Abstract
Problem
Implantation remains the rate-limiting step for the success of in vitro fertilization (IVF). Appropriate models to study the molecular aspects of human implantation are necessary in order to improve fertility.
Methods
First trimester trophoblast cells are differentiated into blastocyst-like spheroids (BLS) by culturing them in low attachment plates. Immortalized human endometrial stromal cells (hESC) and epithelial cells (ECC-1) were stably transfected with GFP or tdTomato. Co-culture experiments were monitored using Volocity imaging analysis system.
Results
This method demonstrates attachment and invasion of BLS, formed by trophoblast cells, into stromal cells but not to uterine epithelial cells.
Conclusion
We have developed an in vitro model of uterine implantation. The manipulation of this system allows for dual color monitoring of the cells over time. Additionally, specific compounds can be added to the culture media to test how this may affect implantation and invasion. This model is a helpful tool in understanding the complexity of human implantation.
Introduction
Approximately half of all human embryo implantations results in failed pregnancy1-3. Although many factors may contribute to this problem, many cases of implantation failure are attributed to poor uterine receptivity 4. Indeed, implantation remains the rate-limiting step for the success of in vitro fertilization (IVF) 5, 6. This lack of progress is the result of a still limited understanding of the molecular and cellular aspects associated with the process of implantation.
The uterine endometrium consists of two distinct cellular components, the stromal cells and the surface epithelium 7. In a receptive uterus, blastocyst apposition and its attachment to the endometrial epithelial cells are the initial steps required for embryo implantation. In order for the blastocyst to implant, the endometrium needs to undergo a series of changes that will facilitate its attachment and invasion 5. In humans, the uterus becomes receptive during the mid-secretory phase (days 19-23) of the menstrual cycle, commonly known as the window of implantation (WOI) 4.
The cellular changes during the WOI include the transformation of the fibroblast-like endometrial stromal cells into larger and rounder decidual cells (decidualization), as well as the growth and development of secretory glandules and the emergence of large apical protrusions (pinopodes) and microvilli on the luminal epithelium. In parallel, modulations in the expression of different cytokines, chemokines, growth factors, and adhesion molecules takes place 8-10. The initial contact of the blastocyst with the uterine wall, termed apposition, is a very loose connection between the blastocyst and the endometrium 11. Apposition is followed by a much stronger attachment of the trophoblast cells to the uterine epithelium termed adhesion, which allows for subsequent blastocyst invasion 12. The blastocyst then reaches the uterine stroma and invades until the embryo is implanted within the endometrium 13. Thus, successful implantation requires efficient apposition, attachment and invasion 13-15. Understanding the factors regulating each of these steps is critical for improving implantation.
Implantation is a complex process that involves a delicate coordination and a dialogue between the rolling blastocyst and the receptive endometrium 16. This dialogue is mediated by factors secreted by the maternal reproductive tract and the implanting blastocyst.
Extensive studies on implantation were performed in rodents 17, 18, 15, 19. However, the implantation process in humans is different from that of mice and rats; therefore, limiting the application of findings from animal studies to human fertility 20, 21. Recent studies have attempted to use human embryos or stem cells to understand the mechanism of embryo adhesion and invasion in the uterus 22-24. Unfortunately, regulations in many countries, including the US, prohibit in vitro studies using human blastocysts.
The goal of this study was to establish an in vitro implantation model mimicking the human uterine/trophoblast interactions and the environment of implantation. Additionally, we describe a multi-color cellular model that allows the evaluation of each of the major cellular players during the process of implantation.
