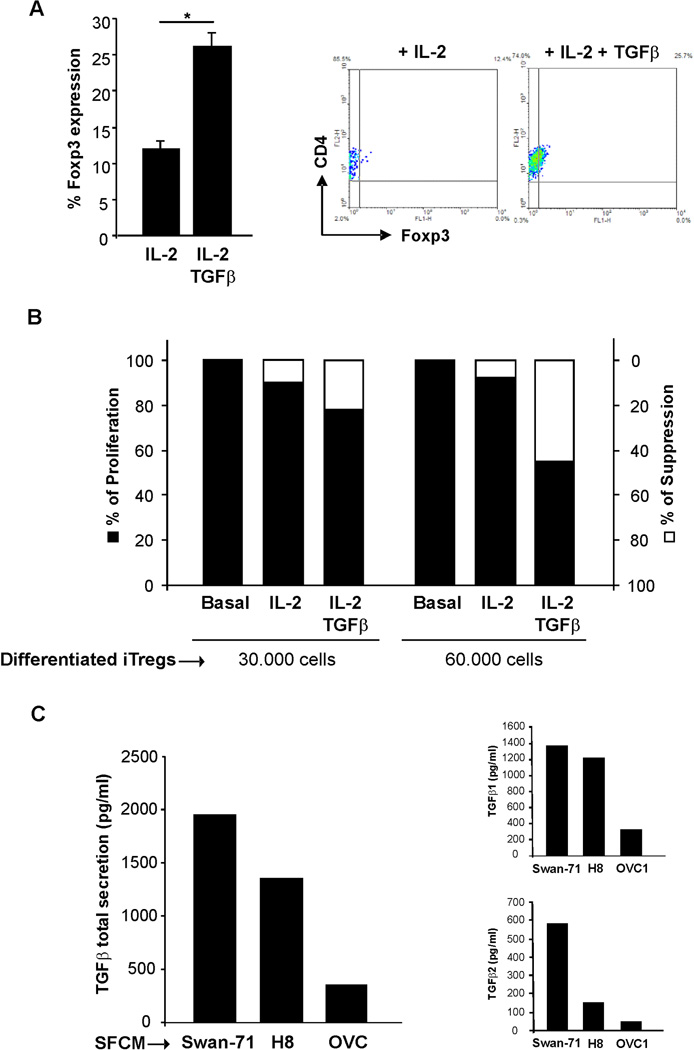
Human iTregs were differentiated as described in Methods section. As depicted in Figure 1A, we obtained a mix of differentiated iTregs (26 ± 4% of CD4+Foxp3+ cells) and non-differentiated T cells. Since the hallmark of Tregs is their ability to suppress immune responses by inhibiting the proliferation of effector T cells25 we next confirmed if the in vitro differentiated iTregs displayed suppressive ability. Suppression assays were performed using the “responder cells and “stimulator cells”, as detailed in the Materials and Methods section. We could observe a significant decrease in proliferation of the allogeneic response when co cultures were performed in the presence of 60 × 103 responder cells/well (Figure 1B). Moreover, when naïve T cells were maintained only with IL-2, we did not observe a decrease in the proliferation rate for both concentrations of cells tested. These results support the fact that the increase in the frequency of CD4+Foxp3+ after in vitro differentiation also correlates with their suppressive ability.
Figure 1. Differentiation of Tregs from maternal naïve T cells.
(A) In vitro differentiation of iTregs was performed using human naïve CD45RA+CCR7+ obtained from fertile women PBMCs. This subpopulation was maintained in pre coated plates with αCD3+αCD28 and media supplemented with IL-2 and TGFβ. After five days we obtained a mix of differentiated-iTregs (26 ± 4% of CD4+Foxp3+ cells) and non-differentiated T cells. Figure shows representative dot plots and the frequency of the CD4+Foxp3+ population. Results are representative of five independent experiments using different fertile women PBMCs (*p<0.05, Student T-test). (B): Maternal naïve cells were differentiated in the presence of IL-2 or IL-2 + TGFβ and after five days cells were recovered and 30 × 103 or 60 × 103 cells/well were supplemented with 70 × 103 or 40 × 103 syngenic PBMCs/well respectively (the responder cells). The responder cells were co cultured with PBMCs from a mismatched allogenic healthy donor, previously treated with mitomycin C (the stimulator cells). The mixture of responder and stimulator cells was incubated in a U-shape microtiter plate, in the presence of 10% human AB serum. After five days, [3H]TdR was added for 18 h and uptake was determined using a β-scintillation counter. Results are expressed as the % of proliferation and % of suppression as mentioned in the M&M section, at least 3 independent experiments run in triplicate (*p<0.05, Student T-test). (C) TGFβ production was quantified in the SFCM from trophoblast cells lines (Swan-71 and H8) using the Bio-Plex® commercial kit. As negative control we used a low TGFβ producer ovarian cancer cell line (OVC1). Figure shows the total TGFβ, TGFβ1 and TGFβ2 production expressed in pg/ml as the mean ± S.E.M (*p<0.05, Student T-test). TGFβ3 was not detected under the mentioned conditions.
