Abstract
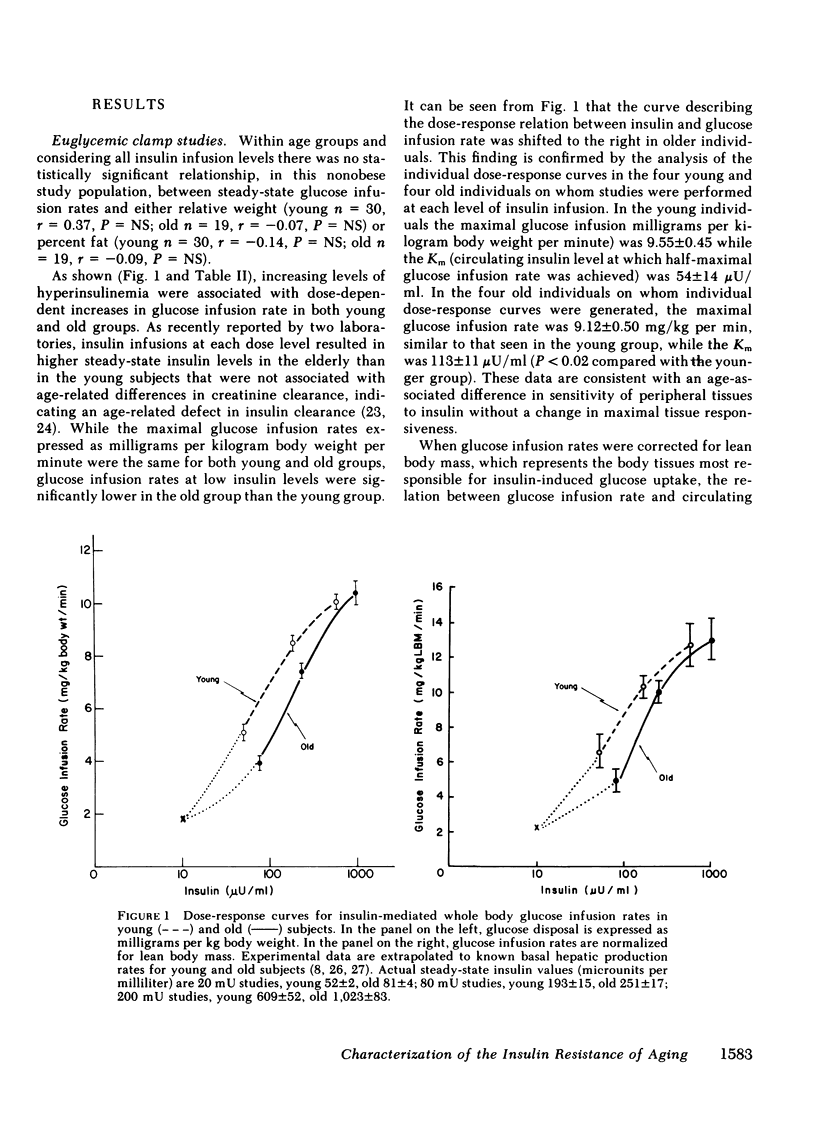
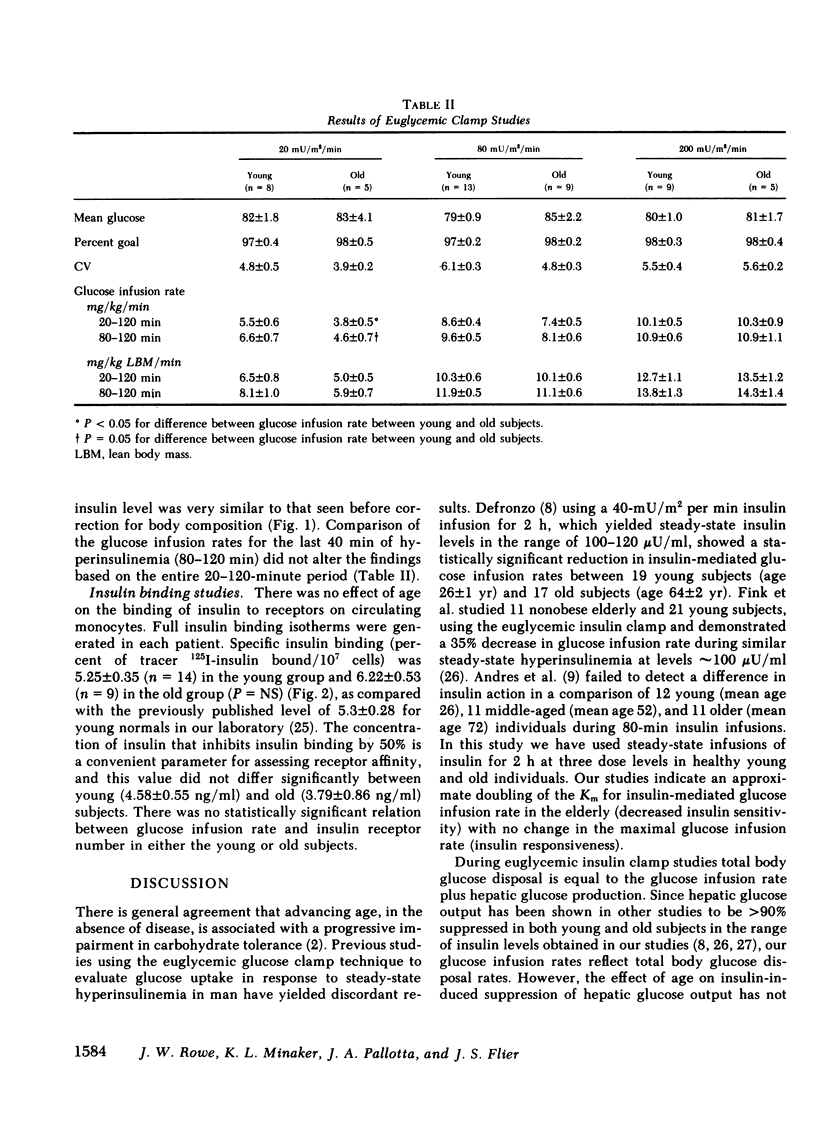
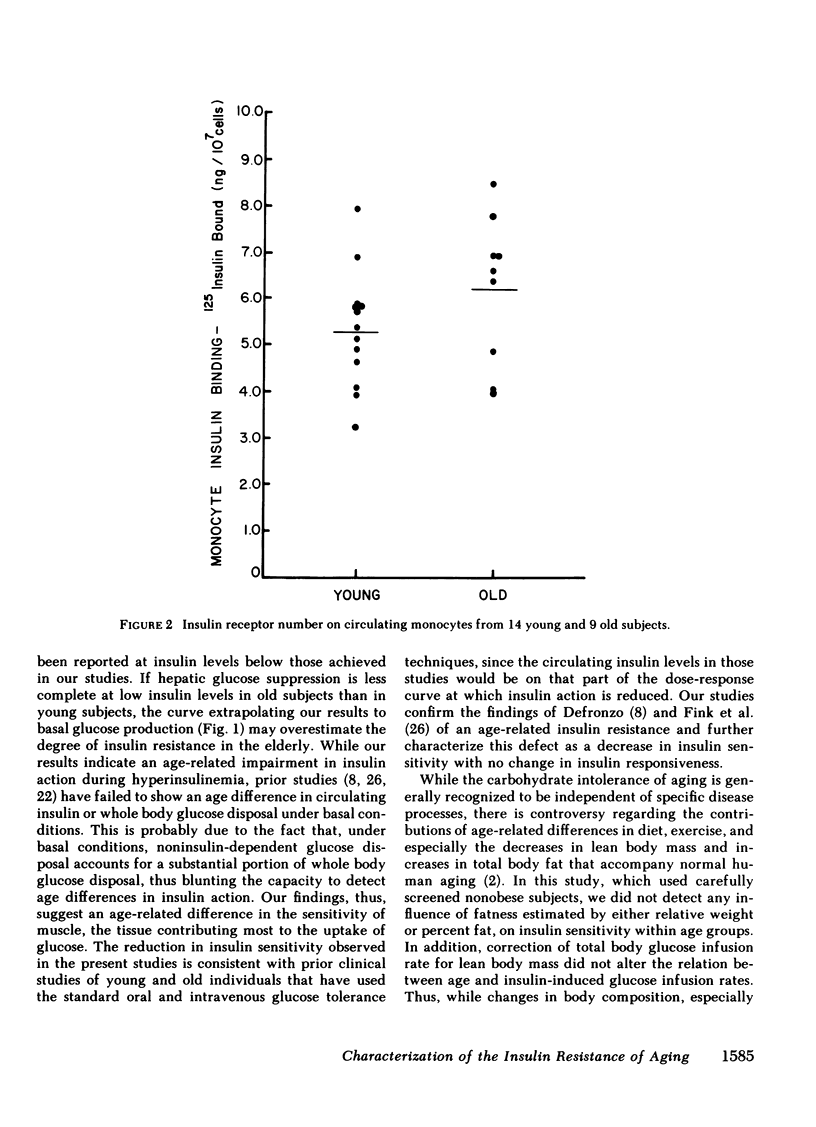
To clarify the nature of the insulin resistance of aging we studied the dose response for insulin-induced glucose disposal and the binding of insulin to circulating monocytes in healthy young and old men. A total of 49 two-hour euglycemic insulin clamp studies were performed in 17 young and 10 old healthy nonobese subjects. While the old group had lower estimates of lean body mass and greater estimates of total body fat than the young group, these differences did not exceed 5% and did not reach statistical significance. Insulin was infused at 20 mU/m2 per min (young = 8, old = 5); 80 mU/m2 per min (young = 13, old = 9); 200 mU/m2 per min (young = 9, old = 5). Increasing levels of hyperinsulinemia were associated with dose-dependent increases in steady-state glucose infusion rates in young and old. The maximal glucose infusion rates (milligrams per kilogram body weight per minute) were the same for young and old. However, the dose-response curve was shifted to the right in the old subjects. In the four individuals in each age group in whom studies were performed at each dose level, the Km was 54 +/- 14 microU/ml in the young and 113 +/- 11 microU/ml in the old (P less than 0.02). Correction of glucose infusion rate for lean body mass had no effect on comparisons between age groups. These data indicate an age-associated decline in sensitivity of peripheral tissues to insulin without a change in maximal tissue responsiveness. Studies of insulin binding with 14 young and 9 old subjects indicated no effect of age on the insulin binding to receptors on circulating monocytes (young = 5.25 +/- 0.35; old = 6.22 +/- 0.53% of 125I-insulin bound/10(7) cells). These studies suggest that aging may be associated with a postreceptor defect in insulin action manifested by decreased whole-body tissue sensitivity to insulin without a change in tissue responsiveness.