Methods
Cell lines
Human first trimester trophoblast cells (Sw.71) 25, human endometrial stromal cells (hESC) 26, and human endometrial epithelial cells (ECC-1, a gift from Charles R Wira, Dartmouth Medical School, USA)27 were used in these studies. All cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated FBS (Gemini Bio-Products, West Sacramento, CA, USA). Decidualization of hESC was done as previously described 26
Generation of Sw.71-GFP and hESC-tom cell lines
Sw.71 trophoblast cells were infected with a lentivirus expressing GFP (Lois et al. Science 2002 Vol 295). Lentivirus was produced using a polyethylenimine (PEI) protocol. HEK 293T cells were seeded in a 10cm plate at a density of 5 × 106 cells. When cells were 80% confluent, medium was changed 30 min prior to transfection and 10 mg of plasmid DNA (5:3:2, psPAX:FUGW:pMD2G) and 30 ul of PEI solution (1mg/ml) was added. Virus was harvested at 48-72 hours and concentrated by ultracentrifugation. Viral infection was done in suspension using 106 cells in a sterile Eppendorf with concentrated viral particles and 1 ml of fresh DMEM. The cells were incubated 1-2 hours at 37°C, shaking intermittently. After incubation, the cells were spun at 2000 rpm for 5 minutes and transferred to a 75cm2 tissue culture flask with 6ml of fresh media containing viral particles and placed in the incubator overnight. The next day, the cells were inspected for fluorescence infection efficiency by microscopy and flow cytometry. The viral media was removed and replaced with fresh DMEM to allow the cells to recover. A similar procedure was used to generate hESC-tom, but using the tdtomato gene inserted in place of GFP.
Formation of BLS from Sw.71-GFP trophoblast cells
A confluent T75 flask of first trimester trophoblast Sw.71-GFP cells was trypsinized and divided equally into 3 wells of a Costar ultra low attachment 6-well plates (Corning Incorporated, Corning, NY USA) or in rotating glass tubes. Formation of spheroids was monitored until they reached a compact morphology.
Co-culture of Sw.71-GFP BLS with stromal and epithelial cells
hESCs or ECC1s were grown to confluence in 4-chamber tissue culture treated glass slides (BD Biosciences, Bedford, MA, USA). Sw.71-GFP spheroids (5 spheroids per chamber) were transferred using a transfer pipette and a dissecting microscope. They were co-cultured with confluent hESCs or ECC-1s. Co-cultures were maintained in 1 ml of DMEM supplemented with 10% FBS. All co-cultures were monitored using a Zeiss microscope and Volocity software.
Results and Discussion
Formation of Blastocyst-like Spheroids (BLS)
As the blastocyst travels from the fallopian tube to the uterine cavity, the surface epithelium of the uterus functions as the first contact responsible for adequate attachment of the trophectoderm to the epithelium 19. Therefore, our first objective was to develop a blastocyst-like structure that would contain only trophoblast cells to be used in coordination with endometrial epithelial and stromal cells.
We used the first trimester human trophoblast cell line Sw.71, which has characteristics of trophoblast stem cells 25. These trophoblast cells were cultured in low attachment tissue culture plates or in rotating glass tubes for several days (24-96h). Under these conditions, Sw.71 trophoblast cells form spheroids that continue to replicate and increase in number and size over time (Fig 1A-C). The capacity of Sw.71 trophoblast cells to form spheroids further suggests that these cells have properties characteristic of stem cells, as spheroid formation can be associated with the maintenance of stemness (28; 29, 30. These spheroids morphologically resemble the external trophoectoderm layer of a blastocyst. Since these spheroids interact with stromal and epithelial cells in a manner similar to that of a blastocyst as described in detail here, we refer to them as “blastocyst-like-spheroids” (BLS).
