Short abstract
In this paper, we had four primary objectives. (1) We reviewed a brief history of the Lissner award and the individual for whom it is named, H.R. Lissner. We examined the type (musculoskeletal, cardiovascular, and other) and scale (organism to molecular) of research performed by prior Lissner awardees using a hierarchical paradigm adopted at the 2007 Biomechanics Summit of the US National Committee on Biomechanics. (2) We compared the research conducted by the Lissner award winners working in the musculoskeletal (MS) field with the evolution of our MS research and showed similar trends in scale over the past 35 years. (3) We discussed our evolving mechanobiology strategies for treating musculoskeletal injuries by accounting for clinical, biomechanical, and biological considerations. These strategies included studies to determine the function of the anterior cruciate ligament and its graft replacements as well as novel methods to enhance soft tissue healing using tissue engineering, functional tissue engineering, and, more recently, fundamental tissue engineering approaches. (4) We concluded with thoughts about future directions, suggesting grand challenges still facing bioengineers as well as the immense opportunities for young investigators working in musculoskeletal research. Hopefully, these retrospective and prospective analyses will be useful as the ASME Bioengineering Division charts future research directions.
Keywords: musculoskeletal; Lissner award winners, tendon, ligament, ligament replacement, mechanobiology strategies to treat soft tissue injuries, tissue engineering, functional tissue engineering, fundamental tissue engineering
Introduction
The Bioengineering Division established the H. R. Lissner Award in 1977 as an annual divisional award to honor Herbert R. Lissner, Professor of Engineering Mechanics at Wayne State University in Detroit, Michigan. Dr. Lissner and a neurosurgeon, Dr. E. S. Gurdjian, began conducting pioneering biomechanics research in 1939 that sought to understand the mechanisms of blunt head trauma and skull fracture. Beginning in 1962, Dr. Lissner also served as the first director of the Bioengineering Center at Wayne State University. The Lissner award was then elevated to an ASME society award that included the Lissner Medal in 1987 as a result of a donation from Wayne State University. The ASME Lissner Medal, “recognizes significant, career-long achievement in the field of biomechanics and is the highest award offered by the society in the field of biomedical engineering.” ASME has awarded 36 winners since 1977 in numerous areas of bioengineering.
As the most recent winner of the Lissner Medal, the first author thought it would be beneficial to review the prior Lissner awardees and the temporal pattern and scale of their pioneering research. His assumption in this retrospective review was that patterns of research by Lissner winners could be a valuable barometer of our field of bioengineering and might even provide a metric for predicting where our field is heading.
The objectives of this paper were fourfold. (1) We quantified the temporal and spatial patterns of research by Lissner awardees. While space limitations prevent us from detailing the contributions of each awardee, we have highlighted their successes over length scales and time. Several winners are noted as role models for their research, leadership, and service (see Acknowledgments). (2) We compared the musculoskeletal (MS) research of a subset of Lissner awardees with our research at the University of Cincinnati during nearly the same time period (1976–present). (3) Given the multidisciplinary nature of our work, we examined how clinical, biomechanical, and biological considerations have shaped our mechanobiology strategies for treating musculoskeletal injuries. (4) We took a peek into the future by proposing grand challenges still facing bioengineers along with unique opportunities for young investigators in our field. While this award has been given to a single individual, the research we are presenting recognizes the contributions of both the coauthors of this paper plus many more colleagues listed in the Acknowledgments. Their collaborations have been invaluable and truly appreciated!
A Retrospective Review of Research by Prior Lissner Awardees
General Background of Prior Winners.
Nearly all of the 36 Lissner awardees have been classically trained engineers. These engineers from fields including mechanical, aerospace, and electrical engineering as well as engineering mechanics initially populated the relatively young bioengineering field. They utilized engineering approaches to solve important and difficult bioengineering problems. However, Lissner winners also included those not formally trained in engineering. Two prior winners were physician scientists (Robert Rushmer, M.D. (1979) and Alf Nachemson, M.D., Ph.D. (1988)) who regularly collaborated with engineers and fostered these partnerships. Another winner (Gaynor Evans, Ph.D. (1980)) was trained as an anatomist and also served as editor of the Journal of Biomechanics. The recognition of their work suggests that the Bioengineering Division understood early that “nonengineers” were making quite valuable contributions to the field. Such recognition suggests that, looking forward, future winners with additional training in subjects such as molecular biology, nanotechnology, and medical device innovation will also be recognized as new and more high-resolution technologies become more accessible to bioengineers.
Retrospective Analysis of Research.
To try and quantify this retrospective analysis, the research of prior winners was analyzed using temporal and spatial measures applied to their major fields of research.
Temporal Analysis.
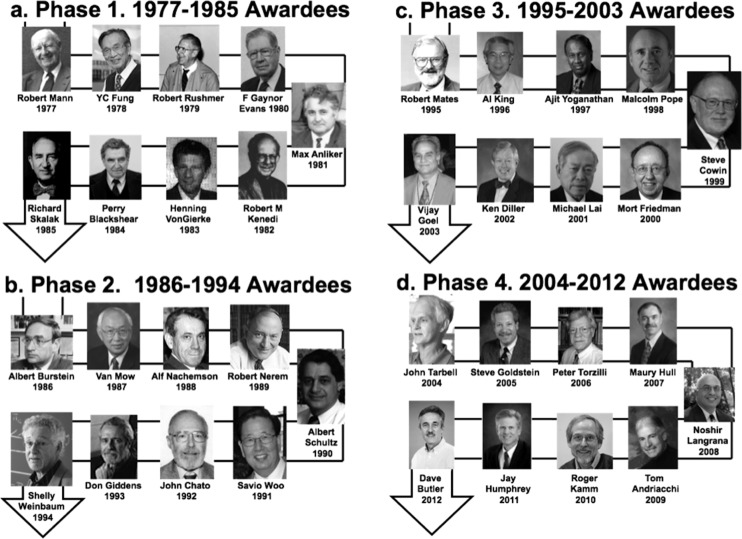
Awardees were first assigned to four 9-year increments (Phases 1–4), based on the assumption that nine years was neither too long nor too short to judge shifting research patterns. Images of all winners are shown for these four phases (Figs. 1(a)–1(d)).
Fig. 1.
(a)–(d) Prior Lissner Award winners assigned to four 9-year phases between 1977 and 2012
Assignment to Major Fields of Research.
The research of each awardee was placed into one major category or field. We recognize the difficulty in partitioning, given the diversity of work performed by these individuals as they crossed disciplinary boundaries to solve important biomedical and biological problems. While we have tracked the evolving research contributions of these individuals, we recognize that other assignments could have equal merit. Given these assumptions, it is noteworthy that among the 36 prior winners, 19 have worked in the musculoskeletal (MS) field and another 15 in the cardiovascular (CV) field. Two other individuals (Robert Kenedi, 1982 and John Chato, 1992) conducted research in more general topics of bioengineering, and their research was not included in the analysis. As a result, we analyzed those working in the MS field separately from those working in the CV field.
Spatial Analysis.
We also conducted a length-scale analysis of the research performed by Lissner awardees. This same analysis was used by the US National Committee on Biomechanics as part of its 2007 Biomechanics Summit in Keystone, Colorado. Summit invitees were assigned to one of five levels (molecular, cellular, tissue, organ, and organism), based on their expertise, and were asked to prepare position papers regarding the future of the field for these levels. Four position papers were subsequently published at the molecular [1], cellular [2], tissue [3], and organ levels [4]. Adopting this same strategy, we made primary and secondary assignments for each Lissner awardee's research in his respective field. Thus, one winner might have conducted musculoskeletal research at the organism (primary) and then organ (secondary) levels, while another performed cardiovascular research at the cellular (primary) and molecular (secondary) levels.
Statistical Analysis.
We then recruited a coauthor, Dr. Marepalli Rao, to examine the temporal and spatial research patterns for those conducting musculoskeletal versus cardiovascular research. Using the R statistical software package, the proportions of Lissner awardees working primarily at larger (organ and organism) and smaller (molecular, cell, and tissue) length scales were compared over time for both the musculoskeletal and cardiovascular fields [5]. While the data collected to conduct this statistical analysis is certainly not free from errors or potential misinterpretations, it is hoped that the spirit of the analysis is still valid and useful.
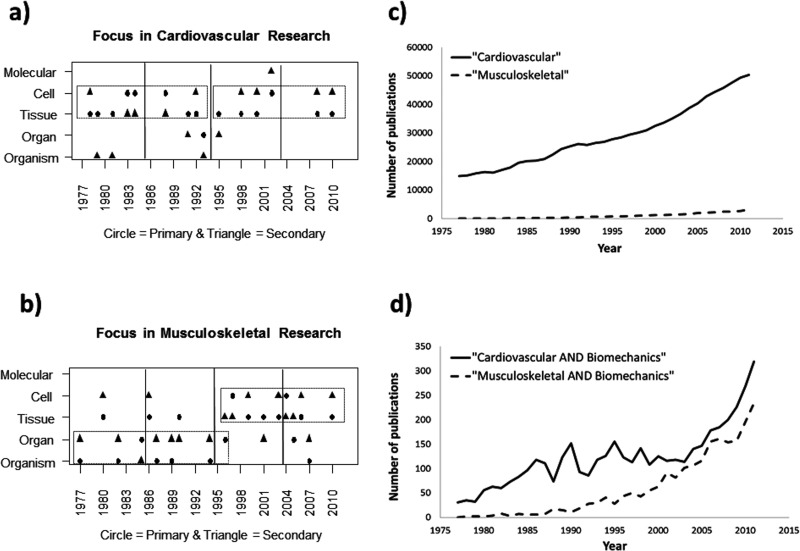
Several intriguing observations can be made from this retrospective analysis. (1) Primary and secondary assignments were typically adjacent to each other for both groups (e.g., organism as primary and organ as secondary). (2) The two fields showed different length scale patterns over the period. Those awardees conducting research in the cardiovascular field (Fig. 2(a)) have generally worked at the tissue and cellular levels for the entire 35-year period. By contrast, winners in the musculoskeletal field (Fig. 2(b)) primarily conducted their studies at the organism and organ levels between 1977 and 1998, after which their research moved to the tissue and cellular levels. This abrupt shift towards smaller length scales was statistically significant (p = 0.05). (3) The number of Lissner winners in the two fields was nearly comparable over the entire period.
Fig. 2.
An analysis of cardiovascular versus musculoskeletal research between 1977 and 2011. (a) Primary and secondary research areas for Lissner awardees working in the cardiovascular area mostly remained at the tissue and cell level during the four 9-year phases. (b) By contrast, primary and secondary areas for Lissner winners working in the musculoskeletal area moved from the organism/organ level to the tissue/cell level around 1998 (p = 0.05). Also interesting to note from PubMed is that, during this same period, (c) the number of cardiovascular publications far exceeded those in the musculoskeletal field (e.g., 50,355 versus 3152 in 2011). (d) Despite this discrepancy, when this data was restricted to biomechanics publications, the two groups were much more similar and began to converge in the late 1990 s.
There could be many possible explanations for these findings. (1) The fact that both musculoskeletal and cardiovascular researchers typically worked at two adjacent length scales of research is not too surprising and suggests that both groups maintained a consistent approach with available tools as they tackled their bioengineering problems. (2) However, the difference in length scale patterns between the two fields is more interesting. It is tempting to link this shift to the beginning of the 5-year doubling of National Institutes of Health (NIH) research funding starting in 1998. Such funding could have led to an influx of new bioengineering students and biological collaborators with novel tools capable of analysis at finer levels. However, this would not explain the absence of a shift in the cardiovascular field. Could it be that the much larger and more mature cardiovascular field actually underwent this transformation to finer levels prior to 1977? Or could it relate to the collaborators and research questions selected by Lissner winners? Cardiovascular winners in the late 1970s (e.g., Dr. Y.C. Fung and Dr. Richard Skalak) were already working with biologists (physiologists) at a finer level, while some musculoskeletal researchers were collaborating with orthopedic surgeons on a larger length scale (thanks to Dr. Jay Humphrey for this insight). Clearly, the recent explosion of high-resolution instrumentation and computing power has enabled bioengineers working in both fields to peer smaller and smaller to answer more fundamental questions than could be addressed before. These technologies, whether developed and refined by bioengineers or made available through collaborations with molecular biologists, geneticists, immunologists, etc., have changed the research questions that bioengineers can now pose. (3) The nearly equivalent number of MS and CV Lissner awardees is less apparent, given the difference in size between the two fields. Using PubMed keyword searches for “cardiovascular” and “musculoskeletal”, one coauthor (S.D.G.) examined the number of publications in both fields between 1977 and 2011. While the total number of cardiovascular publications per year far exceeded those in the musculoskeletal field (e.g., 50,355 versus 3152 in 2011; Fig. 2(c)), the numbers were much closer when only publications including the additional keyword “biomechanics” were tracked (e.g., 319 (0.6% of total) versus 236 (7.5% of total) in 2011, respectively; Fig. 2(d)). Also note that the number of biomechanics publications in each field begins to converge in the late 1990s (Fig. 2(d)). Of course, other factors unique to the musculoskeletal field might have also triggered this shift. These patterns need to be discussed and more closely analyzed, as they could provide insight into future trends for bioengineering.
Chronology and Patterns of our Musculoskeletal Research
Our research has generally paralleled the patterns observed for those Lissner awardees working in the musculoskeletal (MS) field. Since 1977, our work has undergone four phases that have progressively grown smaller in scale. Phase 1: Between 1977 and 1985, our organ/tissue level research focused on human cadaveric knee and ligament function as well as factors affecting anterior cruciate ligament structure-function relationships and reconstruction [6–14]. Phase 2: Between 1986 and 1994, our focus turned more to the tissue level, where we studied structure-function relationships [15–24] and soft tissue healing [25–31]. Phase 3: Between 1995 and 2003, our work moved to the tissue/cell level, as we and our collaborators continued to record in vivo tissue forces [32–38] and initiated studies to identify novel therapies in tissue engineering and functional tissue engineering (FTE) [39–49]. Phase 4: Our most recent work, between 2004 and 2012, has progressed even smaller to the tissue/cell/molecular levels as we have sought to develop not only design criteria for tissue-engineered tendon and ligament repairs compared to normal tissues [50–74] but also new research directions in “fundamental tissue engineering” at the interface of FTE and developmental biology [75–82]. What follows are brief summaries of the four phases of our musculoskeletal research.
Phase 1: Organ/Tissue Level.
Our knee and ligament research in the late 1970s began on a larger scale at the intersection of biomechanical and clinical disciplines. Our group (Frank Noyes, M.D., Edward Grood, Ph.D., and David Butler, Ph.D.) sought to use biomechanical concepts to help explain knee ligament function during a range of activities, including the clinical examination. The work then evolved in the early 1980s with efforts to develop more rational treatment plans after ligament injury. We recognized the significant frequency and associated cost of ligament, tendon, and joint injuries [9,10,83] that have been estimated to involve 16 million patients per year at a cost of $30 billion [84]. It was also estimated that the number of patients sustaining tears to the anterior cruciate ligament (ACL) would continue to grow and could exceed 250,000 per year [27,85,86]. A prior study using human cadaveric donors ranging in age from 16 to 86 years [87] had also demonstrated significant 40% to 65% reductions in the material properties of the anterior cruciate ligament-bone unit between 20 and 50 years of age. The increased tissue vulnerability to injury as a result of aging further emphasized the importance of understanding the forces that this tissue might encounter on a daily basis.