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar R. S., Gorden P., Roth J., Kahn C. R., De Meyts P. Fluctuations in the affinity and concentration of insulin receptors on circulating monocytes of obese patients: effects of starvation, refeeding, and dieting. J Clin Invest. 1976 Nov;58(5):1123–1135. doi: 10.1172/JCI108565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M. B. The effect of aging on carbohydrate metabolism: a review of the English literature and a practical approach to the diagnosis of diabetes mellitus in the elderly. Metabolism. 1979 Jun;28(6):688–705. doi: 10.1016/0026-0495(79)90024-6. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Defronzo R. A. Glucose intolerance and aging: evidence for tissue insensitivity to insulin. Diabetes. 1979 Dec;28(12):1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- Elahi D., Muller D. C., Tzankoff S. P., Andres R., Tobin J. D. Effect of age and obesity on fasting levels of glucose, insulin, glucagon, and growth hormone in man. J Gerontol. 1982 Jul;37(4):385–391. doi: 10.1093/geronj/37.4.385. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Minaker K. L., Landsberg L., Young J. B., Pallotta J., Rowe J. W. Impaired in vivo insulin clearance in patients with severe target-cell resistance to insulin. Diabetes. 1982 Feb;31(2):132–135. doi: 10.2337/diab.31.2.132. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Minaker K. L., Landsberg L., Young J. B., Pallotta J., Rowe J. W. Impaired in vivo insulin clearance in patients with severe target-cell resistance to insulin. Diabetes. 1982 Feb;31(2):132–135. doi: 10.2337/diab.31.2.132. [DOI] [PubMed] [Google Scholar]