Taking into account that TGFβ is a key cytokine that contributes to the differentiation of iTregs, we evaluated TGFβ production in the supernatant from first trimester trophoblast cells lines (Swan-71 and HTR8) and an epithelial ovarian cancer cell24. As shown in Figure 1C, trophoblast cells, Swan-71 and HTR8, express high levels of the main TGFβ isoforms, TGFβ1 and TGFβ2 (Figure 1C inserts); TGFβ3, was not detected under the mentioned conditions. These data suggest that trophoblast cells might influence iTreg differentiation.
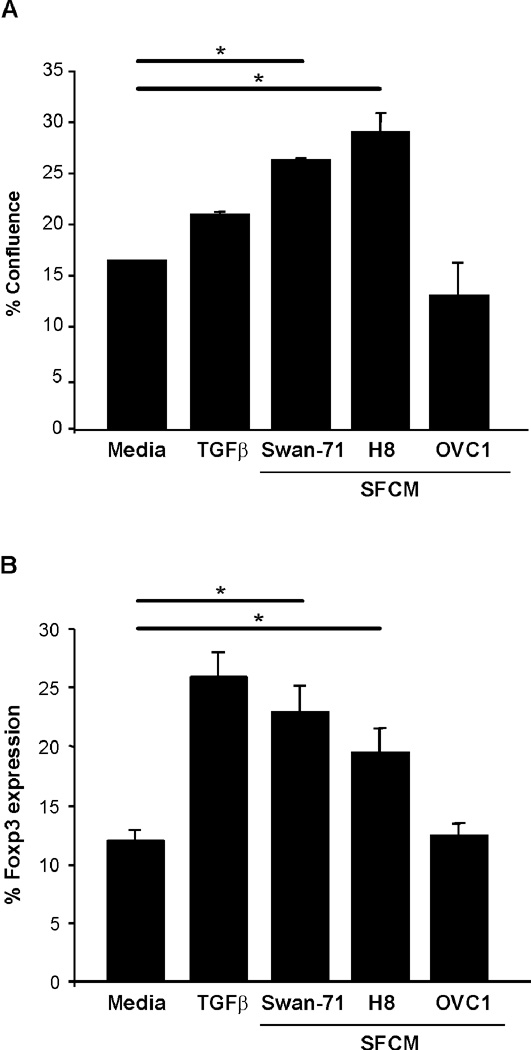
To test this hypothesis, CD4+ T cells were incubated in the presence trophoblast cells’ LSCM or recombinant TGFβ, as a positive control. Trophoblast secreted factors present in the LSCM from Swan-71 and HTR8 cells, induced a significant increase of Foxp3 expression evaluated by FACS analysis (p<0.05, Student t-test), accompanied by an increase in cell growth evidenced by Incucyte measurement (Figures 2A & 2B). However, when differentiation was performed in the presence of LSCM from OVC1 cells, which do not express TGFβ, we did not observe any change in the frequency of CD4+Foxp3+ cells (Figure 2A and B).
Figure 2. Trophoblast cells contribute to the differentiation of iTregs from maternal naïve T cells.
(A) In vitro differentiation of iTregs was performed in the presence of LSCM from Swan-71 and H8. As positive controls we performed the differentiation in the presence of recombinant TGFβ, and in the presence of media (just with IL-2) or LSCM from OVC1 as negative controls. LSCM from Swan and H8 cells induce a significant increase of Foxp3 expression evaluated by FACS analysis (*p<0.05, Student T-test); LSCM=CM. (B) Cellular proliferation was measured with an IncuCyte™ Long-term live cell imaging system and results are expressed as the percentage of confluence based in the image metrics data.
