Abstract
The secretion of the oxalate anion by intestinal epithelia is a functionally significant component of oxalate homeostasis and hence a relevant factor in the etiology and management of calcium oxalate urolithiasis. To test the hypothesis that human cystic fibrosis transmembrane conductance regulator (hCFTR) can directly mediate the efflux of the oxalate anion, we compared cAMP-stimulated 36Cl−, 14C-oxalate, and 35SO42− efflux from Xenopus oocytes expressing hCFTR with water-injected control oocytes. hCFTR-expressing oocytes exhibited a large, reversible cAMP-dependent increase in whole cell conductance measured using a two-electrode voltage clamp and a 13-fold increase in rate of cAMP-stimulated 36Cl− efflux. In contrast, the rate constants of oxalate and sulfate efflux were low and unaffected by cAMP in either control or hCFTR-expressing oocytes. We conclude that the human CFTR gene product does not directly mediate oxalate efflux in secretory epithelia and hence is not directly involved in oxalate homeostasis in humans.
Keywords: Anion selectivity, Excretion, Renal stones, Sulfate, Xenopus oocyte
Introduction
The importance of the intestine in overall oxalate balance is continually emerging and it is apparent that enteric secretion/excretion of oxalate occurs in both pathological and non-pathological conditions [1–5]. The healthy kidney excretes most of the oxalate derived from dietary or endogenous sources, but ~6% may be excreted into the intestinal lumen when renal function is normal [1]. In contrast, when renal function is compromised, as much as 50% of the oxalate load can be excreted enterically and this also occurs in rats challenged by an oxalate load [1, 4]. It was also recently shown that the oxalate-specific, gut resident bacterium, Oxalobacter formigenes can modulate intestinal oxalate transport by inducing enteric oxalate secretion and a positive consequence of this bacterium—enterocyte interaction is a significant reduction in urinary oxalate due to the induced enteric oxalate shunt [5]. Thus, the elucidation of apical and basolateral membrane secretory pathways is critical to our overall understanding of intestinal oxalate homeostasis.
We have previously reported cAMP-dependent secretion of the oxalate anion across isolated, short-circuited segments of intestinal epithelia from rats and rabbits [6, 7]. cAMP-dependent oxalate secretion exhibits some characteristics reminiscent of the cAMP-dependent chloride secretion through CFTR chloride channels in whole epithelia [8] and isolated brush border membrane vesicles [9]. The conductance of non-halide anions through the CFTR channel may have physiological significance; for example, in the secretion of bicarbonate or other anionic metabolites [10, 11]. Consequently, we postulated [8, 9] that some fraction of enteric oxalate secretion may be mediated by an oxalate conductance through CFTR channel, given the broad selectivity of this channel [10, 12]. In the present report we directly assess whether CFTR permits the permeation of the oxalate anion using the wild-type ortholog of human CFTR (hCFTR) expressed in Xenopus oocytes.
Materials and methods
Solutions
The standard bathing solution employed in the efflux and voltage clamp experiments was a calcium-free ND96 buffer containing (in mM): 96.0 Na+, 2.0 K+, 1.0 Mg2+, 100.0 Cl−, 5.0 HEPES titrated to pH 7.5 with NaOH. The oocyte maintenance ND96 included 1.8 mM CaCl2, 2.0 mM sodium pyruvate, and 50 mg/l gentamycin. To activate CFTR expressed in Xenopus oocytes, intracellular cAMP was elevated using the Ca2+-free ND96 saline containing 10 μM forskolin, 250 μM 8-bromoadenosine 3′,5′-cyclic monophosphate, and 100 μM 3-isobutyl-1-methylxanthine. This cAMP stimulating cocktail was prepared daily.
Oocyte preparation and CFTR cRNA injection
Oocytes were harvested from Xenopus laevis (Xenopus Express, Plant City, FL) following protocols approved by the Institutional Animal Care and Use Committee of the University of Florida. Briefly, ovarian lobes were surgically removed from frogs anesthetized in ND96 saline containing 0.12% Tricaine and titrated with Tris base to pH 7.5. Each lobe was teased apart, washed (2×) with calcium-free ND96, and then shaken/incubated in calcium-free ND96 containing 0.7 mg/ml collagenase for 2–3 h. Dispersed oocytes were collected and washed twice with calcium-free ND96 and transferred to sterile petri dishes containing sterile ND96 maintenance saline. All oocytes were maintained in an incubator at 17°C and transferred to fresh sterile media and containers daily.