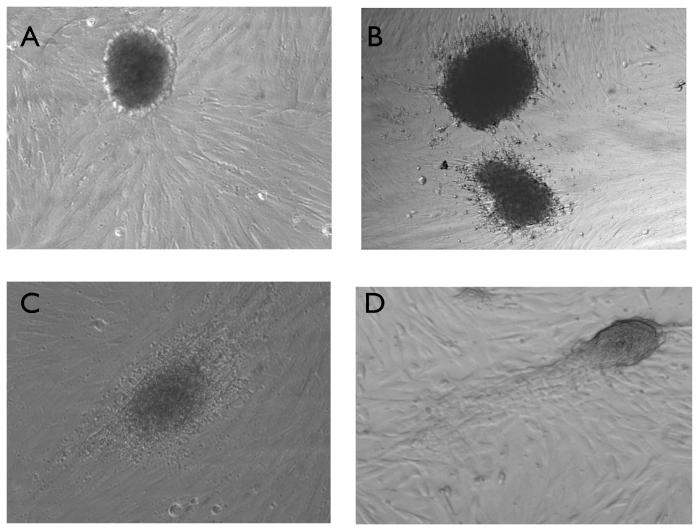
Figure 1. Formation of BLS from Sw.71 trophoblast cells.
Sw.71 cells were trypsinized and placed in a low attachment plates and visualized under 10X magnification after: A) 24 hours of culture in low attachment conditions, B) 48 hours and C) 72 hours. Note the formation of compact spheroids after 72 hours (D).
When we compared the two methods inducing the formation of BLS, low attachment plates yielded spheroids that were more consistent in terms of size and structure than the rotating glass; therefore we used only plates for the remainder of the experiments.
Trophoblast-stroma Interaction
Invasion of the trophoblast through the endometrial stroma or decidua is critical for the formation of the placenta and the implantation site 31. Therefore, our next objective was to determine whether the BLS would have the capacity to attach and potentially invade into stromal cells in culture. For this purpose, we used monolayers of telomerase-immortalized human endometrial stromal cells (hESCs) 26. These cells have the characteristics of uterine stromal cells and, following treatment with estrogen and progesterone, undergo differentiation into decidua-like cells 26. Stromal cells were plated in 4 well chamber slides. Once the cells reached 100% confluence, BLS were transferred to the culture using a dissection microscope and monitored using the Volocity imaging analysis system (ImproVision). It was critical that the stromal monolayer be completely confluent to prevent the attachment of the BLS to the tissue culture plate.
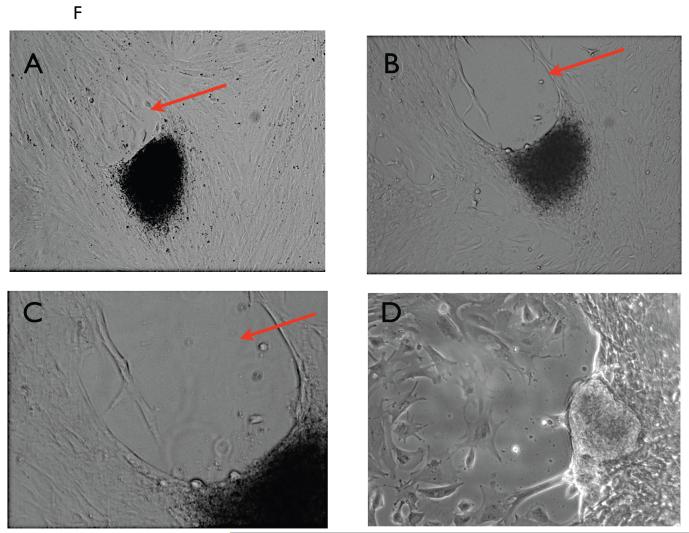
Two hours after transfer BLS were observed on the surface of the stromal cells (Fig. 2A); subsequently, the BLS attached to the stroma and trophoblast cells migrated out towards the stromal cells (Fig 2B). Interestingly, we observed that the migration of trophoblast cells is polar; they invade the stromal cells only from one side of the BLS (Fig. 2C-D). After 72 hours, there is a process of stromal cell removal on the opposite side of the attached BLS, creating an empty space (Fig. 3A-C) similar to the process of lacunae formation during the development of the interstitial space of the placenta (LOOK FOR HUPPERTZ ref). These lacunae formed in the stromal cell cultures are eventually replaced by trophoblast cells (Fig. 3D).
Figure 2. Attachment and invasion of Sw.71 BLS into hESC monolayer.