The increase in the frequency of CD4+Foxp3+ cells observed when naïve T cells were cultured in presence of LSCM from first trimester trophoblast cells was also accompanied by a specific chemokine and cytokine profile. Figures 3A and 3B summarize the changes observed in the supernatant of T cells after undergoing differentiation. The iTreg culture media was enriched with chemokines such as CCL4 (MIP-1β), CCL5 (RANTES), CXCL1 (GRO-α) and CXCL8 (IL-8) and in growth factors such as GM-CSF, G-CSF and IL-6.
Figure 3. iTregs from maternal naïve T cells produce a specific chemokine and cytokine profile.
(A) LSCM from Swan-71, H8 and OVC1 cell lines and (B) supernatant recovered after differentiation of naïve T cells during five days were quantified using Bio-Plex® commercial kit. Data from the reaction were then acquired using the Luminex 200 system and results were expressed in pg/ml ± S.E.M (*p<0.05, Student T-test); LSCM=CM.
Trophoblast cells specifically recruit iTregs and this process is increased in the presence of microbial factors
Our next step was to determine if trophoblast cells have the ability to recruit iTregs in a specific-manner from peripheral blood. To answer this question we performed migration assays using a multi-chamber system. In vitro differentiated iTregs were seeded into 8µm pore-inserts, allowing for cell migration towards the LSCM, used as a chemotactic stimuli, in the lower compartment. After 48h of culture, cells were recovered from the lower compartment and Foxp3 expression was quantified by FACS analysis. As depicted in Figure 4A, the LSCM from Swan-71 and HTR8 cells significantly increased the frequency of Foxp3+ cells compared to the migration observed in the presence of human serum (2.5 fold for Swan-71 and 2 fold for H8 cells in relation to the positive control, p<0.05 Student t-test). However, this behavior was not observed for the LSCM obtained from OVC1 cells. In addition, we did not observe differences in the migration rate when we analyzed the Foxp3 negative population, suggesting a specific recruitment of iTregs toward trophoblast cells (Figure 4B).
Figure 4. Trophoblast cells specifically recruit iTregs.
The naïve T cells after five days of differentiation with IL-2 + TGFβ were seeded in 8µm-inserts and then put in a 24-plate-well containing the LSCM from Swan-71, H8, and OVC1. After 48 hours, the cells were recovered from the lower compartment and the frequency of CD4+Foxp3+ cells were quantified by FACS analysis. As a positive control we used human serum. Results are expressed as the folds change in the migration of the (A) CD4+Foxp3+ cells or (B) CD4+Foxp3− cells with respect to the control positive. LSCM=CM.
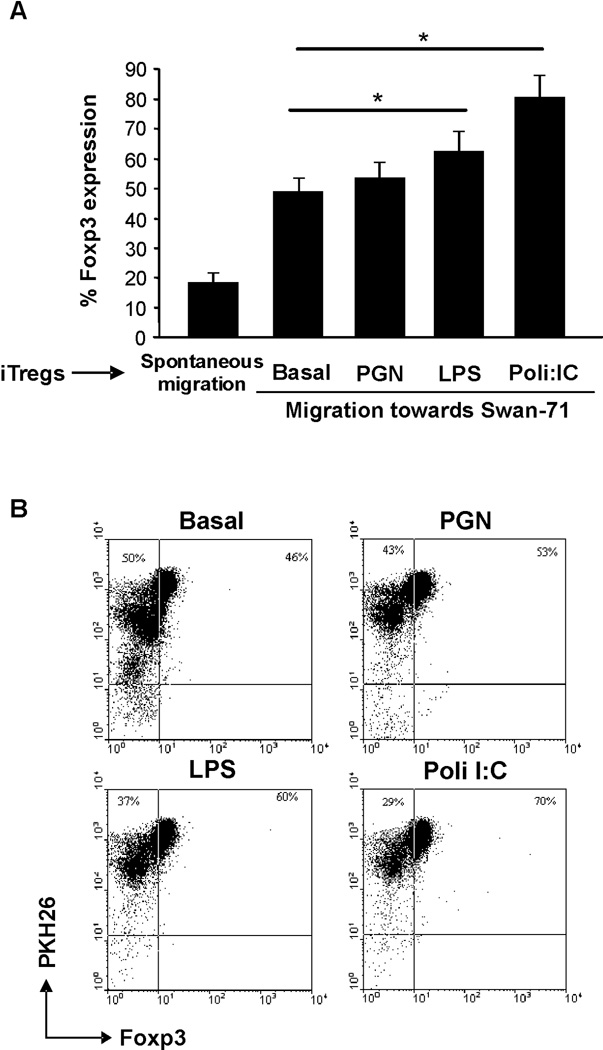
As trophoblast are able to respond to pathogens present at their the microenvironment, and this has been shown to modulate their cytokine profile26, we determined whether the presence of bacterial or viral products could alter the trophoblast induced migration of iTreg. For that purpose, we performed co-cultures of iTregs, stained with PKH26, seeded in the transwell and Swan-71 cells at 70% confluence in the lower compartment in the absence or presence of PGN (10 ug/ml), LPS (10 ug/ml) and Poly[I:C] (10 ug/ml), representing different ligands from bacteria or dsRNA from a virus infection. After 48h of culture, FACS analysis revealed a significant increase in the number of PKH26+Foxp3+ cells that had migrated towards the trophoblast incubated in the presence of LPS and Poly[I:C] (60% and 70% respectively), reflecting that first trimester trophoblast Swan-71 cells not only could selectively recruit iTregs, but this recruitment is enhanced when the trophoblast is exposed to microorganism products (Figure 5A and B).