Human CFTR (hCFTR) cRNA was prepared from a full-length clone (pBQ 4.7) kindly provided by Dr. David Dawson (Department of Physiology and Pharmacology, Oregon Health and Science University, Portland, OR). Linearized hCFTR plasmid DNA was transcribed in vitro using the mMessage mMachine T7 kit (Ambion, Austin, TX) resulting in a single cRNA product of the expected size of CFTR cRNA (4.7 kb) as judged by electrophoresis on a 1% agarose/formaldehyde gel.
One to two days after isolation, Stage V–VI oocytes (average diameter 1.2 ± 0.05 mm) were selected and injected with 50 nl hCFTR cRNA (1.2–12.0 ng/oocyte) or 50 nl sterile H2O using a Nanoliter 2,000 Injector/Micro4 Controller (World Precision Instruments, Sarasota, FL). Borosilicate microinjection pipettes were drawn with a P97 puller (Sutter Instruments, Novato, CA) and had tips broken to diameter of 25 μm. Viability of CFTR- and water-injected oocytes was >96% and all were used 2–6 days post-injection.
Two-electrode voltage clamp
Whole cell current (Im)– voltage(Vm) relations were determined using a two-electrode voltage clamping technique. Microelectrodes were fabricated on the P97 puller using borosilicate glass capillaries, backfilled with 3 M KCl, and had tip resistances of 0.5–2 MΩ; when immersed in standard ND96 saline. The current and voltage microelectrodes were connected to the respective headstages of a GeneClamp 500 amplifier (Molecular Devices, Sunnyvale, CA) via Ag–AgCl electrodes. A virtual ground headstage (VG-2A, Molecular Devices) was employed to actively control bath potential just downstream from the oocyte and measure bath current. Oocytes were clamped at −40 mV with voltage clamp protocols generated and controlled by pClamp software (v. 8.02, Molecular Devices). Resultant I–V relations were analyzed using Clampfit (v. 8.02, Molecular Devices) and membrane conductance (Gm, μS) was computed from the slope of the I–V relation between −20 and −60 mV. Oocytes were studied in a recording chamber (RC-11, Warner Instruments, Hamden, CT) and superfused by a gravity feed system at 1 ml/min with room temperature (21–22°C) buffers.
Efflux of radioisotopes
cAMP-dependent changes in oocyte plasma membrane anion permeability were assessed from efflux rate constants measured isotopically. Oocytes were microinjected with radiolabeled anion and allowed to recover for 5–10 min in calcium-free ND96. Fifty nl of a stock potassium 14C-oxalate solution (Amersham, Piscataway, NJ; 50 μCi/ml; 0.43 mM) was injected into each egg, resulting in an intraoocyte 14C activity of ~5,500 dpm and an oxalate concentration of 21 μM (assuming an oocyte volume of 1 μl). Similarly, Na35SO4 injections (PerkinElmer, Boston, MA; 100 μCi/ml; 0.18 mM) gave a 35SO42− activity of ~11,000 dpm and a SO42− concentration of 10 mM while K36Cl injections (MP Biomedicals, Solon, OH; 120 μCi/ml; 210 mM) resulted in a 36Cl− activity of about 13,000 dpm and an additional Cl− concentration of 10 mM.
An individual injected oocyte was then transferred to a 6 ml test tube containing 1.0 ml calcium-free ND96 buffer and 0.95 ml of the media was removed for scintillation counting and replaced at 4 min intervals. The initial 5–6 samples were replaced with calcium-free ND96 (control period), while the subsequent 5–6 samples were replaced with calcium-free ND96 containing cAMP cocktail (cAMP-activation period). After the final sampling the oocyte was rinsed in standard buffer, placed in a 4 ml scintillation vial, and lysed in 50 μl of 1% SDS to measure remaining oocyte isotope activity. Sample isotope activity was measured in 4.0 ml Ecoscint (National Diagnostics, Atlanta, GA) by liquid scintillation spectrometry (LS6500, Beckman Coulter, Fullerton, CA) with quench correction and each sample was corrected for carry over from the previous sampling. The total dpm/oocyte was calculated and isotope efflux was then expressed as the fraction of activity remaining in the oocyte at each sampling interval. The ensuing series of results (fraction of isotope remaining) were plotted as a function of sampling time as depicted in Figs. 2, 3, and 4. For each oocyte, a control efflux rate constant was determined from the slope obtained by regression analysis of the first 4–5 samples and a cAMP-activated efflux rate constant was estimated from the slope of the 3–4 points following addition of cAMP cocktail.