Several BLSs were transferred to a confluent monolayer of hESCs. The cells were imaged after A) 2 hours, B) 12 hours, C) 24 hours and D) 48 hours.
Figure 3. Formation of cavity opposite the invading BLS.
BLS was placed in co-culture with a hESC monolayer. Note the formation of a cavity in the opposite pole of invasion. A-C show the lack of cells in the cavity (arrow) while in D the cavity is replaced by growing trophoblast cells.
These data provide evidence that the trophoblast cells forming the spheroids are capable of invading the stroma. The formation of the cavity at one of the poles of the BLS is puzzling, but extremely interesting, since it represents a physiological process occurring during placentation REF.. Therefore, the model provides a potential basis to further elucidate the cellular and molecular events during the lacunar stage.
Establishment of a Multicolor Trophoblast-Endometrial Cell Interaction Model
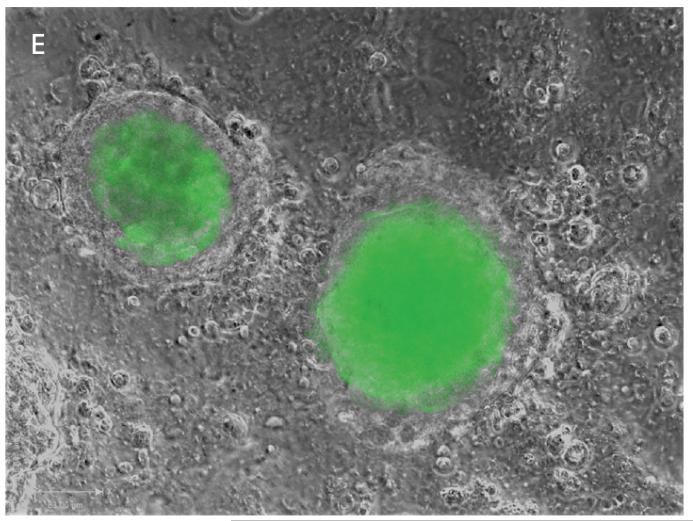
In order to provide a clear definition of the interaction between trophoblast and stromal cells, we established GFP-Sw.71 trophoblast cells by lentiviral infection 32. The cells positive for GFP were selected to obtain a 100% GFP-trophoblast SW.71 culture (Fig. 4A-B) which produced 100% GFP-BLS (Fig. 4C-D). We then tested the ability of the trophoblast cells to invade by transferring GFP-BLS into the stromal monolayer. As shown in Fig. 5, the GFP-BLS that were initially observed floating in the supernatant of the culture, attached to the stroma after 24h and started the process of invasion, which is characterized by its polarity. This is further demonstrated when we use a two color system consisting of GFP-BLS and tdtomato-stromal (hESC-tom) cells. In Fig. 6 A and B we can observe the green trophoblast attaching to the red stroma, and by 24h, GFP-trophoblast move forward between the red labeled stromal cells (Fig. 6C-D). This model provides an excellent tool to identify the function and characteristics of each cellular component during the process of trophoblast invasion.
Figure 4. Establishment of GFP-Sw.71 cells and BLS by lentiviral infection of GFP.
(A, B) Sw.71 trophoblast cell monolayer was infected with GFP-lentivirus and imaged post infection. (C, D) Sw.71-GFP monolayer form GFP-BLS.