- Gold G., Karoly K., Freeman C., Adelman R. C. A possible role for insulin in the altered capability for hepatic enzyme adaptation during aging. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1003–1010. doi: 10.1016/0006-291x(76)90222-9. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Martin F. I., Melick R. A. Correlation between insulin receptor binding in isolated fat cells and insulin sensitivity in obese human subjects. J Clin Invest. 1976 Dec;58(6):1435–1441. doi: 10.1172/JCI108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helderman J. H. Constancy of pharmacokinetic properties of the lymphocyte insulin receptor during aging. J Gerontol. 1980 May;35(3):329–334. doi: 10.1093/geronj/35.3.329. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Schneider E. L. Receptors for insulin and epidermal growth factor-urogastrone in adult human fibroblasts do not change with donor age. Mech Ageing Dev. 1979 Aug;11(1):37–43. doi: 10.1016/0047-6374(79)90062-9. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Blix P. M., Matthews J. A., Hamling J. B., Din B. M., Brown D. C., Belin J., Rubenstein A. H., Nabarro J. D. Influence of ageing on glucose homeostasis. J Clin Endocrinol Metab. 1982 Nov;55(5):840–848. doi: 10.1210/jcem-55-5-840. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978 Dec;27(12 Suppl 2):1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson L. G., Bobrycki V. A., Bush M. J., Tietjen G. E., Yoon A. Insulin release in aging: studies on adenylate cyclase, phosphodiesterase, and protein kinase in isolated islets of Langerhans of the rat. Endocrinology. 1981 Feb;108(2):620–624. doi: 10.1210/endo-108-2-620. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975 Dec;56(6):1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Effects of age and obesity on insulin binding to isolated adipocytes. Endocrinology. 1975 Jun;96(6):1486–1498. doi: 10.1210/endo-96-6-1486. [DOI] [PubMed] [Google Scholar]
- Pagano G., Cassader M., Diana A., Pisu E., Bozzo C., Ferrero F., Lenti G. Insulin resistance in the aged: the role of the peripheral insulin receptors. Metabolism. 1981 Jan;30(1):46–49. doi: 10.1016/0026-0495(81)90217-1. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Ensinck J. W. Acute-phase insulin secretion and glucose tolerance in young and aged normal men and diabetic patients. J Clin Endocrinol Metab. 1975 Sep;41(3):498–503. doi: 10.1210/jcem-41-3-498. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Gold G., Reaven G. M. Effect of age on glucose-stimulated insulin release by the beta-cell of the rat. J Clin Invest. 1979 Aug;64(2):591–599. doi: 10.1172/JCI109498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven G. M., Greenfield M. S., Mondon C. E., Rosenthal M., Wright D., Reaven E. P. Does insulin removal rate from plasma decline with age? Diabetes. 1982 Aug;31(8 Pt 1):670–673. doi: 10.2337/diab.31.8.670. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982 Jan;54(1):131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- Robert J. J., Cummins J. C., Wolfe R. R., Durkot M., Matthews D. E., Zhao X. H., Bier D. M., Young V. R. Quantitative aspects of glucose production and metabolism in healthy elderly subjects. Diabetes. 1982 Mar;31(3):203–211. doi: 10.2337/diab.31.3.203. [DOI] [PubMed] [Google Scholar]
- Rosenbloom A. L., Goldstein S., Yip C. C. Insulin binding to cultured human fibroblasts increases with normal and precocious aging. Science. 1976 Jul 30;193(4251):412–415. doi: 10.1126/science.180604. [DOI] [PubMed] [Google Scholar]
- Rowe J. W., Young J. B., Minaker K. L., Stevens A. L., Pallotta J., Landsberg L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981 Mar;30(3):219–225. doi: 10.2337/diab.30.3.219. [DOI] [PubMed] [Google Scholar]
- Sorrentino R. N., Florini J. R. Variations among individual mice in binding of growth hormone and insulin to membranes from animals of different ages. Exp Aging Res. 1976 May;2(3):191–205. doi: 10.1080/03610737608257176. [DOI] [PubMed] [Google Scholar]
- Swerdloff R. S., Pozefsky T., Tobin J. D., Andres R. Influence of age on the intravenous tolbutamide response test. Diabetes. 1967 Mar;16(3):161–170. doi: 10.2337/diab.16.3.161. [DOI] [PubMed] [Google Scholar]