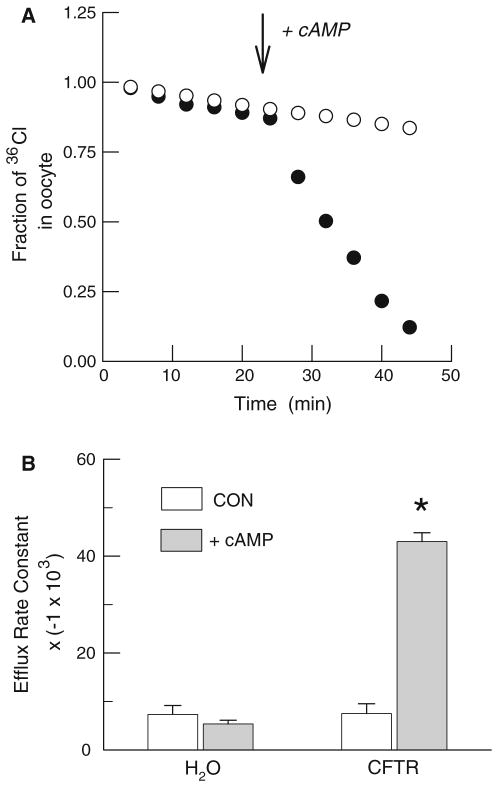
Fig. 2.
Cyclic AMP stimulation of 36Cl− efflux from water- and hCFTR-injected Xenopus oocytes. a. Representative individual efflux experiments using a water-injected oocyte (open circles) and an hCFTR expressing oocyte (filled circles). Efflux is presented as the fraction of the initial 36Cl− content remaining in the oocyte. b Average rate constants for 36Cl− efflux before (open boxes) and during stimulation with cAMP cocktail in water-injected (n = 10) and hCFTR-injected (n = 13) oocytes. An asterisk represents a significant difference (P < 0.05) between rates before and after cAMP stimulation. Note that the values on the ordinate have been multiplied by a constant (−1 × 103) for presentation
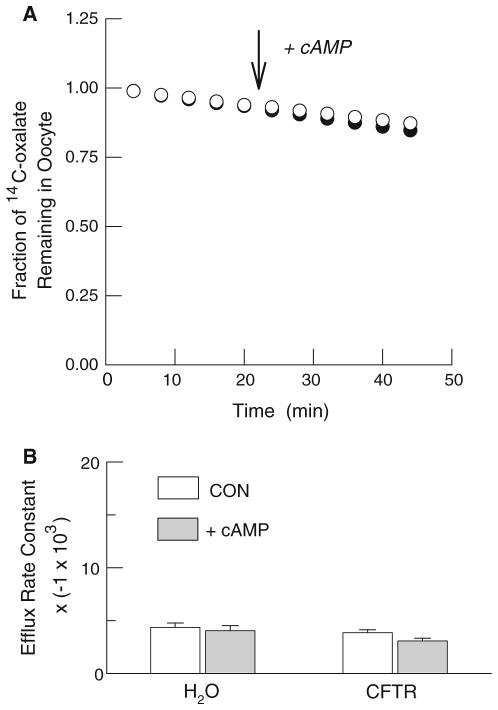
Fig. 3.
Failure of cAMP to stimulate 14C-oxalate efflux from water-and hCFTR-injected Xenopus oocytes. a Representative individual efflux experiments using a water-injected oocyte (open circles) and an hCFTR expressing oocyte (filled circles). Efflux is presented as the fraction of the initial 14C-oxalate content remaining in the oocyte. b Average rate constants for 14C-oxalate efflux before (open boxes) and during stimulation with cAMP cocktail in water-injected (n = 8) and hCFTR-injected (n = 11) oocytes. Note that the values on the ordinate have been multiplied by a constant (−1 × 103) for presentation
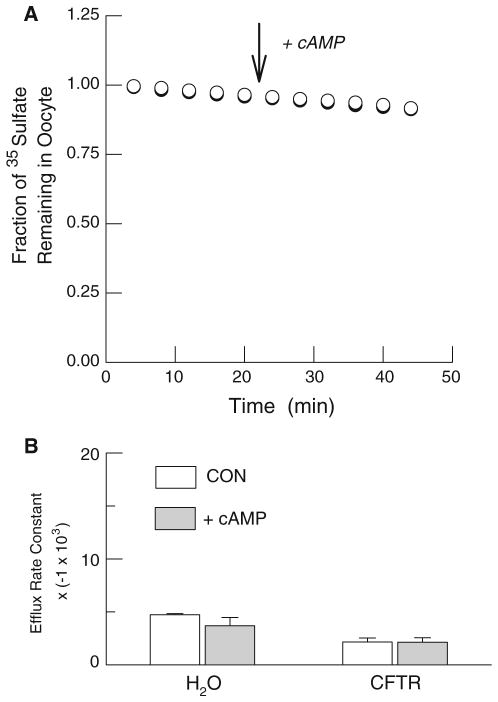
Fig. 4.