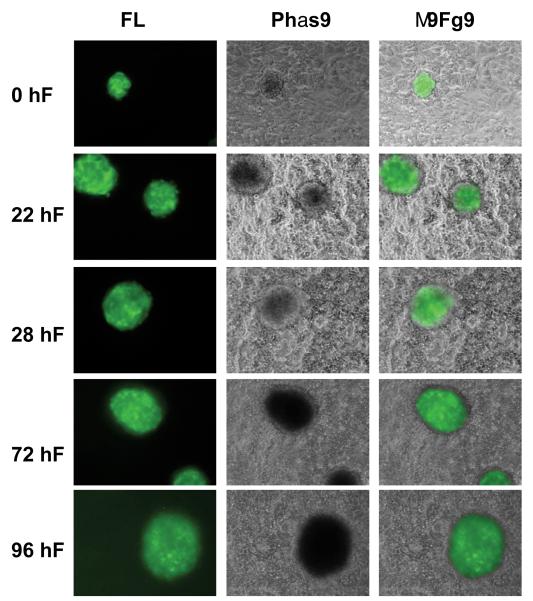
Figure 5.
Invasion of GFP-BLS into hESC monolayer. GFP-BLS was placed in co-culture with a confluent hESC monolayer. Fluorescent and phase contrast images were taken at 0, 24, 48, 72, and 96 hours.
Figure 6. A two-color system for detecting stromal invasion of BLS.
A GFP-BLS was co-cultured with hESC-tom. Fluorescent and phase contrast images were taken at A,B) 0 hours and C,D) 24 hours.
Trophoblast-epithelial cells Interaction
Between the stroma and the trophoblast there is a layer of epithelial cells that constitutes the first barrier for implantation 33, 34. As the blastocyst travels from the fallopian tube to the uterine cavity, the surface epithelium of the uterus functions as the first contact responsible for adequate attachment of the trophectoderm to the epithelium and the subsequent trophoblast invasion and placentation 35, 36. The interaction between the trophoectoderm and the epithelium is a critical step in the process of implantation; failure to interact would lead to infertility 37.
In spite of its relevance for human reproduction, implantation is one of the most difficult stages to evaluate 6. Therefore we tested the interaction between our GFP-BLS and an endometrial epithelial cell line (ECC-1). These ECC-1 cells were created from the luminal epithelium of an endometrial adenocarcinoma and have been shown to express estrogen receptors alpha and beta, progesterone receptors and androgen receptors while maintaining a luminal phenotype 38. Importantly, Mo et al. have shown in these cells the expression of CD55, which is expressed during implantation 38. This cell line has been widely used to study several aspects of the biology of the endometrial epithelium 39.
As it has been suggested that ECC-1 have the capacity to acquire a receptive phenotype 24, we used these cells to determine whether we could identify a receptive and non-receptive epithelium within our model. By culturing the cells in media containing a modulation in cytokines, we tested the ability of the BLS and the ECC-1 cells to form a significant interaction.
GFP-BLS were transferred to the ECC-1 monolayer and monitored in a similar manner to that used for the stromal cells. As shown in Fig. 7, GFP-BLS remained floating in the supernatant of the EEC-1 cell culture which was maintained in complete media and 10% FBS. The GFP-BLS did not attach to the surface of ECC-1 cells, remaining round and compact even after 96h of co-culture.
Figure 7. Lack of attachment of GFP-BLS in co-culture with ECC-1.
GFP-BLS was placed in co-culture with a confluence ECC-1 monolayer. Fluorescent and phase contrast images were taken at 0, 24, 48, 72, 96 hours.
We then wished to determine whether the ECC-1 cells could display characteristics of a receptive epithelium by forming a significant reaction with the BLS. When we added the GFP-BLS to ECC-1 cells treated with a combination of inflammatory cytokines (manuscript in preparation), we observed the attachment of the GFP-BLS to the epithelium and the development of an epithelial reaction forming an epithelial-trophoblast synapsis (Fig. 8 A-C)
Figure 8. Attachment of a GFP-BLS to the epithelium and subsequent reaction surrounding the BLS.
A-C. GFP-BLS attach to the epithelium and form a trophoblast epithelium synapsis characterized by the formation of an epithelial reaction (arrows) that supports the attachment.