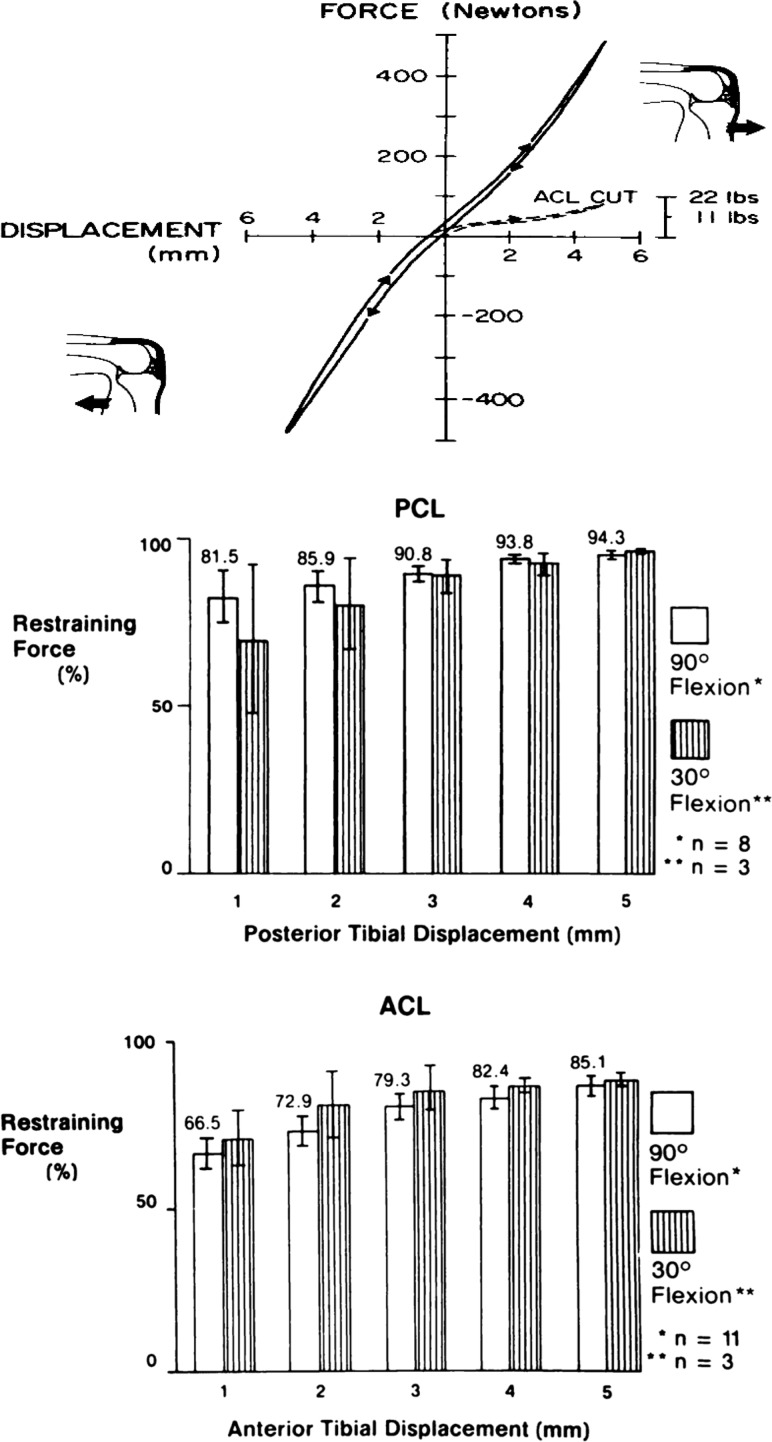
We specifically sought to explain a clinical paradox in cruciate ligament function. At the time, some knee surgeons believed that the posterior cruciate ligament (PCL) rather than the ACL was resisting anterior tibial translation (ATT) relative to the femur. However, anatomical dissections demonstrated that the ACL and not the PCL was properly oriented to resist this motion [9]. Specifically, the PCL, a tensile-bearing tissue, would be expected to show reduced loading during this anterior translation motion. Human cadaveric knees (n = 14) with an average donor age of 42 years (range of 18–65 years) were dissected, mounted in a materials testing machine, and subjected to a controlled displacement profile while recording anterior and posterior restraining forces. The tibia was moved at a constant displacement rate up to 5 mm of anterior translation (the so-called anterior drawer test), returned to neutral, and then moved 5 mm posteriorly and returned to neutral (Fig. 3). We then performed a selective cutting procedure, whereby each tissue was sectioned and the displacement profile repeated. This stiffness-based approach to selective cutting permitted an independence of cutting order. After transecting the anterior cruciate ligament (ACL), anterior restraining force declined dramatically, with no change in posterior restraining force (Fig. 3). The ACL was found to be the primary ligamentous restraint to ATT, providing up to 85% of total restraining force. In a similar way, the PCL was found to be the primary restraint up to 5 mm of posterior tibial translation, providing 94%–96% of total restraining force (Fig. 3) [9]. The paradox of the PCL resisting anterior tibial translation could be explained if the clinical test began more posteriorly and moved toward a “neutral” position, thus producing a “false” anterior drawer sign [9]. The smaller forces applied during this clinical examination could have also contributed to the misdiagnosis.
Fig. 3.
The ACL (bottom graph) is the primary ligamentous restraint up to 5 mm of anterior tibial translation (85% of total anterior restraining force). The PCL (middle graph) is the primary restraint up to 5 mm of posterior tibial translation (94%–96% of total restraining force). Adapted with permission from Ref. [9].
This study was important in the 1970s, especially without the benefits of magnetic resonance imaging, because it (1) helped to change diagnostic tests and treatment strategies after knee injury, (2) led to new terms like “primary” and “secondary” ligamentous restraints as well as “independence of cutting order” during testing, and (3) provided a new way to examine activity level and anterior knee laxity (translation).
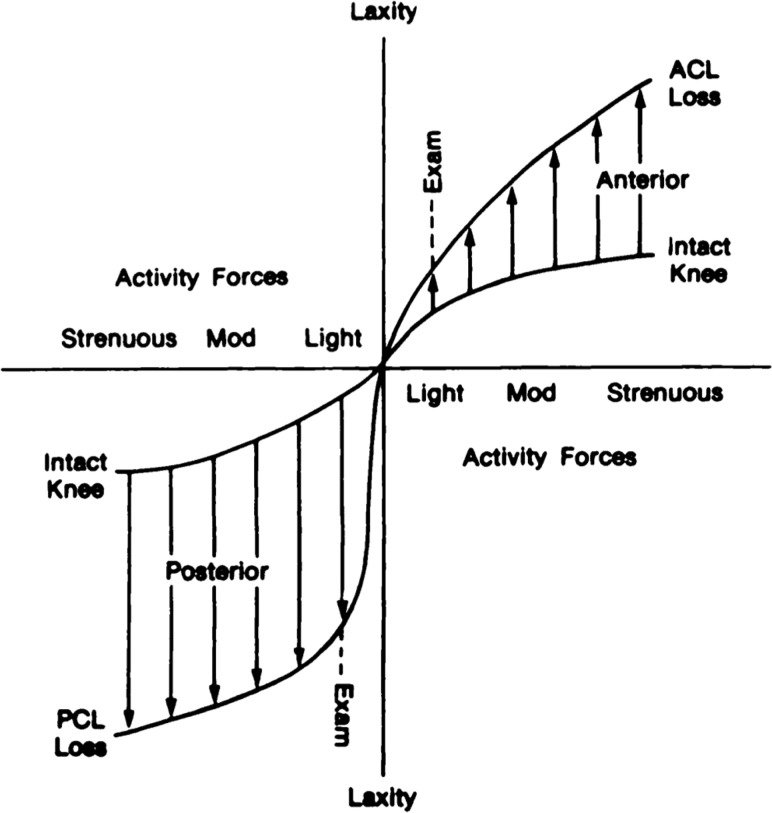
To illustrate this latter point, shown in Fig. 4 is anterior knee laxity plotted against activity forces for the intact cadaveric knee and following individual sectioning of the ACL and the PCL [9]. Note the small increase in anterior knee laxity that the surgeon might not be able to detect under the “light” forces of the clinical exam. Then notice the much larger increases in laxity that the patient would definitely sense for more strenuous ADLs. Finally, note that the increase in anterior laxity under light forces associated with cutting the ACL is much smaller than the corresponding increase in posterior laxity after cutting the PCL. This suggests that the surgeon would have a greater likelihood of diagnosing loss of the posterior cruciate ligament during the clinical exam than the anterior cruciate ligament [9].
Fig. 4.
Anterior knee laxity versus activity forces in intact cadaveric knee and after individual sectioning of the ACL and PCL. The surgeon may not detect a small increase in anterior laxity in the ACL-deficient knee under “light” forces of the clinical exam, but the patient definitely experiences the greater increases in laxity under more strenuous forces. The increases in posterior laxity after loss of the PCL are more pronounced at both load levels. Adapted with permission from Ref. [9].
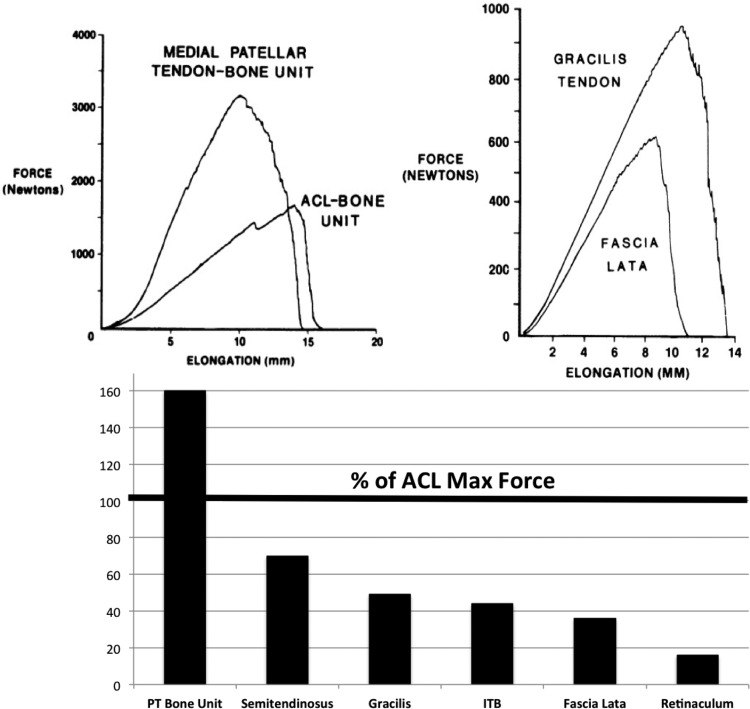
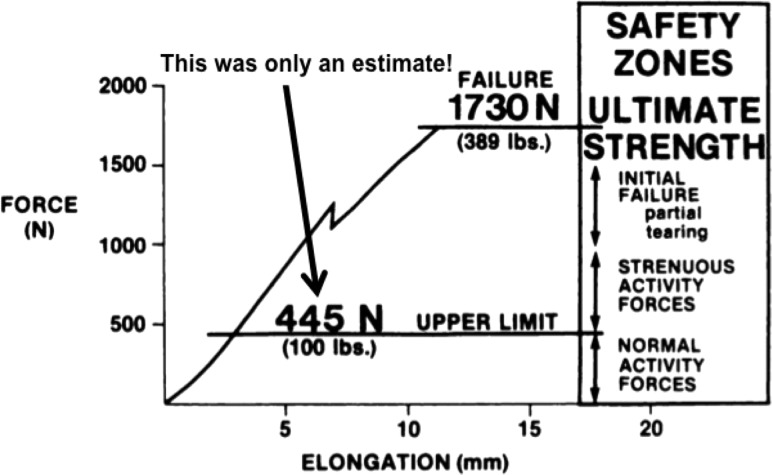
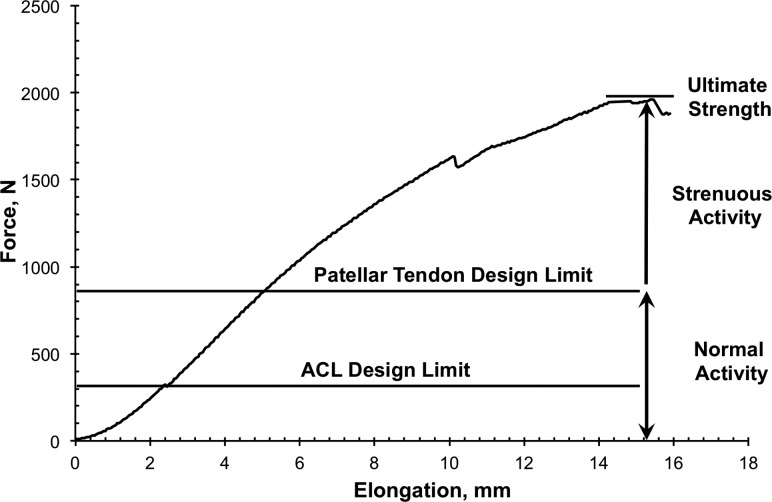
We next used biomechanics concepts to better treat anterior cruciate ligament injuries. Knee surgeons have sought to restore normal anterior knee laxity after ACL surgery. The greatest challenge in the early 1980s was deciding which biological graft to implant to most effectively restore normal laxity. Surgeons were using many ligament grafts, including two tissues on the medial side of the knee (semitendinosus and gracilis tendons) [88,89], two lateral structures (fascia lata and distal iliotibial band) [90], and three anterior tissues (central and medial portions of the patellar tendon-bone unit and quadriceps tendon-prepatellar tissue-patellar tendon or retinaculum) [91,92]. As no generally accepted treatment plan existed to choose among them, we contrasted the initial biomechanical properties of commonly used ACL cadaveric grafts (n = 90 specimens) from 18 young adult donors (26 ± 6 years old; X ± SD). We measured maximum force (strength) and linear stiffness [12] along with maximum stress and linear modulus [13]. Relative to results for young adult ACL-bone units (Fig. 5), the strongest grafts were bone-patellar tendon-bone units (159%–168% of ACL failure force). Semitendinosus and gracilis tendons developed maximum forces of 70% and 49% of ACL maximum force, respectively. All other tissues were weaker, with retinacular tissues transmitting only 14% to 21% of ACL maximum force (Fig. 5). This study was impactful because (1) the bone-central patellar tendon-bone graft became the “gold standard” for ACL ligament reconstruction for the next 20–30 years and (2) the concept of “safety zones” was first introduced for different levels of activities of daily living or ADLs (Fig. 6 [12]). While designing a ligament graft to withstand failure forces of 1700 N would be ideal, more important might be to design grafts to tolerate normal and even strenuous ADLs. Unfortunately, at that time, the field could only estimate what fraction of failure force those ADLs might be [12]!
Fig. 5.
Maximum forces generated by graft tissues compared to the young adult anterior cruciate ligament-bone unit. Central- and medial bone-patellar tendon-bone units were the strongest tissues (159%–168% of ACL failure force). The semitendinosis (70%) and gracilis (49%) tendons were somewhat weaker than the ACL. All other structures were still weaker, with the retinacular tissues transmitting only 14%–21% of ACL maximum force. Adapted with permission from Ref. [12].
Fig. 6.
Designing a graft to withstand normal ligament failure forces is ideal. However, designing grafts within “safety zones” for normal and strenuous ADLs might matter more. Unfortunately, researchers in the mid-1980s could only estimate these force limits. Adapted with permission from Ref. [12].
Phase 2: Tissue Level.