Figure 5. Swan-71 cells specifically recruit iTregs and this process is increased in the presence of an infection.
(A) Trophoblast cells were cultured in a 24-well flat bottom polystyrene plates in complete DMEM 10% FCS. Upon reaching 70% confluency, cells were treated in the absence or presence of LPS, PGN, or Poly[I:C] representing a bacterial or viral infection. Then, 8µm inserts were added containing the differentiated naïve T cells labeled with PKH26. After 48 h of culture, cells from the lower compartment were recovered and analyzed by FACS. The spontaneous migration was considered as the % of PKH26+Foxp3+ cells recovered from the lower compartment without attraction-stimuli (*p<0.05 Student T-test). (B) Figure shows representative dot plots from one of three experiments and shows the frequency of the migrated CD4+Foxp3+ population expressed as a percentage.
Taken together, these data suggest that trophoblast cells may contribute to the recruitment and local differentiation of iTregs. More interestingly, this mechanism is maintained and intensified in the presence of bacterial or viral products.
DISCUSSION
While the appropriate generation of a pro-inflammatory response is a prerequisite for successful implantation1, 2, 4, 5, and immune cells are critical for decidual and trophoblast development in an early inflammatory environment, multiple regulatory mechanisms are also required to balance local immune cell-trophoblast interaction throughout gestation6, 8.
In line with the strict regulation that Treg cells have in the control of the effector immune responses during pregnancy, we analyzed the potential role of trophoblast cells to induce iTregs and to recruit them as local regulators of the maternal-placental interaction. For that purpose, we developed an in vitro differentiation model of iTregs from naïve CD45RA+CCR7+ obtained from fertile women PBMCs cultured in the presence of IL-2 and TGFβ and activated by anti-CD3 Ab and anti-CD28 Ab. After differentiation, we observed not only an increase in the frequency of CD4+Foxp3+ cells, but also an increase in suppressor activity, as evidenced by the reduction in the proliferation of the allogeneic response.
The results presented in this study suggest that trophoblast derived factors present in the CM are able to induce a significant increase in Foxp3 expression by CD4+ cells, accompanied with a specific cytokine and growth factor production profile. Moreover, trophoblast cells are able to recruit iTregs in a specific-manner, as shown by transwells assays, which correlates with TGFβ expression. The role of TGFβ was demonstrated by the lack of effect (neither induced Foxp3 expression nor recruited iTregs) observed when we used an ovarian cancer cell line, which produce several cytokines but TGFβ.
Trophoblast cells not only could contribute to iTregs differentiation, but also could selectively recruit them. Our conclusions are based on several observations: First, the CM from first trimester trophoblast Swan71 and HTR8 cells doubled Foxp3+ cell migration with respect to the human serum used as positive control. Second, in the presence of either bacterial or viral stimuli, we observed a significant increase in the frequency of Foxp3+ migration toward trophoblast cells. Finally, this data also correlates with previously reported observations in murine models11, 27, 28 showing the ability of trophoblast cells to secrete chemokines such as CCL4, CCL5, CXCL1 and CXCL8, which are capable of recruiting iTregs.