When a mammalian blastocyst enters the uterine cavity, the surface epithelium of the uterus is coated by molecules such as Mucin 1 (MUC1) that prevent the attachment of the highly adhesive blastocyst to an improper site. Indeed, in the human endometrium MUC1 is upregulated during the implantation period 11, 12, 40. This suggests that the human endometrial surface epithelium prevents blastocyst adhesion, except for the precise spot were the embryo attaches 31. In the present in vitro model we have an epithelium that does not promote trophoblast attachment (non-receptive epithelium), and an epithelium capable of initiating both attachment and a cellular reaction that strengthens the interaction between the epithelium and the trophoblast. The non-receptive epithelium described in our model presents an opportunity to evaluate factors responsible for the preparation of the epithelium for implantation 41.
In conclusion, we demonstrate the formation of blastocyst-like-spheroids and their ability to mimic attachment and invasion that occurs during human uterine implantation. The interaction with the epithelial monolayer and invasion into the stromal monolayer displays the discrete function of the BLS depending on the cellular environment 42. Additionally, these cells can be labeled for distinct visualization using fluorescent microscopy (see model Fig. 9). The extensive utility of this in vitro method lies in the ability to adjust the culture conditions including to specific hormones, cytokines or compounds that may be present in the uterus during the window of implantation.
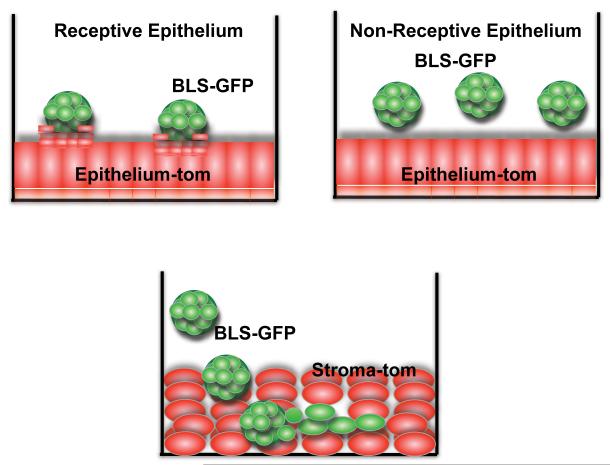
Figure 9.
Schematic of the utility of the in vitro implantation model of GFP-BLS, epithelial and stromal cells. A) Model of Receptive Epithelium: GFP-BLS are able to interact with epithelial cells forming a trophoblast-epithelial synapsis. B) Model of non-receptive epithelium: GFP-BLS are observed floating in the media and do not interact with the epithelium. C) Model of trophoblast invasion in the stoma: GFP-BLS migrate through the stroma cells establishing a layer of trophoblast cells.
Acknowledgments
We would like to thank Dr. Wange Lu at the University of Southern California Keck School of Medicine for the lentiviral plasmids. This study was supported in part by grants from NIH, NICHD P01-HD054713 and the US-Israel Binational Science Foundation (#2009125).