Over the next 9 years, our research moved from the organ/tissue level to the tissue level. We established structure-function relationships for normal and healing tendon and ligament and determined in vivo tendon and ligament forces for various ADLs. While conducting anterior tibial displacement (drawer) tests on human cadaveric knees, we noted a much higher restraining load from the anteromedial band (AMB) of the ACL than the posterolateral band (PLB). Subsequent analysis suggested significantly different material properties for these bundles, consistent with the belief that the AMB is the more frequently loaded group of fascicles in the ACL. We previously found that bone-fascicle-bone subunits from young human cadaveric knee ligaments (ACL, PCL, and lateral collateral ligament) displayed significantly lower material properties than values for subunits from patellar tendon [15], which we suggested was caused by differences in typical in vivo force levels. On closer analysis of ACL data, we found the anteromedial band of the ACL exhibited significantly greater load-related material properties (linear modulus, maximum stress, and strain energy density) than did the posterolateral band [20]. Race and Amis observed similar spatial differences for subunits from human PCL [93]. While we could not conclude that these spatial variations were a result of differing in vivo loads, the data suggested that tendons and ligaments are load sensitive and that measuring these in vivo forces could help address this question.
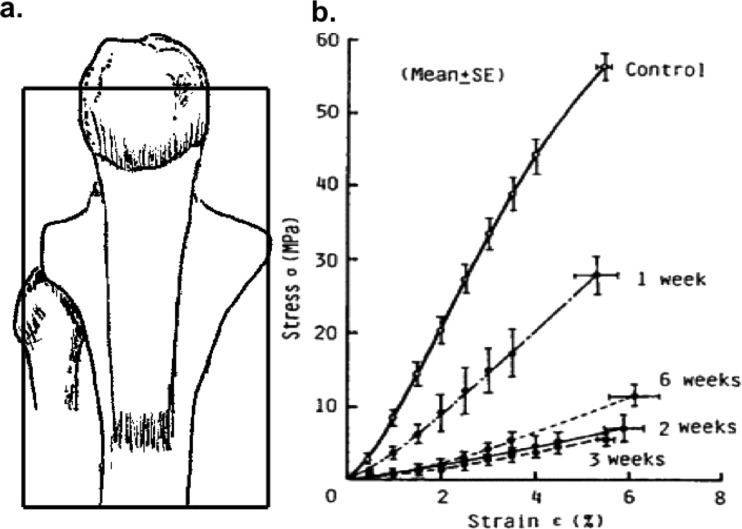
In the early to mid-1990s, investigators were seeking to control in vivo forces in tendons and ligaments. Yamamoto and coworkers [94] unloaded rabbit patellar tendon at surgery using transpatellar and transtibial pins connected by surgical suture (Fig. 7(a)). The group showed that, between 1 and 6 weeks after unloading, patellar tendon linear modulus and maximum stress declined by 70% to 80% (Fig. 7(b)). Bush-Joseph and Grood, in our lab, unloaded the goat ACL by isolating and posteriorly translating its tibial plateau closer to the PCL [34]. After 6 months, the unloaded ACL-bone unit provided only 58% and 34% of unoperated tissue stiffness and maximum force, respectively. These results emphasized the need to measure in vivo forces in the ACL to help researchers (1) design more effective repair strategies, (2) establish benchmarks of success, and (3) discover preconditioning protocols for tissue-engineered constructs.
Fig. 7.
Unloading the rabbit PT with K-wire and sutures produced 70%–80% reductions in tissue material properties by 6 weeks postsurgery. Adapted with permission from Ref. [94].
We then developed techniques to measure in vivo signals to compute in vivo forces after calibration. We designed implantable force transducers (IFTs) to insert in pockets within ligaments and tendons [95,96]. These stainless steel and titanium curved beams were instrumented with strain gauges. After insertion at surgery, the displaced tissue fibers transmitted forces that deflected the IFT and created a voltage change. Calibration of the instrumented tissue after sacrifice allowed us to compute tissue force from these voltage readings. Using these devices, we computed peak in vivo forces to quantify functional design limits or benchmarks for improving tissue repairs.
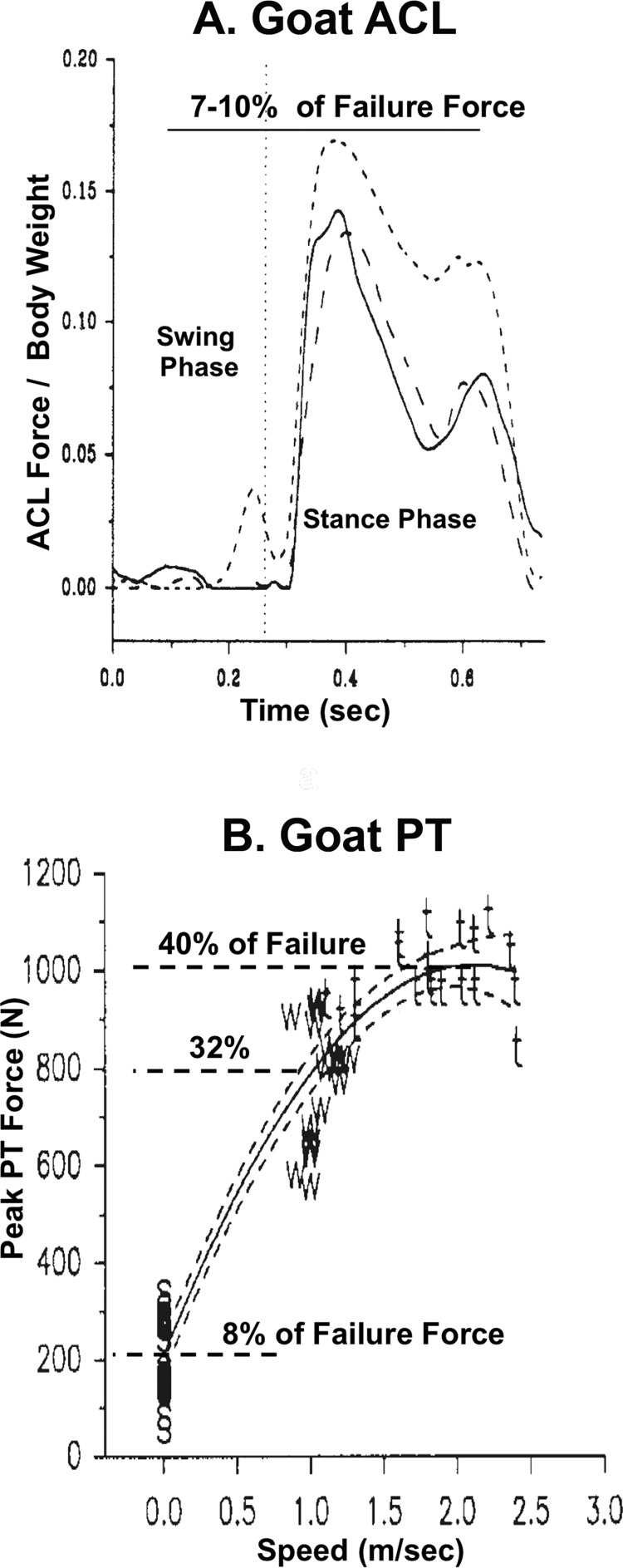
This strategy was then used to determine in vivo forces in goat and rabbit tendons and ligaments. Quite different peak in vivo forces were found in the goat ACL [97] and patellar tendon (PT) [33] for a range of ADLs. While the ACL never developed more than 7%–10% of the tissue's failure force (Fig. 8(a)) [97], the PT transmitted 8% of failure force during quiet standing (Fig. 8(b)) that increased to 32%–40% of failure force at gait speeds of 2.0 to 2.5 m/s (Fig. 8(b) [33]). We concluded that tendons, such as the patellar tendon, possess higher functional design limits than do ligaments like the ACL (Fig. 9). However, even these design limits varied widely among tendons. Voltage recordings in the rabbit patellar [37], Achilles [38], and flexor digitorum profundus tendons [35] revealed that peak forces and stresses achieved a wider range of 11%–29% of corresponding failure values. This variability in peak forces (and stresses) with animal and tissue model suggested that, to improve tissue repair outcome, we needed to select an animal and tissue model system where these forces could be more tightly controlled.
Fig. 8.
Peak in vivo forces in the patellar tendon are larger than those in the anterior cruciate ligament. (a) Note that peak IVFs in the goat ACL are negligible during the swing phase of gait, increasing rapidly during stance but never exceeding 7%–10% of the tissue's failure force. (b) Peak PT force is 8% of failure force during stance phase, increasing rapidly during gait to 32%–40% of failure force at 2.0–2.5 m/s. Adapted with permission from Refs. [97] and [33].
Fig. 9.
Functional design limits for the goat anterior cruciate ligament were found to be less than those for the goat patellar tendon. Adapted with permission from Ref. [12].
Phase 3: Tissue/Cell Level.
In 1995, we began studies in tissue engineering and functional tissue engineering. In collaboration with Dr. Arnold Caplan at Case Western Reserve University and with Osiris Therapeutics, Inc., we found that we could improve rabbit Achilles tendon (AT) defect repair using autologous mesenchymal stem cells (MSCs) in a collagen gel [39,55]. However, the AT injury site was not easily accessible and tissues were challenging to fail in tension. We thus decided to continue our work using only the full-thickness central defect in the rabbit patellar tendon (PT). This model had several advantages. We could evaluate mesenchymal stem cell therapies in an easily accessible and reproducible “load-protected” repair environment with intact adjacent tendon struts. This injury model permitted us to successfully isolate the repair tissue and reliably grip patella and tibia during failure testing [46]. This same method was continually applied in our later tissue engineering studies [50,54–57,68,69]. However, before beginning this next series of studies, we needed a more logical and functional strategy to properly evaluate tissue-engineered construct (TEC)-based repairs for tendon and other load-bearing tissues.
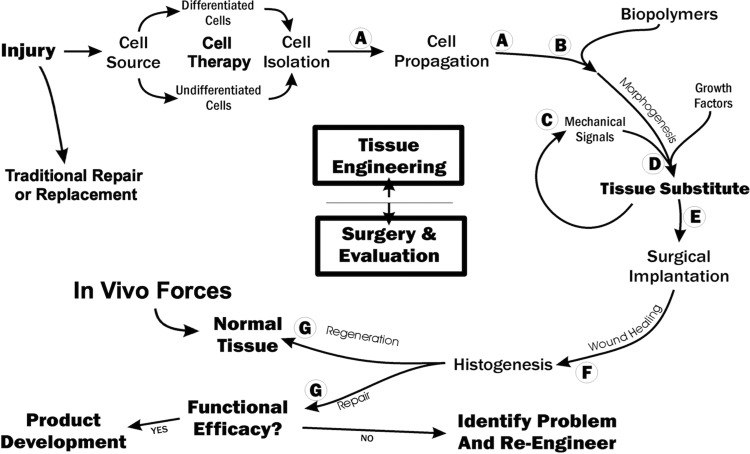
Working as part of the US National Committee of Biomechanics in 1998 and 1999, the first author (D.L.B.), Dr. Steve Goldstein from the University of Michigan, and Dr. Farsh Guilak from Duke University published the first paper describing functional tissue engineering (FTE) [42]. We then published a second paper describing functional tissue engineering of articular cartilage [44] and held a NIH-funded workshop [45]. Three key principles of FTE that helped direct our own research included the need to (1) measure normal in vivo stress/strain histories for a variety of activities; (2) set standards by answering the question, “How good is good enough?”; and (3) determine whether cell-matrix implants could be mechanically stimulated before surgery to improve repair. With regards to “how good is good enough,” the group recognized the need for minimum mechanical (peak forces, tangent stiffness, and safety factor) and biological standards (cell density, growth factor stimulation, etc.) that are not yet known for all tissues and that might interact with each other! Our group then developed a FTE “roadmap” to better track the progress of our in vitro tissue engineering studies as well as our surgery and evaluation efforts (Fig. 10) [47]. We also proposed functional tissue engineering parameters for frequently loaded musculoskeletal tissues based on increasing tissue complexity and knowledge of in vivo forces and displacements [48].
Fig. 10.
Functional tissue engineering roadmap. Shown are the in vitro, tissue engineering phase required to create a tissue engineering substitute or construct as well as the important surgery and evaluation phase to determine if the repair regenerates the tissue to exceed in vivo forces or at least repairs to achieve functional efficacy. Adapted from Ref. [47].
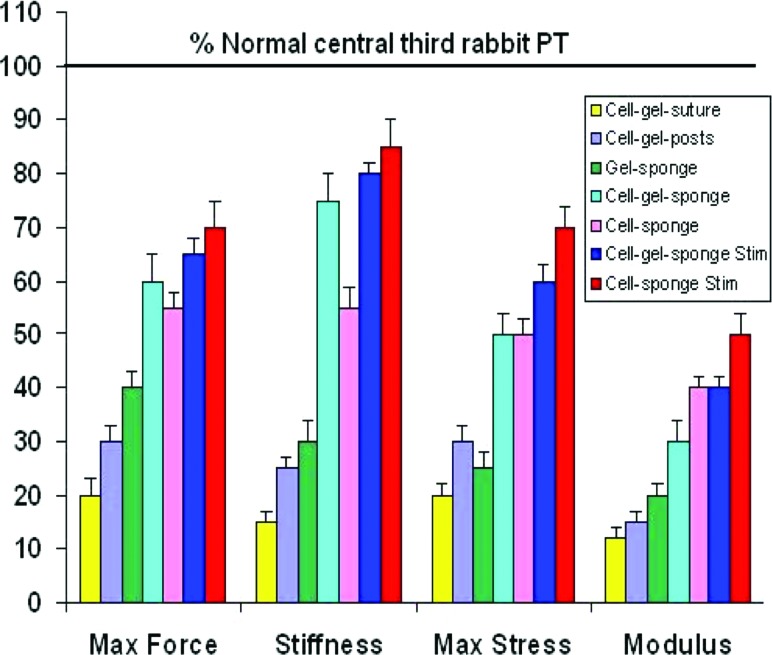
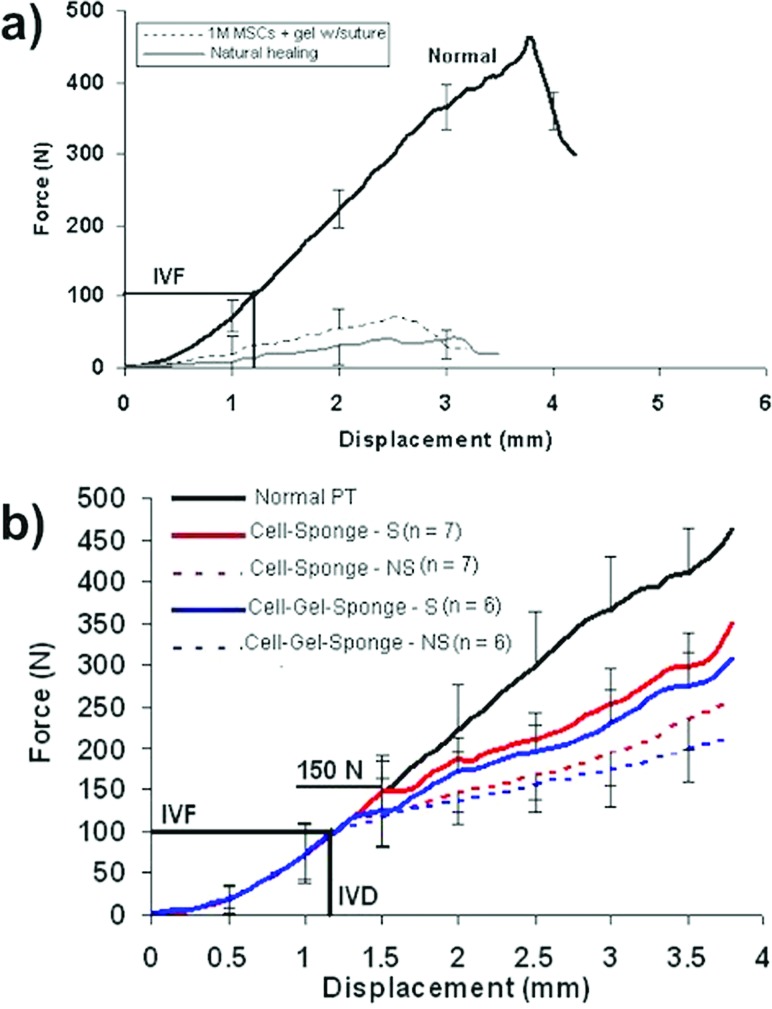
Over the next 8 years, we applied this FTE paradigm [69] to our rabbit PT defect model. We continuously improved repair biomechanical properties, including maximum force and stiffness as well as maximum stress and linear modulus (Fig. 11). We initially investigated whether increasing the density of MSCs in the tissue-engineered constructs would improve central PT repair at three time periods (6, 12, and 26 weeks postsurgery) [46]. DiI-labeled MSCs were contracted in a collagen gel around a central, load-bearing suture for up to 72 h before surgery [43]. While all cell densities (1, 4, and 8 × 106 cells/ml) significantly improved repair biomechanics compared to natural healing at 12 and 26 weeks after surgery, the failure curves for these repairs did not resemble the failure curve for the normal central PT (Fig. 12(a)) [37,46]. The failure curves did not exceed peak in vivo forces (IVFs) for the tendon nor did they match normal PT stiffness (slope) up to these peak values. The improvements were also independent of cell density and ectopic bone formed in 28% of repair sites, regardless of cell density and time postsurgery. Cells within these bone spicules contained the DiI label used to mark the implanted MSCs. A follow-up study in culture showed that alkaline phosphatase, an early bone marker, was elevated after the MSCs contracted the gel around the suture [49]. We concluded that we had failed both in achieving two mechanical design targets (to exceed IVF and to match tangent stiffness) and in our biological outcome (to avoid ectopic bone and to achieve aligned collagen fibers anchored into bone through a fibrocartilage zonal insertion site). Before proceeding, we chose to decrease the cell density in our TECs and remove the stiff, potentially stress-shielding suture.