Taken together these results support the hypothesis that trophoblast cells may contribute to the recruitment of T cells towards the placental-maternal interface and to induce the local differentiation of iTregs through TGFβ production. Interestingly, this mechanism is enhanced by the presence of microbial products; suggesting that the trophoblast cells could limit the inflammatory response to microorganism by enhancing the recruitment and differentiation of Tregs.
The strategy used by trophoblast cells to control an exacerbated inflammatory response at the local interface to avoid tissue damage was previously reported, this included several mechanisms, such as the regulation of protein inhibitors of the complement system, the selective expression of HLA-G, the inhibition of the cytotoxic activity of NK cells and IDO (indolamine 2,3 di-oxigenase)29. To our knowledge, this is the first report showing an additional mechanism where trophoblast cells are able to control damage that can be deleterious through the selective recruitment of iTregs.
New evidences have confirmed that the Th1/Th2 paradigm has expanded into the Th1/Th2/Th17/Treg paradigm, and implies a new reconsideration of cytokine production in the placental-maternal microenvironment6, 30. In this sense, an interesting observation is that iTregs differentiated in the presence trophoblast CM produce IL-6, probably at levels necessary to support implantation. Additional studies are required in order to determine whether those levels of IL-6 are able to induce differentiation into Th17.
Previous reports pointed to the contribution of different antigens, such as seminal fluid, which can drive iTregs expansion prior to implantation31, and then the continuous release of the placental antigens into the maternal circulation would further maintain a Treg population specific against paternal antigens which would mediate tolerance towards the semiallogeneic fetus until birth32. Our data suggest that trophoblast cells, through the secretion of cytokines and chemokines, actively participate in the process of recruitment and differentiation of iTregs.
Regarding the contribution of Tregs in regulating the immune response during gestation, several studies suggested an association between alterations in Treg number or function and pregnancy complications such as unexplained infertility, recurrent spontaneous miscarriages and pre-eclampsia33–35. With the goal of generating therapeutic strategies, it seems reasonable that boosting the number and/or the activity of Treg cells reactive with appropriate conceptus antigens should confer stronger immune tolerance.
In this sense, recent reports have shown that induction of Treg cells might protect against fetal loss in the abortion-prone CBA/J × DBA/2J mouse model36. Moreover, exogenous TGFβ delivered at conception, could induce an increase in vaginal Treg cell numbers and also reduce fetal loss in the same mice model37. Another strategy is the generation of Foxp3 cells from naive peripheral T cells by stimulation with low levels of antigens38 or by delivering antigens in association with DCs39. However, further studies are required to assess the therapeutic applications of iTregs in the human placental-fetal interface in order to maintain the maternal tolerogenic state that is crucial during pregnancy.
Acknowledge
This study was supported in part by grants to GM from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, (NICDH) P01HD054713 and to RR (short-term fellowship for the Young Researcher Program from the National Research Council of Argentina).
References
- 1.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175:8096–8104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 2.Makrigiannakis A, Karamouti M, Drakakis P, Loutradis D, Antsaklis A. Fetomaternal immunotolerance. Am J Reprod Immunol. 2008;60:482–496. doi: 10.1111/j.1600-0897.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 3.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16:216–229. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mor G. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- 5.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaouat G, Petitbarat M, Dubanchet S, Rahmati M, Ledee N. Tolerance to the foetal allograft? Am J Reprod Immunol. 2010;63:624–636. doi: 10.1111/j.1600-0897.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 7.Terness P, Kallikourdis M, Betz AG, Rabinovich GA, Saito S, Clark DA. Tolerance signaling molecules and pregnancy: IDO, galectins, and the renaissance of regulatory T cells. Am J Reprod Immunol. 2007;58:238–254. doi: 10.1111/j.1600-0897.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshinaga K. Research on Blastocyst Implantation Essential Factors (BIEFs) Am J Reprod Immunol. 2010;63:413–424. doi: 10.1111/j.1600-0897.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- 9.Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323–6327. doi: 10.4049/jimmunol.171.12.6323. [DOI] [PubMed] [Google Scholar]