Lack of cAMP stimulation of 35SO42− efflux from water- and hCFTR-injected Xenopus oocytes. a Typical individual efflux experiments using a water-injected oocyte (open circles) and an hCFTR expressing oocyte (filled circles). Efflux is presented as the fraction of the initial 35SO42− content remaining in the oocyte. b Average rate constants for 35SO42− efflux before (open boxes) and during stimulation with cAMP cocktail in water-injected (n = 8) and hCFTR-injected (n = 7) oocytes. Note that the values on the ordinate have been multiplied by a constant (−1 × 103) for presentation
Statistics
Results are presented as the mean ± SE for n number of oocytes from at least three different donor frogs. A two-tailed paired t-test was used to establish significance of cAMP-stimulated changes in conductance or anion efflux with P ≤ 0.05.
Results
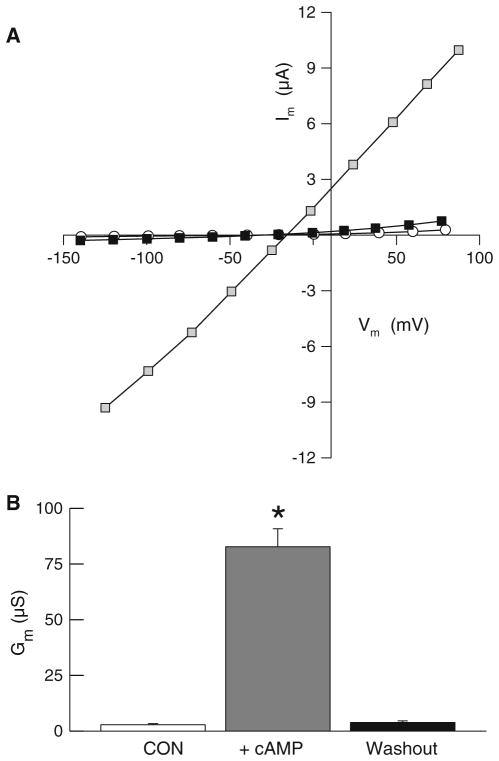
Anion conductance through CFTR chloride channel is reversibly increased by phosphorylation subsequent to elevations in intracellular cAMP [13–15]. We first established the efficacy of our CFTR expression system in Xenopus oocytes by comparing cAMP-dependent changes in the electrical properties of water-injected and hCFTR-injected oocytes. Water-injected control oocytes (n = 8) had a mean resting membrane potential (Vm) of −42.1 ± 3.6 mV and mean membrane conductance (Gm) of 1.68 ± 0.41 μS before superfusion with cAMP cocktail. Superfusion of these control oocytes with the cAMP cocktail did not significantly alter Vm (40.9 ± 4.5 mS) or Gm (1.78 ± 0.38 μS) indicating that the native oocyte does not express a significant cAMP-dependent conductance. Oocytes injected with hCFTR RNA exhibited mean pre-stimulation Vm of −40.3 ± 1.9 mV and Gm of 2.75 ± 0.52 μS (n = 10, Fig. 1); these initial values were not significantly different from those of the water-injected oocytes. However, as shown in Fig. 1, superfusion of hCFTR-injected oocytes with cAMP cocktail promoted a significant increase in Gm (to 82.6 ± 12.1 μS) and a depolarization of Vm (to −32.1 ± 1.9 mV). These electrophysiological changes were reversible; within 15 min after removal of the cAMP cocktail from the superfusate, both Gm and Vm returned to values that were not significantly different from those measured pre-stimulation. In chloride-free solutions, Gm of cAMP-stimulated hCFTR oocytes was reduced to 29.1 ± 8.7 μS (n = 3), which indicates that chloride ion contributes substantially to the cAMP-dependent conductance mediated by hCFTR. These large, reversible, and cAMP-dependent changes in whole cell anion conductance in Xenopus oocytes injected with hCFTR cRNA confirm the robust expression of functional CFTR channels [14–16].