Fig. 11.
Continuous improvement in traditional biomechanical properties, including maximum force, stiffness, maximum stress, and linear modulus. These improvements involved changes in cell density, collagen scaffold stiffness, and the use of mechanical preconditioning of the TEC before surgery. Adapted from Ref. [69].
Fig. 12.
Tissue-engineered constructs containing MSCs in a collagen scaffold improve central rabbit PT repair. (a) Constructs containing a high cell density (1 × 106 cells/ml) produce a small but significant improvement in the force-displacement repair curve compared to natural healing. Not only does the failure curve for the TEC repair not match that for the normal unoperated PT, the 12-week repair also does not reach the peak in vivo forces (IVFs) acting on the normal central PT or match normal tangent stiffness. (b) Lowering the cell density, stiffening the collagen scaffold, and mechanically preconditioning the constructs before surgery resulted in improvements in failure properties as well as functional parameters (exceeding peak IVFs and matching normal PT tangent stiffness with a 50% safety factor). Adapted from Ref. [56].
We then systematically modified our TECs to improve repair. We reduced the cell density by tenfold (to 100 K cells/ml), replaced the suture with a silicone dish outfitted with two vertical posts that allowed for matrix (and cellular) deformation [54,59,61], stiffened the TECs by replacing the collagen gel with a collagen sponge scaffold [54,64], and mechanically stimulated (preconditioned) the TECs before implantation [56,60,62,64,68,69]. All of these changes resulted in failure curves for repairs that greatly exceeded the peak in vivo forces level for two different sponge scaffolds (Fig. 12(b)) with tangent stiffness values matching the normal PT up to 50% beyond peak IVFs [56]. These improvements provided a buffer beyond the peak values recorded in the rabbit PT, which could provide protection should larger in vivo forces further challenge the repairs. By reducing cell density and eliminating the suture, we observed no further ectopic bone in any tendon repairs. Our strategy of harvesting bone marrow-derived MSCs had one other benefit: it permitted us to create enough constructs to directly compare the stiffness and modulus of the in vitro TEC with the stiffness and modulus of the in vivo repair [56]. The significant correlations between these in vitro and in vivo measures (i.e., increasing TEC stiffness/modulus in vitro leads to improved repair tissue stiffness/modulus at 12 weeks) allowed us to perform less costly and more rapid in vitro TEC screening and optimization experiments [56,57,68,69]. We also employed response surface methodologies [98] to optimize preconditioning parameters, such as the peak strain and number of cycles per day necessary to improve TEC stiffness before surgery [62]. We found that the highest in vitro stiffness was achieved when constructs were exposed to 2.4% peak strain for 3000 cycles per day [62].
Although such strategies for optimization to achieve mechanical design limits are critical, we also recognized the need to better understand biological design limits. The goal of Phase 4 thus became to learn how to improve the biology of our TECs and the resulting repairs.
Phase 4: Tissue/Cell/Molecular Level.
Beginning in 2004, we sought to incorporate more biological response measures into the tendon tissue engineering design process [57,65,72]. We needed a mechanobiology paradigm [80,81] to identify biological design targets to accompany the biomechanical ones (matching tangent stiffness, exceeding peak IVFs, and incorporating a safety factor). This process also required multidisciplinary collaborations. To achieve these objectives:
(1) We first needed a more comprehensive set of design targets for tendon and other load-bearing tissues. Three investigators (Cy Frank, M.D., Jack Lewis, Ph.D., and David Butler, Ph.D.) organized a NIH-sponsored conference in 2007 to develop mechanical, structural, biological, and clinical evaluation criteria for musculoskeletal and craniofacial load-bearing tissues. A small group of bioengineers, biologists, material scientists, and surgeons were recruited from academia, industry, and government. The group used a “reverse tissue engineering strategy” to carefully define two important clinical problems for each tissue type (ligament, articular cartilage, bone, etc.) [70]. Multidisciplinary teams were then assembled to identify design parameters and their minimally acceptable values for each tissue type and clinical problem. Preclinical studies were discussed that would support these clinical studies. Only then did the group propose in vitro laboratory studies to complement these preclinical and clinical studies. The resulting publication [70] summarized conference findings and was useful as our research team expanded our criteria for tendon tissue-engineered constructs.
(2) We needed to expand our preclinical models and link those species amenable for biological studies with those more suited for translational studies [99]. At this stage, our tendon and ligament structure-function, repair, and replacement studies had been conducted in larger rabbit [24,35–40,46,49–51, 54–58,62,65,67–69,73], canine [100–103], goat [18,19,21,23,25, 30,31,33,34,66,97,104–106], and nonhuman primate [26] models. These models permitted (a) the surgeon to more reproducibly create and repair the injuries and (b) the bioengineer to more easily determine in vivo forces and measure repair biomechanics relative to nonoperated controls. However, taking advantage of powerful genetic tools required that we move to smaller murine models. While these models had clear advantages (e.g., transgenic and knockout models to better understand developmental and repair mechanisms), they also presented challenges due to their small size (e.g., performing reproducible surgeries, directly recording in vivo mechanical signals, and accurately measuring structural and material properties in normal and repair tissues). The question we now faced was choosing the most effective “biological” or “mechanobiological” path to create suitable repairs. Should biological design limit mimic successful adult repair? Should we actually regenerate the tissue by mimicking normal tissue development? Or would we choose some combination of mechanical and biological strategies? We chose a mechanobiology strategy to mimic tissue development [75,79].
We next expanded our research team to study how normal tendon development might improve tissue repair in the adult [57,75,79,80]. Working with Dr. Christopher Wylie and coworkers at Cincinnati Children's Hospital Medical Center, we sought to link mechanical and biological indicators of success. We proposed a new subfield called “fundamental tissue engineering” or FdTE, which merged functional tissue engineering (FTE) and developmental biology [82]. Since then, the goals of FdTE have been to (1) expand our mechanical design criteria, (2) seek biological signals mimicking normal tissue development, and (3) meld these data sets using novel statistical methods, such as multiresponse surface methodology that weighs quantitative and semiquantitative outcomes [98]. FdTE would use development to improve repair by (a) systematically studying normal cell signaling and gene expression during late embryonic and early postnatal murine development [79,80], (b) contrasting these results with adult natural healing [81], (c) utilizing signals from development in combination with mechanical loading to better precondition our TECs, and (d) linking these patterns across species to improve TEC design in culture and to more rapidly repair or regenerate the damaged tendon tissue and insertion site into bone.
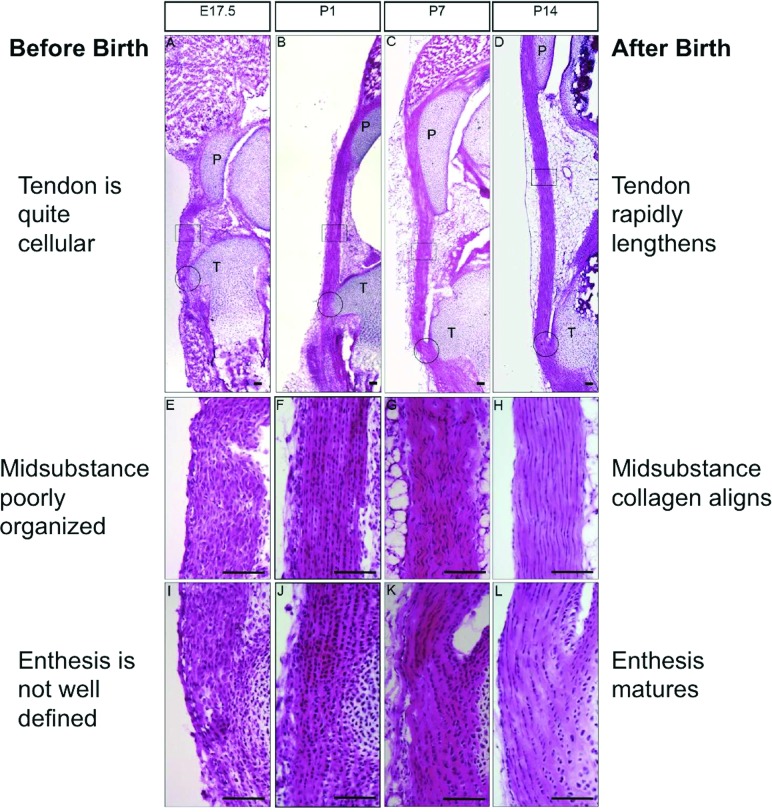
Our studies began by characterizing the histology and immunochemistry of the murine patellar tendon during late fetal life to two weeks after birth [80]. Three to four days before birth (at E17.5), the tendon midsubstance was quite cellular and its extracellular matrix rather poorly organized (Fig. 13). The cartilaginous insertion was also not well defined. Postnatally, the tendon rapidly lengthened from P1 to P14, its cellularity decreased, the midsubstance collagen aligned, and the insertion formed and began to mature into fibrocartilage and bone (Fig. 13). The number of cycling cells also decreased over time based on Ki67 results showing cell proliferation. Immunohistochemistry revealed that, from late fetal life to two weeks after birth, collagen I, tenomodulin, and fibromodulin were expressed rather uniformly throughout the tendon, while biglycan, COMP, and tenascin-C expression was highly enriched in the attachment site [80]. Cell signaling pathways, such as transforming growth factor β (TGFβ) and bone morphogenetic protein (BMP) were activated throughout the developing tendon, while cells responding to hedgehog (Hh) signaling were restricted to the insertion [80]. These results have led us to try and (1) identify which markers and signaling pathways are useful biological design targets for therapeutic intervention and (2) find other differentially expressed genes using whole transcriptome technologies, like RNA sequencing (RNA-Seq).
Fig. 13.
The murine patellar tendon rapidly changes its structure and cellularity from late fetal life to 2 weeks after birth. The tendon midsubstance and insertion are cellular and their extracellular matrices are rather poorly aligned at E17.5. Postnatally, the tissue midsubstance shows decreasing cellularity and increasing collagen alignment from P1 to P14. The insertion is also maturing into fibrocartilage and bone. Adapted with permission from Ref. [80].
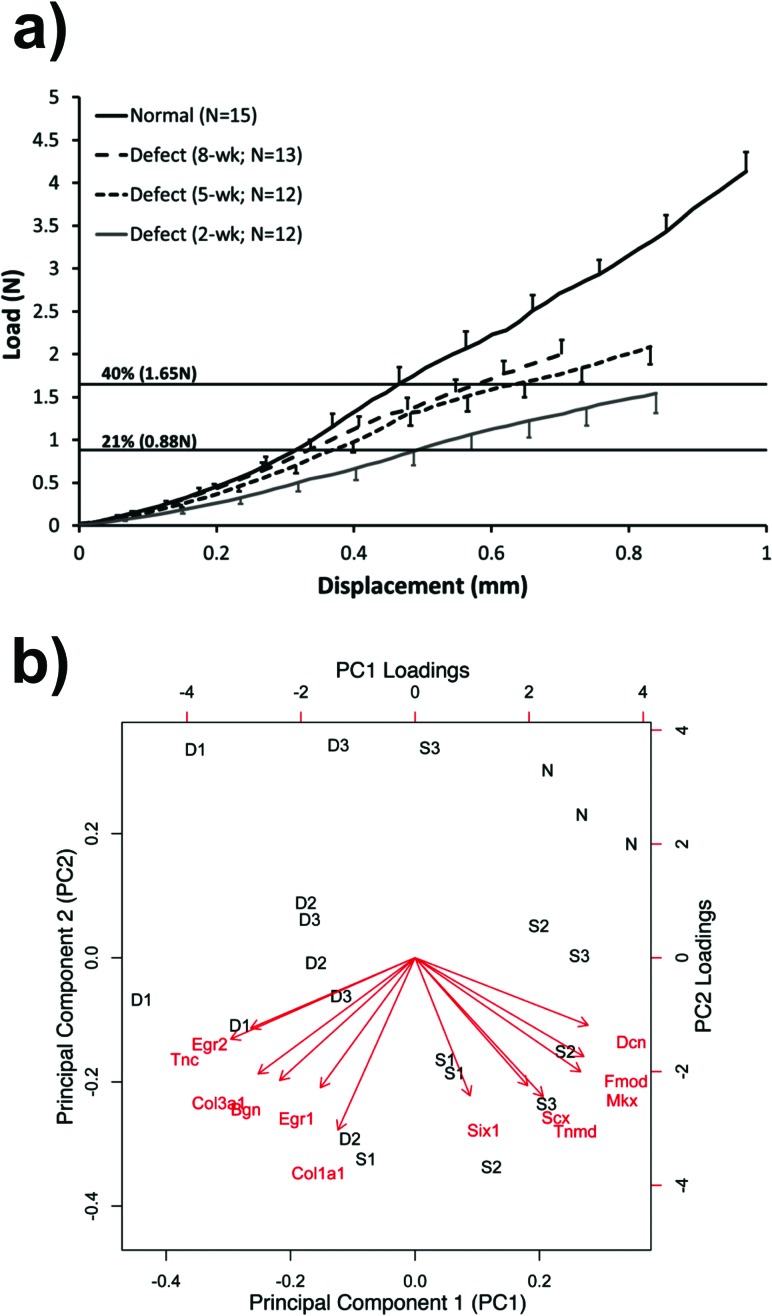
This same murine model could also be useful for studying natural tendon healing in the adult and possibly establishing homology with the rabbit patellar tendon defect injury model [46,50,54,55,57,68]. Like the rabbit, central full-thickness defects in the murine PT heal slowly compared to normal, up to 8 weeks after injury (Fig. 14(a) [81]). However, unlike the rabbit, the murine tissue was too small to directly determine in vivo forces [37]. Instead, we used peak IVF force design limits from the rabbit PT (21% of failure force [37]) and goat PT (40% of failure force [33]) to set lower and upper force limits, respectively (Fig. 14(a) [81]). We are also using quantitative real-time polymerase chain reaction (qRT-PCR), immunohistochemistry, and green fluorescent protein (GFP) reporters to measure expression patterns of tenogenic markers and the origin and phenotype of the progenitor cells that replace the damaged tissue. Principal component analysis [98] is providing panels of genes to contrast defect healing with shams and normal PTs to establish biological design thresholds that link with our mechanical thresholds (Fig. 14(b)). When established, these biological targets could be useful predictors of later mechanical outcomes.