References
- 1.Pellicer A, Rubio C, Vidal F, Minguez Y, Gimenez C, Egozcue J, Remohi J, Simon C. In vitro fertilization plus preimplantation genetic diagnosis in patients with recurrent miscarriage: an analysis of chromosome abnormalities in human preimplantation embryos. Fertility and sterility. 1999;71:1033–1039. doi: 10.1016/s0015-0282(99)00143-0. [DOI] [PubMed] [Google Scholar]
- 2.Simon C, Landeras J, Zuzuarregui JL, Martin JC, Remohi J, Pellicer A. Early pregnancy losses in in vitro fertilization and oocyte donation. Fertility and sterility. 1999;72:1061–1065. doi: 10.1016/s0015-0282(99)00408-2. [DOI] [PubMed] [Google Scholar]
- 3.Simon C, Martin JC, Galan A, Valbuena D, Pellicer A. Embryonic regulation in implantation. Semin Reprod Endocrinol. 1999;17:267–274. doi: 10.1055/s-2007-1016234. [DOI] [PubMed] [Google Scholar]
- 4.Paria BC, Song H, Dey SK. Implantation: molecular basis of embryo-uterine dialogue. Int J Dev Biol. 2001;45:597–605. [PubMed] [Google Scholar]
- 5.Paria BC, Lim H, Das SK, Reese J, Dey SK. Molecular signaling in uterine receptivity for implantation. Semin Cell Dev Biol. 2000;11:67–76. doi: 10.1006/scdb.2000.0153. [DOI] [PubMed] [Google Scholar]
- 6.Edwards RG. Human implantation: the last barrier in assisted reproduction technologies? Reprod Biomed Online. 2006;13:887–904. doi: 10.1016/s1472-6483(10)61039-5. [DOI] [PubMed] [Google Scholar]
- 7.Lim H, Song H, Paria BC, Reese J, Das SK, Dey SK. Molecules in blastocyst implantation: uterine and embryonic perspectives. Vitam Horm. 2002;64:43–76. doi: 10.1016/s0083-6729(02)64002-6. [DOI] [PubMed] [Google Scholar]
- 8.Meseguer M, Pellicer A, Simon C. MUC1 and endometrial receptivity. Mol Hum Reprod. 1998;4:1089–1098. doi: 10.1093/molehr/4.12.1089. [DOI] [PubMed] [Google Scholar]
- 9.Simon C, Martin JC, Pellicer A. Paracrine regulators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:815–826. doi: 10.1053/beog.2000.0121. [DOI] [PubMed] [Google Scholar]
- 10.Simon C, Moreno C, Remohi J, Pellicer A. Cytokines and embryo implantation. J Reprod Immunol. 1998;39:117–131. doi: 10.1016/s0165-0378(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 11.Meseguer M, Aplin JD, Caballero-Campo P, O’Connor JE, Martin JC, Remohi J, Pellicer A, Simon C. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biol Reprod. 2001;64:590–601. doi: 10.1095/biolreprod64.2.590. [DOI] [PubMed] [Google Scholar]
- 12.Simon C, Martin JC, Meseguer M, Caballero-Campo P, Valbuena D, Pellicer A. Embryonic regulation of endometrial molecules in human implantation. J Reprod Fertil Suppl. 2000;55:43–53. [PubMed] [Google Scholar]
- 13.Simon C, Dominguez F, Remohi J, Pellicer A. Embryo effects in human implantation: embryonic regulation of endometrial molecules in human implantation. Ann N Y Acad Sci. 2001;943:1–16. doi: 10.1111/j.1749-6632.2001.tb03785.x. [DOI] [PubMed] [Google Scholar]
- 14.Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci U S A. 1996;93:3115–3120. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Illera MJ, Juan L, Stewart CL, Cullinan E, Ruman J, Lessey BA. Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertil Steril. 2000;74:41–48. doi: 10.1016/s0015-0282(00)00552-5. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez F, Pellicer A, Simon C. The chemokine connection: hormonal and embryonic regulation at the human maternal-embryonic interface--a review. Placenta. 2003;24(Suppl B):S48–55. doi: 10.1016/s0143-4004(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Paria BC. Importance of uterine cell death, renewal, and their hormonal regulation in hamsters that show progesterone-dependent implantation. Endocrinology. 2006;147:2215–2227. doi: 10.1210/en.2005-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paria BC, Sengupta J, Manchanda SK. Involvement of lysosomal enzymes in mouse embryo implantation: effect of the anti-oestrogen, CI-628 citrate. J Endocrinol. 1981;90:83–88. doi: 10.1677/joe.0.0900083. [DOI] [PubMed] [Google Scholar]