Fig. 1.
Reversible, cAMP-dependent increases in whole cell conductance of oocytes expressing hCFTR. a Representative I–V relations of an oocyte before (open circles), during (shaded boxes), and after (black boxes) activation with a cAMP cocktail. b Average whole cell conductance of hCFTR expressing oocytes (n = 10) before (open box), during (gray box), and after (black box) superfusing with cAMP cocktail. Average conductance of water-injected oocytes was low (1.68 ± 0.41, n = 8) and not significantly different before, during, or after cAMP superfusion. An asterisk represents a significant difference (P < 0.05)
To directly test the hypothesis that oxalate can permeate hCFTR we examined cAMP-dependent changes in radiolabeled oxalate efflux in control and hCFTR expressing oocytes using the same frogs employed in the electrophysiological experiments. Included in these studies were positive controls using 36Cl− efflux and negative controls using 35SO42− efflux, since an earlier report indicated that sulfate is impermeable [13]. In the first series, we compared 36Cl− efflux from water-injected oocytes with hCFTR-injected oocytes. As shown in Fig. 2a, 36Cl− efflux was negligible in control oocytes and was not altered by cAMP. However, chloride efflux from oocytes expressing hCFTR was rapidly and strongly enhanced by cAMP as evidenced by the significant increase in the mean rate constant for 36Cl− efflux (Fig. 2b). In marked contrast, efflux of both 14C-oxalate (Fig. 3) and 35SO42− (Fig. 4) from either water- or hCFTR-injected oocytes was low and not altered by cAMP. The latter results plainly show that both oxalate and sulfate are impermeable to the wild-type human ortholog of the CFTR chloride channel.
Discussion
Interest in the mediators of intestinal oxalate transport is based on the fact that the relative magnitudes of enteric oxalate absorption and secretion impact the renal load of this anion which, in turn, is an important component in renal calcium oxalate stone formation [7]. Two sets of observations prompted the current study of oxalate permeability of the CFTR chloride channel. First, rat and rabbit large intestinal epithelia exhibit cAMP-stimulated secretion of oxalate resembling electrogenic, CFTR-mediated chloride secretion [4, 6]. Additionally, oxalate uptake into rabbit ileal brush border membrane vesicles exhibits a voltage-sensitive component that is poorly saturable and has a low energy of activation; all suggestive of a channel-like permeation mechanism [8]. Second, the CFTR anion channel is activated by cAMP and, like other chloride channels, has a rather broad selectivity [10, 16]. Besides admitting the halide anions, it has been reported that solutes as diverse as formate [13], HCO3− [11], and glutathione [17] exhibit a finite permeability to the CFTR chloride channel. Consequently, we proposed that some component of transepithelial oxalate secretion, particularly that stimulated by cAMP, might be mediated by CFTR channels in the apical membranes of secretory enterocytes [8]. The present findings show that direct mediation of oxalate flux by the hCFTR anion channel does not occur. Accordingly, the phenomenological observations of cAMP-dependent oxalate secretion in rat and rabbit intestines [4, 6, 8] and voltage-sensitive oxalate transport in apical membrane vesicles from rabbit ileal enterocytes [8] are not explained as an oxalate conductance through CFTR chloride channels, at least in humans. Thus, at the present time, PAT-1 (slc26a6) is the only protein that has been experimentally established as a mediator of apical oxalate efflux (secretion) from enterocytes [18]. It should be noted that CFTR could be indirectly involved in the regulation of epithelial oxalate transport since anion exchanger proteins like PAT-1 have been reported to interact in a regulatory manner with CFTR [11, 19]. Whether there are additional apical conductive pathways that contribute to apical oxalate efflux and intestinal secretion of oxalate remains to be determined.
Acknowledgments
Dr. David Dawson (Department of Physiology/ Pharmacology, Oregon Health & Science University) kindly provided the wild-type hCFTR cDNA and Dr. M. Green (University of Florida) prepared the cRNA. Amanda Morris assisted in maintenance and surgical procedures. Supported by NIH DK060544 (RWF), DK056245 (MH), and the Oxalosis and Hyperoxaluria Foundation.