Fig. 14.
Natural healing of murine central patellar tendon defect injury. (a) Healing occurs slowly between 2 and 8 weeks postinjury when compared to the normal tendon failure curve. Estimated upper and lower peak in vivo force bounds are shown (using rabbit results from Ref. [37] and goat results from Ref. [33]) (from Ref. [81]). (b) Panels of genes for normal, sham, and defect healing groups at 1, 2, and 3 weeks postsurgery analyzed using principal component analysis.
Clinical, Biomechanical, and Biological Considerations in Mechanobiology Strategies for Treating Musculoskeletal Injuries
These developmental and adult natural healing studies are now driving our efforts to create more effective tissue-engineered constructs (TECs) in the murine model that can be translated to larger species. The biological response of these TECs will require fine-tuning the biological components of the collagen sponge. For example, researchers may want to incorporate glycosaminoglycans into the collagen sponge to improve gene expression of tendon genes of interest [72] or optimize pore structure in the collagen sponge to improve expression of tendon genes [107]. Alternatively, we could use acelluar extracellular matrix (ECM) scaffolds with normal 3D architecture and glycosaminoglycans (GAGs) and growth factors as long as porosity permits cellular and vascular infiltration. Ultimately, we will need candidate tenogenic markers important in normal tendon development that are insufficiently expressed in adult healing to be applied across model systems.
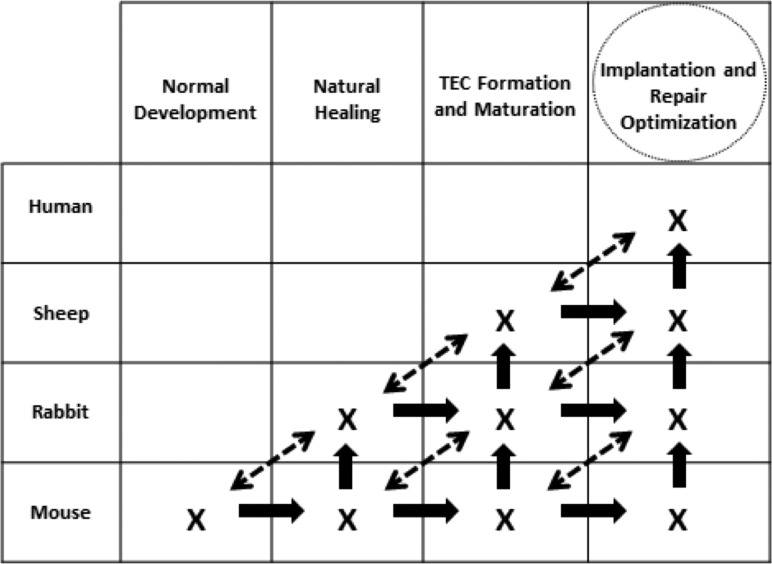
We hope to use murine normal development and natural healing to form and mature TECs for implantation and repair optimization (Fig. 15). This strategy will be most effective if we (a) move horizontally, vertically, and even obliquely across species and (b) create multidisciplinary teams in multiple sites using common model and tissue systems. Clearly translating FdTE discoveries to larger models will require (1) preclinical model systems with common markers across species; (2) agreement on clinically relevant injuries to pursue; (3) a systematic transition to larger models, where surgeries are better controlled but repairs experience greater mechanical demands; and (4) small initial successes that can be applied to other load-bearing musculoskeletal and cardiovascular tissue systems.
Fig. 15.
A fundamental tissue engineering strategy that seeks to more rapidly design, evaluate, and optimize tissue-engineered constructs using normal tissue development, natural healing, and TEC manipulation across species. Adapted from Ref. [82].
Developing a comprehensive treatment strategy will involve clinicians, bioengineers, biologists, and material scientists from industry, academia, and government laboratories [70]. This plan should use reverse tissue engineering that clearly defines the clinical problem before initiating preclinical and then in vitro studies. If properly conducted, the plan could engage many investigators and secure the necessary funding to make rapid advances in identifying successful tissue engineering therapies.
Future efforts during the next wave of tissue engineering will require a balance to find the “sweet spot” positioned among clinical, biomechanical, and biologic needs. Tissue engineers will need to tackle clinical problems using biological and biomechanical strategies with rational design criteria and values that reflect a successful outcome. This remains one of our greatest challenges 25 years after the field was first defined [108].
Future Grand Challenges and Opportunities for Young Bioengineers
There are many grand challenges and opportunities for young bioengineers as they advance the field of tissue engineering. Particularly important are those listed below.
Better Understand the Biomechanics and Biology of “Normal.”
A difficult task still facing the field is establishing what is normal. For example, finding the mechanical design limits for a repair means that we determine peak in vivo forces for actual ADLs for relevant and frequently injured tissues, like the ACL. Many investigators have estimated these forces by measuring surface skin motions or bone-to-bone motions and then modeling joint structures to compute individual tissue forces. Our approach in the sheep has been to directly monitor vertical ground reaction forces during controlled gait speeds and inclinations [109] followed by recordings of 3D kinematics and ACL transducer voltages after surgery. Similar to sheep studies performed by the Calgary group [110,111], we have then used the instrumented limb to drive a robot to replicate these motions in order to calibrate the transducer and to compute 3D ligament forces and joint contact loads [109,112,113]. Once successful, this strategy sets the mechanical design targets for even complex structures, like the ACL. Once these tissue-specific limits are known, investigators can (a) better judge the merits of primary ligament repairs and replacements in models like the sheep and (b) begin to estimate ADL-related forces in humans [114–123]. In a similar manner, developing complementary methods to monitor real-time biological markers for various activities would be equally impactful in formulating a more comprehensive set of benchmarks for promising tissue engineering therapies.
Understand the Fundamentals and Fate of “Natural Healing.”
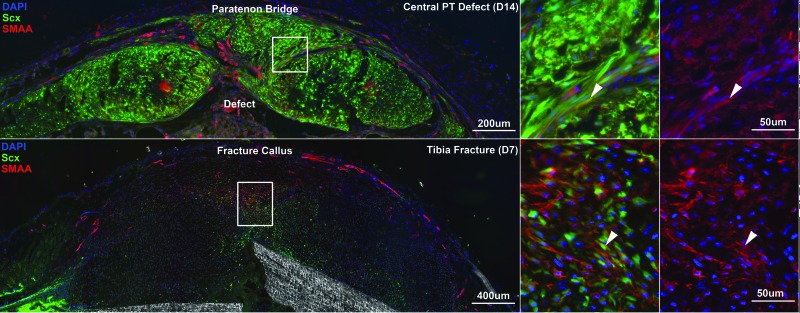
A natural temptation in tissue engineering is to immediately seek cell- and/or scaffold-based therapies. However, it is probably more important to first understand the natural healing capacity of the injured tissue in the preclinical model of choice. If spontaneous healing occurs in this simulation of the clinical injury, it becomes hard to justify its use to study new and novel treatments. Current efforts by one coauthor (N.A.D.) are tracking the source, path, and timing of cells, attempting to fill the natural healing defect in the murine PT (Fig. 16, top panels). These studies can identify intrinsic versus extrinsic cell sources and how they contribute to natural healing and how they compare to cells that drive development and growth. It will be important to determine conditions in which specific genes are either upregulated or not present while bridging the defect gap. Linking these findings to the biomechanical results also firmly establishes the degree to which the tissue is capable of healing. As shown in Figs. 12 and 14, establishing the envelope between “normal” and “natural healing” highlights opportunities to improve outcome and reveals new biological (and biomechanical) therapies to more rapidly and completely repair the wound site.
Fig. 16.
Tendon healing shares similar characteristics with bone healing. Central PT healing in the mouse (upper panel, cross-sectional view) results in paratenon progenitor cells proliferating and migrating to form a bridge over the anterior surface of the defect space. This response is similar to fracture callus formation in tibial fractures (lower panel). Scleraxis (Scx) GFP reporter expression and smooth muscle actin α (SMAA) immunostaining (red) label potential early progenitor cells in these healing scenarios. White arrows indicate coexpressing cells.
Look for “Similarities” and Not Just Differences.
Those in tissue engineering most often customize or individualize their approaches to treating injury. For example, tissue engineers working to understand natural healing and to improve ligament repair might adopt different strategies than those focused in tendon, bone, or cartilage applications. Clearly, differences exist. Cartilaginous tissues may be the most problematic to successfully repair and bone and muscle (with more pronounced vascularity) the easiest. However, there are likely connections between these tissue subsystems that might warrant closer inspection. For example, we are finding that “extrinsic” paratenon healing of patellar tendon defect injuries (Fig. 16, top panels) resembles, in some respects, “extrinsic” periosteal healing following creation of subcritical defects in the midshaft of the murine femur (Fig. 16, bottom panels). Cells migrate along the paratenon and the periosteum and express scleraxis (Scx) and smooth muscle α actin (SMAA) in a specific order over time. Knowing results from one subsystem could benefit those working in the other. Those working in multiple models or who collaborate with those who do improve their chances of finding promising candidates to heal more than one wound type. Such connections may extend beyond the musculoskeletal field as well.
Test New Paradigms Using More Complete Roadmaps.
Future breakthroughs in tissue engineering will require revolutionary as well as evolutionary paradigms with more comprehensive roadmaps to guide the field. Many examples will certainly be forthcoming. (1) While we have chosen to compare development with natural healing to direct our TE strategies, there likely exists a significant gap between the phenotypes of the cells contributing to these two vastly different processes and between the mechanical environments acting on these cells. It could prove useful to generate TE strategies that move beyond development to incorporate tissue growth and maturation effects. The biological and mechanical environments change rapidly during these phases, and understanding the direction and rate of these effects could lead to new therapies for improved healing in the adult. (2) Tissue engineering may also be quite valuable in studying developmental biology, given its ability to control matrix chemistry and mechanics combined with construct loading. (3) With regard to finding similarities rather than differences, why not more fully compare models of successful adult healing? What mechanobiological features permit bone to remodel and regenerate while ligament and articular cartilage heal poorly or not at all? Closer communication among investigators working in these fields might lead to new discoveries. (4) Do we need to develop more in vivo incubator systems for creating novel tissue-engineered constructs rather than in vitro chambers that slow the process and create less realistic environments for TEC maturation? (5) How will novel therapies discovered in the laboratory become more readily available to patients while still satisfying US Food and Drug Administration panels that have tended to approve more conventional approaches? (6) Scale-up and cost containment strategies are also needed where cross-species connections are made, and large numbers of constructs are created of sufficient size and scale to meet surgeon and patient expectations.
Tissue engineering remains a complex and quite interactive process [69,74]. Cells and biomaterials are mixed in different configurations to create a tissue-engineered construct that is often mechanically and/or chemically stimulated before surgery. After surgery, various outcome measures (clinical, mechanical, biological, and structural) are then used to assess the resulting repair. Unfortunately, interactions among these steps means that changes in any of them (e.g., cell density or material type) can dramatically alter the final result. Utilizing a reverse tissue engineering approach [70] that first broadly defines biomechanical and biological repair functionality and design limits before creating clinically relevant preclinical models and beginning in vitro experiments offers hope that tissue engineers will strategically assess individual and combined effects of these important TE factors. Bioengineers and future Lissner Medal winners will and should play a critical role in this evolving design process!
Acknowledgment
The authors wish to acknowledge support from the following sources: NIH Grants NIH AR46574, AR56943, AR47054, AR56660, EB002361, EB004859, and T32 GM 063483 to the University of Cincinnati Medical Scientist Training Program; NSF IGERT Training Program 333377; Cincinnati Sportsmedicine Research and Education Foundation; University Orthopaedic Research and Education Foundation; and the Veteran's Administration.
Several individuals have had a very important influence in the evolution of this research. The first author is particularly grateful to two long-time collaborators and friends, Dr. Edward Grood and Dr. Frank Noyes, who were so important during the initial two phases of our research. He has also had the good fortune to interact with and know most of the prior winners who have influenced his career and who have greatly impacted the Bioengineering Division. Among those working in the cardiovascular field, Dr. Y. C. Fung, Dr. Robert Nerem, and Dr. Richard Skalak were incredible role models for the next generation of bioengineers by providing insight about research direction along with repeated encouragement and technical expertise that greatly shaped the engineering and science performed by those who followed. And in the musculoskeletal field, colleagues like Dr. Savio Woo, Dr. Albert Burstein, Dr. Albert Schultz, and Dr. Van Mow have contributed and guided so many of us interested in ligament, cartilage, and bone tissue applications. The dedication, leadership, and mentorship of all of these individuals and others cannot be overstated.
The authors also want to recognize the many other collaborators over the past 36 years who have contributed to aspects of this work. These individuals include bioengineering colleagues at the University of Cincinnati (Dr. Hani Awad, Dr. Aditya Chaubey, Dr. Kumar Chokalingam, Dr. John Cummings, Dr. Matthew Dressler, Dr. Bala Haridas, Dr. John Holden, Dr. Shawn Hunter, Dr. Natalia Juncosa-Melvin, Dr. Sanjit Nirmalanandhan, and Dr. Donald Stouffer, as well as Mr. David Glos, Mr. Matthew Harris, and Dr. John West); veterinary and human surgery colleagues in Cincinnati (Dr. Greg Boivin, Dr. Chris Casstevens, Dr. Marc Galloway, Dr. Brian Grawe, Dr. Michael Greiwe, Dr. Samer Hasan, Dr. Keith Kenter, and Dr. Donna Korvick); researchers at Cincinnati Children's Hospital (Lindsey Aschbacher-Smith, Jane Florer, Chris Frede, and Dr. Richard Wenstrup); bioengineering, biological, and design collaborators and coauthors in the musculoskeletal field (Ms. Mary Beth Privitera and Dr. Al Banes, Dr. Arnold Caplan, Dr. David Fink, Dr. Steve Goldstein, Dr. Steve Gordon, Dr. Farsh Guilak, Dr. Peter Maye, Dr. Van Mow, Dr. David Mooney, Dr. Heather Powell, Dr. David Rowe, Dr. Jeff Ruberti, Dr. Ronen Schweitzer, Dr. Randall Young, Dr. Christopher Wagner, Dr. Sandy Williams, and Dr. Savio Woo).