- 10.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 11.Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517–535. doi: 10.1093/humupd/dmp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004;136:373–378. doi: 10.1111/j.1365-2249.2004.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraccaroli L, Alfieri J, Larocca L, Calafat M, Mor G, Leiros CP, Ramhorst R. A potential tolerogenic immune mechanism in a trophoblast cell line through the activation of chemokine-induced T cell death and regulatory T cell modulation. Hum Reprod. 2009;24:166–175. doi: 10.1093/humrep/den344. [DOI] [PubMed] [Google Scholar]
- 14.Zhao JX, Zeng YY, Liu Y. Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol. 2007;75:71–81. doi: 10.1016/j.jri.2007.06.052. [DOI] [PubMed] [Google Scholar]
- 15.Darrasse-Jeze G, Klatzmann D, Charlotte F, Salomon BL, Cohen JL. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett. 2006;102:106–109. doi: 10.1016/j.imlet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Kallikourdis M, Andersen KG, Welch KA, Betz AG. Alloantigen-enhanced accumulation of CCR5+ 'effector' regulatory T cells in the gravid uterus. Proc Natl Acad Sci U S A. 2007;104:594–599. doi: 10.1073/pnas.0604268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Nagymanyoki Z, Callahan MJ, Parast MM, Fulop V, Mok SC, Berkowitz RS. Immune cell profiling in normal pregnancy, partial and complete molar pregnancy. Gynecol Oncol. 2007;107:292–297. doi: 10.1016/j.ygyno.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Straszewski-Chavez SL, Abrahams VM, Alvero AB, Aldo PB, Ma Y, Guller S, Romero R, Mor G. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30:939–948. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aplin JD, Straszewski-Chavez SL, Kalionis B, Dunk C, Morrish D, Forbes K, Baczyk D, Rote N, Malassine A, Knofler M. Trophoblast differentiation: progenitor cells, fusion and migration -- a workshop report. Placenta. 2006;27(Suppl A):S141–S143. doi: 10.1016/j.placenta.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 24.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 26.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 27.Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G, Lockwood CJ. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol. 2006;72:60–73. doi: 10.1016/j.jri.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Leber A, Teles A, Zenclussen AC. Regulatory T cells and their role in pregnancy. Am J Reprod Immunol. 2010;63:445–459. doi: 10.1111/j.1600-0897.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 29.Clark DA, Chaouat G, Wong K, Gorczynski RM, Kinsky R. Tolerance mechanisms in pregnancy: a reappraisal of the role of class I paternal MHC antigens. Am J Reprod Immunol. 2010;63:93–103. doi: 10.1111/j.1600-0897.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 30.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 31.Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod. 2009;80:1036–1045. doi: 10.1095/biolreprod.108.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Kampen CA, Versteeg-van der Voort Maarschalk MF, Langerak-Langerak J, van Beelen E, Roelen DL, Claas FH. Pregnancy can induce long-persisting primed CTLs specific for inherited paternal HLA antigens. Hum Immunol. 2001;62:201–207. doi: 10.1016/s0198-8859(01)00209-9. [DOI] [PubMed] [Google Scholar]
- 33.Wicherek L, Basta P, Pitynski K, Marianowski P, Kijowski J, Wiatr J, Majka M. The characterization of the subpopulation of suppressive B7H4(+) macrophages and the subpopulation of CD25(+) CD4(+) and FOXP3(+) regulatory T-cells in decidua during the secretory cycle phase, Arias Stella reaction, and spontaneous abortion - a preliminary report. Am J Reprod Immunol. 2009;61:303–312. doi: 10.1111/j.1600-0897.2009.00696.x. [DOI] [PubMed] [Google Scholar]
- 34.Winger EE, Reed JL. Low Circulating CD4(+) CD25(+) Foxp3(+) T Regulatory Cell Levels Predict Miscarriage Risk in Newly Pregnant Women with a History of Failure. Am J Reprod Immunol. 2011 doi: 10.1111/j.1600-0897.2011.00992.x. [DOI] [PubMed] [Google Scholar]
- 35.Redman CW, Sargent IL. Immunology of pre-eclampsia. Am J Reprod Immunol. 2010;63:534–543. doi: 10.1111/j.1600-0897.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 36.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, Volk HD. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark DA, Fernandes J, Banwatt D. Prevention of spontaneous abortion in the CBA x DBA/2 mouse model by intravaginal TGF-beta and local recruitment of CD4+8+ FOXP3+ cells. Am J Reprod Immunol. 2008;59:525–534. doi: 10.1111/j.1600-0897.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- 38.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]