References
- 1.Costello JF, Smith M, Stolarski C, Sadovnic MJ. Extrarenal clearance of oxalate increases with progression of renal failure in the rat. J Am Soc Nephrol. 1992;3:1098–1104. doi: 10.1681/ASN.V351098. [DOI] [PubMed] [Google Scholar]
- 2.Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol. 1994;5:1339–1343. doi: 10.1681/ASN.V561339. [DOI] [PubMed] [Google Scholar]
- 3.Hatch M, Freel RW. Angiotensin II involvement in adaptive enteric oxalate excretion in rats with chronic renal failure induced by hyperoxaluria. Urol Res. 2003;31:426–432. doi: 10.1007/s00240-003-0367-5. [DOI] [PubMed] [Google Scholar]
- 4.Hatch M, Freel RW. Renal and intestinal handling of oxalate following oxalate loading in rats. Am J Nephrol. 2003;23:18–26. doi: 10.1159/000066300. [DOI] [PubMed] [Google Scholar]
- 5.Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006;69:691–698. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 6.Hatch M, Freel RW, Vaziri ND. Characteristics of the transport of oxalate and other ions across rabbit proximal colon. Pflugers Arch. 1993;423:206–212. doi: 10.1007/BF00374396. [DOI] [PubMed] [Google Scholar]
- 7.Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res. 2005;33:1–16. doi: 10.1007/s00240-004-0445-3. [DOI] [PubMed] [Google Scholar]
- 8.Freel RW, Hatch M, Vaziri ND. Conductive pathways for chloride and oxalate in rabbit ileal brush-border membrane vesicles. Am J Physiol. 1998;275:C748–C757. doi: 10.1152/ajpcell.1998.275.3.C748. [DOI] [PubMed] [Google Scholar]
- 9.Freel RW, Hatch M, Vaziri ND. cAMP-dependent sulfate secretion by the rabbit distal colon: a comparison with electrogenic chloride secretion. Am J Physiol. 1997;273:C148–C160. doi: 10.1152/ajpcell.1997.273.1.C148. [DOI] [PubMed] [Google Scholar]
- 10.Linsdell P. Mechanism of chloride permeation in the cystic fibrosis transmembrane conductance regulator chloride channel. Exp Physiol. 2006;91:123–129. doi: 10.1113/expphysiol.2005.031757. [DOI] [PubMed] [Google Scholar]
- 11.Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- 12.Smith SS, Steinle ED, Meyerhoff ME, Dawson DC. Cystic fibrosis transmembrane conductance regulator. Physical basis for lyotropic anion selectivity patterns. J Gen Physiol. 1999;114:799–818. doi: 10.1085/jgp.114.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohrui T, Skach W, Thompson M, Matsumoto-Pon J, Calayag C, Widdicombe JH. Radiotracer studies of cystic fibrosis transmembrane conductance regulator expressed in Xenopus oocytes. Am J Physiol. 1994;266:C1586–C1593. doi: 10.1152/ajpcell.1994.266.6.C1586. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham SA, Worrell RT, Benos DJ, Frizzell RA. cAMP-stimulated ion currents in Xenopus oocytes expressing CFTR cRNA. Am J Physiol. 1992;262:C783–C788. doi: 10.1152/ajpcell.1992.262.3.C783. [DOI] [PubMed] [Google Scholar]
- 15.McCarty NA. Permeation through the CFTR chloride channel. J Exp Biol. 2000;203:1947–1962. doi: 10.1242/jeb.203.13.1947. [DOI] [PubMed] [Google Scholar]
- 16.Dawson DC, Smith SS, Mansoura MK. CFTR: mechanism of anion conduction. Physiol Rev. 1999;79:S47–S75. doi: 10.1152/physrev.1999.79.1.S47. [DOI] [PubMed] [Google Scholar]
- 17.Linsdell P, Hanrahan JW. Glutathione permeability of CFTR. Am J Physiol. 1998;275:C323–C326. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- 18.Freel RW, Hatch M, Green M, Soleimani M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G719–G728. doi: 10.1152/ajpgi.00481.2005. [DOI] [PubMed] [Google Scholar]
- 19.Shcheynikov N, Ko SB, Zeng W, Choi JY, Dorwart MR, Thomas PJ, Muallem S. Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp. 2006;273:177–186. discussion 186–192, 261–264. [PubMed] [Google Scholar]