Contributor Information
David L. Butler, Tissue Engineering and Biomechanics Laboratories, Biomedical Engineering Program, College of Engineering and Applied Sciences, University of Cincinnati; Cincinnati, OH 45221, e-mail: david.butler@uc.edu.
Nathaniel A. Dyment, Department of Reconstructive Sciences, College of Dental Medicine, University of Connecticut Health Center, Farmington, CT, Farmington, CT 06030
Jason T. Shearn, Tissue Engineering and Biomechanics Laboratories, Biomedical Engineering Program, College of Engineering and Applied Sciences, University of Cincinnati, Cincinnati, OH 45221
Kirsten R. C. Kinneberg, Department of Mechanical Engineering, College of Engineering, University of Colorado, Boulder, CO 80309
Andrea L. Lalley, Tissue Engineering and Biomechanics Laboratories, Biomedical Engineering Program, College of Engineering and Applied Sciences, University of Cincinnati, Cincinnati, OH 45221
Steven D. Gilday, Tissue Engineering and Biomechanics Laboratories, Biomedical Engineering Program, College of Engineering and Applied Sciences, University of Cincinnati; Medical Scientist Training Program, College of Medicine, University of Cincinnati, Cincinnati, OH 45221
Cynthia Gooch, Tissue Engineering and Biomechanics Laboratories, Biomedical Engineering Program, College of Engineering and Applied Sciences, University of Cincinnati, Cincinnati, OH 45221.
M. B. Rao, Department of Environmental Health, College of Medicine, University of Cincinnati, Cincinnati, OH 45267
Christopher Wylie, Division of Developmental Biology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH 45229.
References
- [1]. Bao, G. , Kamm, R. D. , Thomas, W. , Hwang, W. , Fletcher, D. A. , Grodzinsky, A. J. , Zhu, C. , and Mofrad, M. R. , 2010, “Molecular Biomechanics: The Molecular Basis of How Forces Regulate Cellular Function,” Mol. Cell Biomech., 3(2), pp. 91–105. 10.1007/s12195-010-0109-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Discher, D. , Dong, C. , Fredberg, J. J. , Guilak, F. , Ingber, D. , Janmey, P. , Kamm, R. D. , Schmid-Schonbein, G. W. , and Weinbaum, S. , 2009, “Biomechanics: Cell Research and Applications for the Next Decade,” Ann. Biomed. Eng., 37(5), pp. 847–859. 10.1007/s10439-009-9661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Butler, D. L. , Goldstein, S. A. , Guldberg, R. E. , Guo, X. E. , Kamm, R. , Laurencin, C. T. , McIntire, L. V. , Mow, V. C. , Nerem, R. M. , Sah, R. L. , Soslowsky, L. J. , Spilker, R. L. , and Tranquillo, R. T. , 2009, “The Impact of Biomechanics in Tissue Engineering and Regenerative Medicine,” Tissue Eng. Part B Rev., 15(4), pp. 477–484. 10.1089/ten.teb.2009.0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Ateshian, G. A. , and Friedman, M. H. , 2009, “Integrative Biomechanics: A Paradigm for Clinical Applications of Fundamental Mechanics,” J. Biomech., 42(10), pp. 1444–1451. 10.1016/j.jbiomech.2009.04.001 [DOI] [PubMed] [Google Scholar]
- [5]. Dalgaard, P. , 2008, Introductory Statistics With R, Springer, New York, p. 364. [Google Scholar]
- [6]. Butler, D. L. , Noyes, F. R. , and Grood, E. S. , 1978, “Measurement of the Biomechanical Properties of Ligaments,” CRC Handb. Eng. Biol., 279(1), pp. 279–314. [Google Scholar]
- [7]. Butler, D. L. , Zernicke, R. F. , Grood, E. S. , and Noyes, F. R. , 1978, “Biomechanics of Ligaments and Tendons,” Exerc. Sports Sci. Rev., 125(6), pp. 125–181. [PubMed] [Google Scholar]
- [8]. Noyes, F. R. , Grood, E. S. , and Butler, D. L. , 1978, Mechanical Properties of Soft Tissues, Resources for Basic Science Educators, American Academy of Orthopaedic Surgeons, Park Ridge, IL. [Google Scholar]
- [9]. Butler, D. L. , Noyes, F. R. , and Grood, E. S. , 1980, “Ligamentous Restraints to Anterior-Posterior Drawer in the Human Knee. A Biomechanical Study,” J. Bone Jt. Surg. Am., 62(2), pp. 259–270. [PubMed] [Google Scholar]
- [10]. Noyes, F. R. , Grood, E. S. , Butler, D. L. , and Malek, M. , 1980, “Clinical Laxity Tests and Functional Stability of the Knee: Biomechanical Concepts,” Clin. Orthop. Relat. Res., 146, pp. 84–89. 10.1097/00003086-198001000-00012 [DOI] [PubMed] [Google Scholar]
- [11]. Grood, E. S. , Noyes, F. R. , Butler, D. L. , and Suntay, W. J. , 1981, “Ligamentous and Capsular Restraints Preventing Straight Medial and Lateral Laxity in Intact Human Cadaver Knees,” J. Bone Jt. Surg. Am., 63(8), pp. 1257–1269. [PubMed] [Google Scholar]
- [12]. Noyes, F. R. , Butler, D. L. , Grood, E. S. , Zernicke, R. F. , and Hefzy, M. S. , 1984, “Biomechanical Analysis of Human Ligament Grafts used in Knee-Ligament Repairs and Reconstructions,” J. Bone Jt. Surg. Am., 66(3), pp. 344–352. [PubMed] [Google Scholar]
- [13]. Butler, D. L. , Grood, E. S. , Noyes, F. R. , Zernicke, R. F. , and Brackett, K. , 1984, “Effects of Structure and Strain Measurement Technique on the Material Properties of Young Human Tendons and Fascia,” J. Biomech., 17(8), pp. 579–596. 10.1016/0021-9290(84)90090-3 [DOI] [PubMed] [Google Scholar]
- [14]. Stouffer, D. C. , Butler, D. L. , and Hosny, D. , 1985, “The Relationship Between Crimp Pattern and Mechanical Response of Human Patellar Tendon-Bone Units,” ASME J. Biomech. Eng., 107(2), pp. 158–165. 10.1115/1.3138536 [DOI] [PubMed] [Google Scholar]
- [15]. Butler, D. L. , Kay, M. D. , and Stouffer, D. C. , 1986, “Comparison of Material Properties in Fascicle-Bone Units From Human Patellar Tendon and Knee Ligaments,” J. Biomech., 19(6), pp. 425–432. 10.1016/0021-9290(86)90019-9 [DOI] [PubMed] [Google Scholar]
- [16]. Butler, D. L. , Sheh, M. Y. , Stouffer, D. C. , Samaranayake, V. A. , and Levy, M. S. , 1990, “Surface Strain Variation in Human Patellar Tendon and Knee Cruciate Ligaments,” ASME J. Biomech. Eng., 112(1), pp. 38–45. 10.1115/1.2891124 [DOI] [PubMed] [Google Scholar]
- [17]. Butler, D. L. , and Guan, Y. , 1990, Biomechanics of Diarthroidal Joints, Springer-Verlag, Berlin, pp. 105–154. [Google Scholar]
- [18]. Gibbons, M. J. , Butler, D. L. , Grood, E. S. , Bylski-Austrow, D. I. , Levy, M. S. , and Noyes, F. R. , 1991, “Effects of Gamma Irradiation on the Initial Mechanical and Material Properties of Goat Bone-Patellar Tendon-Bone Allografts,” J. Orthop. Res., 9(2), pp. 209–218. 10.1002/jor.1100090209 [DOI] [PubMed] [Google Scholar]
- [19]. Oster, D. M. , Grood, E. S. , Feder, S. M. , Butler, D. L. , and Levy, M. S. , 1992, “Primary and Coupled Motions in the Intact and the ACL-Deficient Knee: An in vitro Study in the Goat Model,” J. Orthop. Res., 10(4), pp. 476–484. 10.1002/jor.1100100403 [DOI] [PubMed] [Google Scholar]
- [20]. Butler, D. L. , Guan, Y. , Kay, M. D. , Cummings, J. F. , Feder, S. M. , and Levy, M. S. , 1992, “Location-Dependent Variations in the Material Properties of the Anterior Cruciate Ligament,” J. Biomech., 25(5), pp. 511–518. 10.1016/0021-9290(92)90091-E [DOI] [PubMed] [Google Scholar]
- [21]. Feder, S. M. , Butler, D. L. , and Holden, J. P. , 1993, “A Technique for the Evaluation of the Contributions of Knee Structures to Knee Mechanics in the Knee That has a Reconstructed Anterior Cruciate Ligament,” J. Orthop. Res., 11(3), pp. 448–451. 10.1002/jor.1100110318 [DOI] [PubMed] [Google Scholar]
- [22]. Rasmussen, T. J. , Feder, S. M. , Butler, D. L. , and Noyes, F. R. , 1994, “The Effects of 4 Mrad of Gamma Irradiation on the Initial Mechanical Properties of Bone-Patellar Tendon-Bone Grafts,” Arthroscopy, 10(2), pp. 188–197. 10.1016/S0749-8063(05)80092-1 [DOI] [PubMed] [Google Scholar]
- [23]. Salehpour, A. , Butler, D. L. , Proch, F. S. , Schwartz, H. E. , Feder, S. M. , Doxey, C. M. , and Ratcliffe, A. , 1995, “Dose-Dependent Response of Gamma Irradiation on Mechanical Properties and Related Biochemical Composition of Goat Bone-Patellar Tendon-Bone Allografts,” J. Orthop. Res., 13(6), pp. 898–906. 10.1002/jor.1100130614 [DOI] [PubMed] [Google Scholar]
- [24]. Dressler, M. R. , Butler, D. L. , Wenstrup, R. , Awad, H. A. , Smith, F. , and Boivin, G. P. , 2002, “A Potential Mechanism for Age-Related Declines in Patellar Tendon Biomechanics,” J. Orthop. Res., 20(6), pp. 1315–1322. 10.1016/S0736-0266(02)00052-9 [DOI] [PubMed] [Google Scholar]
- [25]. Holden, J. P. , Grood, E. S. , Butler, D. L. , Noyes, F. R. , Mendenhall, H. V. , Van Kampen, C. L. , and Neidich, R. L. , 1988, “Biomechanics of Fascia Lata Ligament Replacements: Early Postoperative Changes in the Goat,” J. Orthop. Res., 6(5), pp. 639–647. 10.1002/jor.1100060504 [DOI] [PubMed] [Google Scholar]
- [26]. Butler, D. L. , Grood, E. S. , Noyes, F. R. , Olmstead, M. L. , Hohn, R. B. , Arnoczky, S. P. , and Siegel, M. G. , 1989, “Mechanical Properties of Primate Vascularized Vs. Nonvascularized Patellar Tendon Grafts: Changes Over Time,” J. Orthop. Res., 7(1), pp. 68–79. 10.1002/jor.1100070110 [DOI] [PubMed] [Google Scholar]
- [27]. Butler, D. L. , 1989, “Kappa Delta Award Paper. Anterior Cruciate Ligament: Its Normal Response and Replacement,” J. Orthop. Res., 7(6), pp. 910–921. 10.1002/jor.1100070618 [DOI] [PubMed] [Google Scholar]
- [28]. Bylski-Austrow, D. I. , Grood, E. S. , Hefzy, M. S. , Holden, J. P. , and Butler, D. L. , 1990, “Anterior Cruciate Ligament Replacements: A Mechanical Study of Femoral Attachment Location, Flexion Angle at Tensioning, and Initial Tension,” J. Orthop. Res., 8(4), pp. 522–531. 10.1002/jor.1100080408 [DOI] [PubMed] [Google Scholar]
- [29]. Butler, D. L. , and Siegel, A. , 1990, “Alterations in Tissue Response: Conditioning Effects at Different Ages,” American Orthopaedic Society for Sports Medicine Symposium, Ledbetter W., Buckwalter J. and Gordon S. L., eds. AAOS, Park Ridge, IL., pp. 713–730. [Google Scholar]
- [30]. Grood, E. S. , Walz-Hasselfeld, K. A. , Holden, J. P. , Noyes, F. R. , Levy, M. S. , Butler, D. L. , Jackson, D. W. , and Drez, D. J. , 1992, “The Correlation Between Anterior-Posterior Translation and Cross-Sectional Area of Anterior Cruciate Ligament Reconstructions,” J. Orthop. Res., 10(6), pp. 878–885. 10.1002/jor.1100100617 [DOI] [PubMed] [Google Scholar]
- [31]. Cummings, J. F. , Grood, E. S. , Butler, D. L. , and Levy, M. S. , 2002, “Subject Variation in Caprine Anterior Cruciate Ligament Reconstruction,” J. Orthop. Res., 20(5), pp. 1009–1015. 10.1016/S0736-0266(02)00034-7 [DOI] [PubMed] [Google Scholar]
- [32]. Ronsky, J. L. , Herzog, W. , Brown, T. D. , Pedersen, D. R. , Grood, E. S. , and Butler, D. L. , 1995, “ In vivo Quantification of the Cat Patellofemoral Joint Contact Stresses and Areas,” J. Biomech., 28(8), pp. 977–983. 10.1016/0021-9290(94)00153-U [DOI] [PubMed] [Google Scholar]
- [33]. Korvick, D. L. , Cummings, J. F. , Grood, E. S. , Holden, J. P. , Feder, S. M. , and Butler, D. L. , 1996, “The Use of an Implantable Force Transducer to Measure Patellar Tendon Forces in Goats,” J. Biomech., 29(4), pp. 557–561. 10.1016/0021-9290(95)00036-4 [DOI] [PubMed] [Google Scholar]
- [34]. Bush-Joseph, C. A. , Cummings, J. F. , Buseck, M. , Bylski-Austrow, D. I. , Butler, D. L. , Noyes, F. R. , and Grood, E. S. , 1996, “Effect of Tibial Attachment Location on the Healing of the Anterior Cruciate Ligament Freeze Model,” J. Orthop. Res., 14(4), pp. 534–541. 10.1002/jor.1100140406 [DOI] [PubMed] [Google Scholar]
- [35]. Malaviya, P. , Butler, D. L. , Korvick, D. L. , and Proch, F. S. , 1998, “ In vivo Tendon Forces Correlate With Activity Level and Remain Bounded: Evidence in a Rabbit Flexor Tendon Model,” J. Biomech., 31(11), pp. 1043–1049. 10.1016/S0021-9290(98)00123-7 [DOI] [PubMed] [Google Scholar]
- [36]. Malaviya, P. , Butler, D. L. , Boivin, G. P. , Smith, F. N. , Barry, F. P. , Murphy, J. M. , and Vogel, K. G. , 2000, “An in vivo Model for Load-Modulated Remodeling in the Rabbit Flexor Tendon,” J. Orthop. Res., 18(1), pp. 116–125. 10.1002/jor.1100180117 [DOI] [PubMed] [Google Scholar]
- [37]. Juncosa, N. , West, J. , Galloway, M. , Boivin, G. , and Butler, D. , 2003, “ In vivo Forces Used to Develop Design Parameters for Tissue Engineered Implants for Rabbit Patellar Tendon Repair,” J. Biomech., 36(4), pp. 483–488. 10.1016/S0021-9290(02)00459-1 [DOI] [PubMed] [Google Scholar]