- 19.Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 20.Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science. 2002;296:2185–2188. doi: 10.1126/science.1071601. [DOI] [PubMed] [Google Scholar]
- 22.Golos TG, Bondarenko GI, Dambaeva SV, Breburda EE, Durning M. On the role of placental Major Histocompatibility Complex and decidual leukocytes in implantation and pregnancy success using non-human primate models. Int J Dev Biol. 2010;54:431–443. doi: 10.1387/ijdb.082797tg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimitriadis E, Nie G, Hannan NJ, Paiva P, Salamonsen LA. Local regulation of implantation at the human fetal-maternal interface. Int J Dev Biol. 2010;54:313–322. doi: 10.1387/ijdb.082772ed. [DOI] [PubMed] [Google Scholar]
- 24.Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod. 2010;82:235–245. doi: 10.1095/biolreprod.109.077800. [DOI] [PubMed] [Google Scholar]
- 25.Straszewski-Chavez SL, Abrahams VM, Aldo PB, Mor G. Isolation and characterization of a novel telomerase-immortalized human first trimester trophoblast cell line. Placenta. 2005;26:A.62. doi: 10.1016/j.placenta.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145:2291–2296. doi: 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- 27.Fahey JV, Wallace PK, Johnson K, Guyre PM, Wira CR. Antigen presentation by human uterine epithelial cells to autologous T cells. Am J Reprod Immunol. 2006;55:1–11. doi: 10.1111/j.1600-0897.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 28.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M, Visintin I, Mor G. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashid NA, Lalitkumar S, Lalitkumar PG, Gemzell-Danielsson K. Endometrial receptivity and human embryo implantation. Am J Reprod Immunol. 2011;66(Suppl 1):23–30. doi: 10.1111/j.1600-0897.2011.01048.x. [DOI] [PubMed] [Google Scholar]
- 32.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 33.Dekel N, Gnainsky Y, Granot I, Mor G. Inflammation and implantation. Am J Reprod Immunol. 2010;63:17–21. doi: 10.1111/j.1600-0897.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118:3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mangale SS, Reddy KV. Expression pattern of integrins and their ligands in mouse feto-maternal tissues during pregnancy. Reprod Fertil Dev. 2007;19:452–460. doi: 10.1071/rd06143. [DOI] [PubMed] [Google Scholar]
- 36.Edwards RG. Human implantation: the last barrier in assisted reproduction technologies? Reprod Biomed Online. 2007;14(Spec No 1):5–22. doi: 10.1016/S1472-6483(10)61454-X. [DOI] [PubMed] [Google Scholar]
- 37.Hartshorne GM, Edwards RG. Role of embryonic factors in implantation: recent developments. Baillieres Clin Obstet Gynaecol. 1991;5:133–158. doi: 10.1016/s0950-3552(05)80075-6. [DOI] [PubMed] [Google Scholar]
- 38.Mo B, Vendrov AE, Palomino WA, DuPont BR, Apparao KB, Lessey BA. ECC-1 cells: a well-differentiated steroid-responsive endometrial cell line with characteristics of luminal epithelium. Biol Reprod. 2006;75:387–394. doi: 10.1095/biolreprod.106.051870. [DOI] [PubMed] [Google Scholar]
- 39.Castro-Rivera E, Wormke M, Safe S. Estrogen and aryl hydrocarbon responsiveness of ECC-1 endometrial cancer cells. Mol Cell Endocrinol. 1999;150:11–21. doi: 10.1016/s0303-7207(99)00041-6. [DOI] [PubMed] [Google Scholar]
- 40.van Mourik MS, Macklon NS, Heijnen CJ. Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol. 2009;85:4–19. doi: 10.1189/jlb.0708395. [DOI] [PubMed] [Google Scholar]
- 41.Yoshinaga K. Research on Blastocyst Implantation Essential Factors (BIEFs) Am J Reprod Immunol. 2010;63:413–424. doi: 10.1111/j.1600-0897.2010.00853.x. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald JS, Germeyer A, Huppertz B, Jeschke U, Knofler M, Moser G, Scholz C, Sonderegger S, Toth B, Markert UR. Governing the invasive trophoblast: current aspects on intra- and extracellular regulation. Am J Reprod Immunol. 2010;63:492–505. doi: 10.1111/j.1600-0897.2010.00824.x. [DOI] [PubMed] [Google Scholar]