- [38]. West, J. , Juncosa, N. , Galloway, M. , Boivin, G. , and Butler, D. , 2004, “Characterization of in vivo Achilles Tendon Forces in Rabbits During Treadmill Locomotion at Varying Speeds and Inclinations,” J. Biomech., 37(11), pp. 1647–1653. 10.1016/j.jbiomech.2004.02.019 [DOI] [PubMed] [Google Scholar]
- [39]. Young, R. G. , Butler, D. L. , Weber, W. , Caplan, A. I. , Gordon, S. L. , and Fink, D. J. , 1998, “Use of Mesenchymal Stem Cells in a Collagen Matrix for Achilles Tendon Repair,” J. Orthop. Res., 16(4), pp. 406–413. 10.1002/jor.1100160403 [DOI] [PubMed] [Google Scholar]
- [40]. Awad, H. A. , Butler, D. L. , Boivin, G. P. , Smith, F. N. L. , Malaviya, P. , Huibregtse, B. , and Caplan, A. I. , 1999, “Autologous Mesenchymal Stem Cell-Mediated Repair of Tendon,” Tissue Eng., 5(3), pp. 267–277. 10.1089/ten.1999.5.267 [DOI] [PubMed] [Google Scholar]
- [41]. Butler, D. L. , and Awad, H. A. , 1999, “Perspectives on Cell and Collagen Composites for Tendon Repair,” Clin. Orthop. Relat. Res., 367(Suppl), pp. S324–S332. 10.1097/00003086-199910001-00031 [DOI] [PubMed] [Google Scholar]
- [42]. Butler, D. L. , Goldstein, S. A. , and Guilak, F. , 2000, “Functional Tissue Engineering: The Role of Biomechanics,” ASME J. Biomech. Eng., 122(6), pp. 570–575. 10.1115/1.1318906 [DOI] [PubMed] [Google Scholar]
- [43]. Awad, H. A. , Butler, D. L. , Harris, M. T. , Ibrahim, R. E. , Wu, Y. , Young, R. G. , Kadiyala, S. , and Boivin, G. P. , 2000, “ In vitro Characterization of Mesenchymal Stem Cell-Seeded Collagen Scaffolds for Tendon Repair: Effects of Initial Seeding Density on Contraction Kinetics,” J. Biomed. Mater. Res., 51(2), pp. 233–240. [DOI] [PubMed] [Google Scholar]
- [44]. Guilak, F. , Butler, D. L. , and Goldstein, S. A. , 2001, “Functional Tissue Engineering: The Role of Biomechanics in Articular Cartilage Repair,” Clin. Orthop. Relat. Res., 391(Suppl), pp. S295–S305. 10.1097/00003086-200110001-00027 [DOI] [PubMed] [Google Scholar]
- [45]. Guilak, F. , Butler, D. L. , Goldstein, S. A. , and Mooney, D. , 2003, Functional Tissue Engineering, Springer-Verlag, New York, p. 426. [Google Scholar]
- [46]. Awad, H. A. , Boivin, G. P. , Dressler, M. R. , Smith, F. N. L. , Young, R. G. , and Butler, D. L. , 2003, “Repair of Patellar Tendon Injuries Using a Cell-Collagen Composite,” J. Orthop. Res., 21(3), pp. 420–431. 10.1016/S0736-0266(02)00163-8 [DOI] [PubMed] [Google Scholar]
- [47]. Butler, D. L. , Juncosa, N. , and Dressler, M. R. , 2004, “Functional Efficacy of Tendon Repair Processes,” Annu. Rev. Biomed. Eng., 6, pp. 303–329. 10.1146/annurev.bioeng.6.040803.140240 [DOI] [PubMed] [Google Scholar]
- [48]. Butler, D. L. , Shearn, J. T. , Juncosa, N. , Dressler, M. R. , and Hunter, S. A. , 2004, “Functional Tissue Engineering Parameters Toward Designing Repair and Replacement Strategies,” Clin. Orthop. Relat. Res., 427(Suppl), pp. S190–S199. 10.1097/01.blo.0000144858.65450.d2 [DOI] [PubMed] [Google Scholar]
- [49]. Harris, M. T. , Butler, D. L. , Boivin, G. P. , Florer, J. B. , Schantz, E. J. , and Wenstrup, R. J. , 2004, “Mesenchymal Stem Cells Used for Rabbit Tendon Repair Can Form Ectopic Bone and Express Alkaline Phosphatase Activity in Constructs,” J. Orthop. Res., 22(5), pp. 998–1003. 10.1016/j.orthres.2004.02.012 [DOI] [PubMed] [Google Scholar]
- [50]. Juncosa-Melvin, N. , Boivin, G. , Galloway, M. , Gooch, C. , West, J. , Sklenka, A. , and Butler, D. , 2005, “Effects of Cell-to-Collagen Ratio in Mesenchymal Stem Cell-Seeded Implants on Tendon Repair Biomechanics and Histology,” Tissue Eng., 11(3-4), pp. 448–457. 10.1089/ten.2005.11.448 [DOI] [PubMed] [Google Scholar]
- [51]. Dressler, M. R. , Butler, D. L. , and Boivin, G. P. , 2005, “Effects of Age on the Repair Ability of Mesenchymal Stem Cells in Rabbit Tendon,” J. Orthop. Res., 23(2), pp. 287–293. 10.1016/j.orthres.2004.06.017 [DOI] [PubMed] [Google Scholar]
- [52]. Sipes, N. S. , Shearn, J. T. , and Butler, D. L. , 2005, “Evaluation of a Sonomicrometry Device for Measuring in vivo Dynamic Joint Kinematics: Applications to Functional Tissue Engineering,” J. Biomech., 38, pp. 2486–2490. 10.1016/j.jbiomech.2004.10.017 [DOI] [PubMed] [Google Scholar]
- [53]. Schuler, N. , Bey, M. , Shearn, J. , and Butler, D. , 2005, “Evaluation of an Electromagnetic Position Tracking Device for Measuring in vivo, Dynamic Joint Kinematics,” J. Biomech., 38(10), pp. 2113–2117. 10.1016/j.jbiomech.2004.09.015 [DOI] [PubMed] [Google Scholar]
- [54]. Juncosa-Melvin, N. , Boivin, G. P. , Gooch, C. , Galloway, M. T. , West, J. R. , Dunn, M. G. , and Butler, D. L. , 2006, “The Effect of Autologous Mesenchymal Stem Cells on the Biomechanics and Histology of Gel-Collagen Sponge Constructs Used for Rabbit Patellar Tendon Repair,” Tissue Eng., 12(2), pp. 369–379. 10.1089/ten.2006.12.369 [DOI] [PubMed] [Google Scholar]
- [55]. Juncosa-Melvin, N. , Boivin, G. , Galloway, M. , Gooch, C. , West, J. , and Butler, D. , 2006, “Effects of Cell-to-Collagen Ratio in Stem Cell-Seeded Constructs for Achilles Tendon Repair,” Tissue Eng., 12(4), pp. 681–689. 10.1089/ten.2006.12.681 [DOI] [PubMed] [Google Scholar]
- [56]. Juncosa-Melvin, N. , Shearn, J. , Boivin, G. , Gooch, C. , Galloway, M. , West, J. , Nirmalanandhan, V. , Bradica, G. , and Butler, D. , 2006, “Effects of Mechanical Stimulation on the Biomechanics and Histology of Stem Cell-Collagen Sponge Constructs for Rabbit Patellar Tendon Repair,” Tissue Eng., 12(8), pp. 2291–2300. 10.1089/ten.2006.12.2291 [DOI] [PubMed] [Google Scholar]
- [57]. Juncosa-Melvin, N. , Matlin, K. , Holdcraft, R. , Nirmalanandhan, V. , and Butler, D. , 2007, “Mechanical Stimulation Increases Collagen Type I and Collagen Type III Gene Expression of Stem Cell-Collagen Sponge Constructs for Patellar Tendon Repair,” Tissue Eng., 13(6), pp. 1219–1226. 10.1089/ten.2006.0339 [DOI] [PubMed] [Google Scholar]
- [58]. Dressler, M. R. , Butler, D. L. , and Boivin, G. P. , 2006, “Age-Related Changes in the Biomechanics of Healing Patellar Tendon,” J. Biomech., 39(12), pp. 2205–2212. 10.1016/j.jbiomech.2005.07.003 [DOI] [PubMed] [Google Scholar]
- [59]. Nirmalanandhan, V. S. , Levy, M. S. , Huth, A. J. , and Butler, D. L. , 2006, “Effects of Cell Seeding Density and Collagen Concentration on Contraction Kinetics of Mesenchymal Stem Cell-Seeded Collagen Constructs,” Tissue Eng., 12(7), pp. 1865–1872. 10.1089/ten.2006.12.1865 [DOI] [PubMed] [Google Scholar]
- [60]. Nirmalanandhan, V. S. , Dressler, M. R. , Shearn, J. T. , Juncosa-Melvin, N. , Rao, M. , Gooch, C. , Bradica, G. , and Butler, D. L. , 2007, “Mechanical Stimulation of Tissue Engineered Tendon Constructs: Effect of Scaffold Materials,” ASME J. Biomech. Eng., 129(6), pp. 919–923. 10.1115/1.2800828 [DOI] [PubMed] [Google Scholar]
- [61]. Nirmalanandhan, V. S. , Rao, M. , Sacks, M. S. , Haridas, B. , and Butler, D. L. , 2007, “Effect of Length of the Engineered Tendon Construct on Its Structure-function Relationships in Culture,” J. Biomech., 40(11), pp. 2523–2529. 10.1016/j.jbiomech.2006.11.016 [DOI] [PubMed] [Google Scholar]
- [62]. Nirmalanandhan, V. S. , Shearn, J. T. , Juncosa-Melvin, N. , Rao, M. , Jain, A. , Gooch, C. , and Butler, D. L. , 2007, “Optimizing the Mechanical Stimulus in Culture to Improve Construct Biomechanics for Tendon Repair,” Mol. Cell. Mech., 3(4), pp. 131–133. 10.3970/mcb.2006.003.129 [DOI] [Google Scholar]
- [63]. Nirmalanandhan, V. , Shearn, J. , Juncosa-Melvin, N. , Rao, M. , Gooch, C. , Jain, A. , Bradica, G. , and Butler, D. , 2008, “Improving Linear Stiffness of the Cell-Seeded Collagen Sponge Constructs by Varying the Components of the Mechanical Stimulus,” Tissue Eng., Part A, 14(11), pp. 1883–1891. 10.1089/ten.tea.2007.0125 [DOI] [PubMed] [Google Scholar]
- [64]. Nirmalanandhan, V. , Rao, M. , Shearn, J. , Juncosa-Melvin, N. , Gooch, C. , and Butler, D. , 2008, “Effect of Scaffold Material, Construct Length and Mechanical Stimulation on the in vitro Stiffness of the Engineered Tendon Construct,” J. Biomech., 41(4), pp. 822–828. 10.1016/j.jbiomech.2007.11.009 [DOI] [PubMed] [Google Scholar]
- [65]. Nirmalanandhan, V. , Juncosa-Melvin, N. , Shearn, J. , Boivin, G. , Galloway, M. , Gooch, C. , Bradica, G. , and Butler, D. , 2009, “Combined Effects of Scaffold Stiffening and Mechanical Preconditioning Cycles on Construct Biomechanics, Gene Expression, and Tendon Repair Biomechanics,” Tissue Eng., Part A, 15(8), pp. 2103–2111. 10.1089/ten.tea.2008.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Schwartz, H. E. , Matava, M. J. , Proch, F. S. , Butler, C. A. , Ratcliffe, A. , Levy, M. , and Butler, D. L. , 2006, “The Effect of Gamma Irradiation on Anterior Cruciate Ligament Allograft Biomechanical and Biochemical Properties in the Caprine Model at Time Zero and at 6 Months After Surgery,” Am. J. Sports Med., 34(11), pp. 1747–1755. 10.1177/0363546506288851 [DOI] [PubMed] [Google Scholar]
- [67]. Butler, D. L. , Juncosa-Melvin, N. , Boivin, G. P. , Galloway, M. T. , Gooch, C. , Shearn, J. T. , Nirmalanandhan, V. S. , Hunter, S. A. , Chokalingam, K. , Frede, C. , Florer, J. , and Wenstrup, R. J. , 2007, “Functional Tissue Engineering to Repair Tendon and Other Musculoskeletal Tissues,” Mol. Cell. Mech., 3(4), pp. 127–129. 10.3970/mcb.2006.003.127 [DOI] [Google Scholar]
- [68]. Shearn, J. T. , Juncosa-Melvin, N. , Boivin, G. P. , Galloway, M. T. , Goodwin, W. , Gooch, C. , Dunn, M. G. , and Butler, D. L. , 2007, “Mechanical Stimulation of Tendon Tissue Engineered Constructs: Effects on Construct Stiffness, Repair Biomechanics, and Their Correlation,” ASME J. Biomech. Eng., 129(6), pp. 848–854. 10.1115/1.2800769 [DOI] [PubMed] [Google Scholar]
- [69]. Butler, D. , Juncosa-Melvin, N. , Boivin, G. , Galloway, M. , Shearn, J. , Gooch, C. , and Awad, H. , 2008, “Functional Tissue Engineering for Tendon Repair: A Multidisciplinary Strategy Using Mesenchymal Stem Cells, Bioscaffolds, and Mechanical Stimulation,” J. Orthop. Res., 26(1), pp. 1–9. 10.1002/jor.20456 [DOI] [PubMed] [Google Scholar]
- [70]. Tissue Engineering Conference Group Functional, 2008, “Evaluation Criteria for Musculoskeletal and Craniofacial Tissue Engineering Constructs: A Conference Report,” Tissue Eng., Part A, 14(12), pp. 2089–2104. 10.1089/ten.tea.2007.0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Butler, D. L. , Hunter, S. A. , Chokalingam, K. , Cordray, M. J. , Shearn, J. , Juncosa-Melvin, N. , Nirmalanandhan, S. , and Jain, A. , 2009, “Using Functional Tissue Engineering and Bioreactors to Mechanically Stimulate Tissue-Engineered Constructs,” Tissue Eng., Part A, 15(4), pp. 741–749. 10.1089/ten.tea.2008.0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Kinneberg, K. R. C. , Nirmalanandhan, V. S. , Juncosa-Melvin, N. , Powell, H. M. , Boyce, S. T. , Shearn, J. T. , and Butler, D. L. , 2010, “Chondroitin-6-Sulfate Incorporation and Mechanical Stimulation Increase MSC-Collagen Sponge Construct Stiffness,” J. Orthop. Res., 28(8), pp. 1092–1099. 10.1002/jor.21095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Kinneberg, K. R. C. , Galloway, M. T. , Butler, D. L. , and Shearn, J. T. , 2011, “Effect of Implanting a Soft Tissue Autograft in a Central-Third Patellar Tendon Defect: Biomechanical and Histological Comparisons,” ASME J. Biomech. Eng., 133(9), p. 091002. 10.1115/1.4004948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Shearn, J. T. , Kinneberg, K. R. , Dyment, N. A. , Galloway, M. T. , Kenter, K. , Wylie, C. , and Butler, D. L. , 2011, “Tendon Tissue Engineering: Progress, Challenges, and Translation to the Clinic,” J. Musculoskeletal and Neuronal Interact., 11(2), pp. 163–173. [PMC free article] [PubMed] [Google Scholar]
- [75]. Ingber, D. , Mow, V. , Butler, D. , Niklason, L. , Huard, J. , Mao, J. , Yannas, I. , Kaplan, D. , and Vunjak-Novakovic, G. , 2006, “Tissue Engineering and Developmental Biology: Going Biomimetic,” Tissue Eng., 12(12), pp. 3265–3283. 10.1089/ten.2006.12.3265 [DOI] [PubMed] [Google Scholar]
- [76]. Chokalingam, K. , Juncosa-Melvin, N. , Hunter, S. , Gooch, C. , Frede, C. , Florer, J. , Bradica, G. , Wenstrup, R. , and Butler, D. , 2009, “Tensile Stimulation of Murine Stem Cell-Collagen Sponge Constructs Increases Collagen Type I Gene Expression and Linear Stiffness,” Tissue Eng., Part A, 15(9), pp. 2561–2570. 10.1089/ten.tea.2008.0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77]. Chokalingam, K. , Hunter, S. , Gooch, C. , Frede, C. , Florer, J. , Wenstrup, R. , and Butler, D. , 2009, “Three-Dimensional in vitro Effects of Compression and Time in Culture on Aggregate Modulus and on Gene Expression and Protein Content of Collagen Type II in Murine Chondrocytes,” Tissue Eng., Part A, 15(10), pp. 2807–2816. 10.1089/ten.tea.2008.0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Maye, P. , Fu, Y. , Butler, D. L. , Chokalingam, K. , Liu, Y. , Florer, J. , Stover, M. L. , Wenstrup, R. , Jiang, X. , Gooch, C. , and Rowe, D. , 2011, “Generation and Characterization of Col10a1-mCherry Reporter Mice,” Genesis, 49(5), pp. 410–418. 10.1002/dvg.20733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Liu, C. , Aschbacher-Smith, L. , Barthelery, N. J. , Dyment, N. , Butler, D. , and Wylie, C. , 2011, “What We Should Know Before Using Tissue Engineering Techniques to Repair Injured Tendons: A Developmental Biology Perspective,” Tissue Eng., Part B Rev., 17(3), pp. 165–176. 10.1089/ten.teb.2010.0662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Liu, C. , Aschbacher-Smith, L. , Bathelery, N. J. , Dyment, N. , Butler, D. L. , and Wylie, C. , 2012, “Spatial and Temporal Expression of Molecular Markers and Cell Signals During Normal Development of the Mouse Patellar Tendon,” Tissue Eng., Part A, 18(5-6), pp. 598–608. 10.1089/ten.tea.2011.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Dyment, N. A. , Kazemi, N. , Aschbacher-Smith, L. E. , Barthelery, N. J. , Kenter, K. , Gooch, C. , Shearn, J. T. , Wylie, C. , and Butler, D. L. , 2011, “The Relationships Among Spatiotemporal Collagen Gene Expression, Histology, and Biomechanics Following Full-Length Injury in the Murine Patellar Tendon,” J. Orthop. Res., 30(1), pp. 28–36. 10.1002/jor.21484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Butler, D. L. , Dyment, N. A. , Shearn, J. T. , Kinneberg, K. R. C. , Breidenbach, A. P. , Lalley, A. L. , Gilday, S. D. , Gooch, C. , Liu, C. , and Wylie, C. , 2012, “Working Across Model Systems at the Interface Between Functional Tissue Engineering and Developmental Biology to Improve Adult Tendon Repair,” International Symposium of Ligaments and Tendons, San Francisco, CA. [Google Scholar]
- [83]. Noyes, F. R. , Bassett, R. W. , Grood, E. S. , and Butler, D. L. , 1980, “Arthroscopy in Acute Traumatic Hemarthrosis of the Knee. Incidence of Anterior Cruciate Tears and Other Injuries,” J. Bone Jt. Surg. Am., 62(5), pp. 687–695, 757. [PubMed] [Google Scholar]
- [84]. Praemer, A. , Furner, S. , and Rice, D. P. , 1999, Musculoskeletal Condition in the United States, American Academy of Orthopaedic Surgeons, Park Ridge, IL, pp. 182. [Google Scholar]
- [85]. Kleipool, A. E. , Zijl, J. A. , and Willems, W. J. , 1998, “Arthroscopic Anterior Cruciate Ligament Reconstruction With Bone-Patellar Tendon-Bone Allograft or Autograft. A Prospective Study With an Average Follow Up of 4 Years,” Knee Surg. Sports Traumatol. Arthrosc., 6(4), pp. 224–230. 10.1007/s001670050104 [DOI] [PubMed] [Google Scholar]
- [86]. Beasley, L. S. , and Chudik, S. C. , 2003, “Anterior Cruciate Ligament Injury in Children: Update of Current Treatment Options,” Curr. Opin. Pediatr., 15(1), pp. 45–52. 10.1097/00008480-200302000-00008 [DOI] [PubMed] [Google Scholar]
- [87]. Noyes, F. R. , and Grood, E. S. , 1976, “The Strength of the Anterior Cruciate Ligament in Humans and Rhesus Monkeys,” J. Bone Jt. Surg. Am., 58(8), pp. 1074–1082. [PubMed] [Google Scholar]
- [88]. Ferretti, A. , Conteduca, F. , De Carli, A. , Fontana, M. , and Mariani, P. P. , 1990, “Results of Reconstruction of the Anterior Cruciate Ligament With the Tendons of Semitendinosus and Gracilis in Acute Capsulo-Ligamentous Lesions of the Knee,” Ital. J. Orthop. Traumatol., 16(4), pp. 452–458. [PubMed] [Google Scholar]
- [89]. Hanley, P. , Lew, W. D. , Lewis, J. L. , Hunter, R. E. , Kirstukas, S. , and Kowalczyk, C. , 1989, “Load Sharing and Graft Forces in Anterior Cruciate Ligament Reconstructions With the Ligament Augmentation Device,” Am. J. Sports Med., 17(3), pp. 414–422. 10.1177/036354658901700317 [DOI] [PubMed] [Google Scholar]
- [90]. Engebretsen, L. , Lew, W. D. , Lewis, J. L. , and Hunter, R. E. , 1990, “The Effect of an Iliotibial Tenodesis on Intraarticular Graft Forces and Knee Joint Motion,” Am. J. Sports Med., 18(2), pp. 169–176. 10.1177/036354659001800210 [DOI] [PubMed] [Google Scholar]
- [91]. Tibone, J. E. , and Antich, T. J. , 1988, “A Biomechanical Analysis of Anterior Cruciate Ligament Reconstruction With the Patellar Tendon. A Two Year Follow-Up,” Am. J. Sports Med., 16(4), pp. 332–335. 10.1177/036354658801600404 [DOI] [PubMed] [Google Scholar]
- [92]. Yasuda, K. , Tomiyama, Y. , Ohkoshi, Y. , and Kaneda, K. , 1989, “Arthroscopic Observations of Autogeneic Quadriceps and Patellar Tendon Grafts After Anterior Cruciate Ligament Reconstruction of the Knee,” Clin. Orthop. Relat. Res., 246, pp. 217–224. 10.1097/00003086-198909000-00031 [DOI] [PubMed] [Google Scholar]
- [93]. Race, A. , and Amis, A. A. , 1994, “The Mechanical Properties of the Two Bundles of the Human Posterior Cruciate Ligament,” J. Biomech., 27(1), pp. 13–24. 10.1016/0021-9290(94)90028-0 [DOI] [PubMed] [Google Scholar]
- [94]. Yamamoto, N. , Ohno, K. , Hayashi, K. , Kuriyama, H. , Yasuda, K. , and Kaneda, K. , 1993, “Effects of Stress Shielding on the Mechanical Properties of Rabbit Patellar Tendon,” ASME J. Biomech. Eng, 115(1), pp. 23–28. 10.1115/1.2895466 [DOI] [PubMed] [Google Scholar]
- [95]. Xu, W. S. , Butler, D. L. , Stouffer, D. C. , Grood, E. S. , and Glos, D. L. , 1992, “Theoretical Analysis of an Implantable Force Transducer for Tendon and Ligament Structures,” ASME J. Biomech. Eng., 114(2), pp. 170–177. 10.1115/1.2891368 [DOI] [PubMed] [Google Scholar]
- [96]. Glos, D. L. , Butler, D. L. , Grood, E. S. , and Levy, M. S. , 1993, “ In vitro Evaluation of an Implantable Force Transducer (IFT) in a Patellar Tendon Model,” ASME J. Biomech. Eng., 115(4A), pp. 335–343. 10.1115/1.2895495 [DOI] [PubMed] [Google Scholar]
- [97]. Holden, J. P. , Grood, E. S. , Korvick, D. L. , Cummings, J. F. , Butler, D. L. , and Bylski-Austrow, D. I. , 1994, “ In vivo Forces in the Anterior Cruciate Ligament: Direct Measurements During Walking and Trotting in a Quadruped,” J. Biomech., 27(5), pp. 517–526. 10.1016/0021-9290(94)90063-9 [DOI] [PubMed] [Google Scholar]
- [98]. Myers, R. L. , Montgomery, D. C. , and Anderson-Cook, C. , 2009, Response Surface Methodology: Process and Product Optimization Using Designed Experiments, Wiley, Hoboken, NJ, p. 704. [Google Scholar]
- [99]. Hunziker, E. , Spector, M. , Libera, J. , Gertzman, A. , Woo, S. L. , Ratcliffe, A. , Lysaght, M. , Coury, A. , Kaplan, D. , and Vunjak-Novakovic, G. , 2006, “Translation From Research to Applications,” Tissue Eng., 12(12), pp. 3341–3364. 10.1089/ten.2006.12.3341 [DOI] [PubMed] [Google Scholar]
- [100]. Stouffer, D. C. , Butler, D. L. , and Kim, H. , 1983, “Tension-Torsion Characteristics of the Canine Anterior Cruciate Ligament–Part I: Theoretical Framework,” ASME J. Biomech. Eng., 105(2), pp. 154–159. 10.1115/1.3138399 [DOI] [PubMed] [Google Scholar]
- [101]. Butler, D. L. , Hulse, D. A. , Kay, M. D. , Grood, E. S. , Shires, P. K. , D'Ambrosia, R. , and Shoji, H. , 1983, “Biomechanics of Cranial Cruciate Reconstruction in the Dog: II. Mechanical Properties,” Vet. Surg., 12, pp. 113–118. 10.1111/j.1532-950X.1983.tb00721.x [DOI] [Google Scholar]
- [102]. Butler, D. L. , and Stouffer, D. C. , 1983, “Tension-Torsion Characteristics of the Canine Anterior Cruciate Ligament–Part II: Experimental Observations,” ASME J. Biomech. Eng., 105(2), pp. 160–165. 10.1115/1.3138400 [DOI] [PubMed] [Google Scholar]
- [103]. Hulse, D. A. , Butler, D. L. , Kay, M. D. , Noyes, F. R. , Shires, P. K. , D'Ambrosia, R. , and Shoji, H. , 1983, “Biomechanics of Cranial Cruciate Reconstruction in the Dog: I. in vitro Laxity Testing,” Vet. Surg., 12, pp. 109–112. 10.1111/j.1532-950X.1983.tb00719.x [DOI] [Google Scholar]
- [104]. Jackson, D. W. , Grood, E. S. , Arnoczky, S. P. , Butler, D. L. , and Simon, T. M. , 1987, “Freeze Dried Anterior Cruciate Ligament Allografts. Preliminary Studies in a Goat Model,” Am. J. Sports Med., 15(4), pp. 295–303. 10.1177/036354658701500401 [DOI] [PubMed] [Google Scholar]
- [105]. Jackson, D. W. , Grood, E. S. , Arnoczky, S. P. , Butler, D. L. , and Simon, T. M. , 1987, “Cruciate Reconstruction Using Freeze Dried Anterior Cruciate Ligament Allograft and a Ligament Augmentation Device (LAD). An Experimental Study in a Goat Model,” Am. J. Sports Med., 15(6), pp. 528–538. 10.1177/036354658701500602 [DOI] [PubMed] [Google Scholar]
- [106]. Jackson, D. W. , Grood, E. S. , Wilcox, P. , Butler, D. L. , Simon, T. M. , and Holden, J. P. , 1988, “The Effects of Processing Techniques on the Mechanical Properties of Bone-Anterior Cruciate Ligament-Bone Allografts. An Experimental Study in Goats,” Am. J. Sports Med., 16(2), pp. 101–105. 10.1177/036354658801600203 [DOI] [PubMed] [Google Scholar]
- [107]. Byrne, E. M. , Farrell, E. , McMahon, L. A. , Haugh, M. G. , O'Brien, F. J. , Campbell, V. A. , Prendergast, P. J. , and O'Connell, B. C. , 2008, “Gene Expression by Marrow Stromal Cells in a Porous Collagen-Glycosaminoglycan Scaffold Is Affected by Pore Size and Mechanical Stimulation,” J. Mater. Sci. Mater. Med., 19(11), pp. 3455–3463. 10.1007/s10856-008-3506-2 [DOI] [PubMed] [Google Scholar]
- [108]. Lysaght, M. J. , and Crager, J. , 2009, “Origins,” Tissue Eng., Part A, 15(7), pp. 1449–1450. 10.1089/ten.tea.2007.0412 [DOI] [PubMed] [Google Scholar]
- [109]. Herfat, S. T. , Shearn, J. T. , Bailey, D. L. , Greiwe, R. M. , Galloway, M. T. , Gooch, C. , and Butler, D. L. , 2011, “Effect of Surgery to Implant Motion and Force Sensors on Vertical Ground Reaction Forces in the Ovine Model,” ASME J. Biomech. Eng., 133(2), p. 021010. 10.1115/1.4003322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110]. Howard, R. , Rosvold, J. M. , and Tapper, J. E. , 2004, “Measurement of Loads in the Ovine Stifle Joint During in vitro Robotic Reproduction of in vivo Kinematics,” Transactions of the 2004 International Symposium on Ligaments and Tendons. [Google Scholar]
- [111]. Howard, R. A. , Rosvold, J. M. , Darcy, S. P. , Corr, D. T. , Shrive, N. G. , Tapper, J. E. , Ronsky, J. L. , Beveridge, J. E. , Marchuk, L. L. , and Frank, C. B. , 2007, “Reproduction of in vivo Motion Using a Parallel Robot,” ASME J. Biomech. Eng., 129(5), pp. 743–749. 10.1115/1.2768983 [DOI] [PubMed] [Google Scholar]
- [112]. Boguszewski, D. V. , Shearn, J. T. , Wagner, C. T. , and Butler, D. L. , 2011, “Investigating the Effects of Anterior Tibial Translation on Anterior Knee Force in the Porcine Model: Is the Porcine Knee ACL Dependent?” J. Orthop. Res., 29(5), pp. 641–646. 10.1002/jor.21298 [DOI] [PubMed] [Google Scholar]
- [113]. Nesbitt, R. J. , Herfat, S. T. , Galloway, M. T. , Gooch, C. , Butler, D. L. , and Shearn, J. T. , 2012, “Effects of Altering Grade on Vertical Ground Reaction Forces and ACL Forces in the Sheep Model,” Transaction of the International Symposium on Ligaments and Tendons - XII, San Francisco, CA. [Google Scholar]
- [114]. Tashman, S. , Kolowich, P. , Collon, D. , Anderson, K. , and Anderst, W. , 2007, “Dynamic Function of the ACL-Reconstructed Knee During Running,” Clin. Orthop. Relat. Res., 454, pp. 66–73. 10.1097/BLO.0b013e31802bab3e [DOI] [PubMed] [Google Scholar]
- [115]. Li, G. , Rudy, T. W. , Sakane, M. , Kanamori, A. , Ma, C. B. , and Woo, S. L. , 1999, “The Importance of Quadriceps and Hamstring Muscle Loading on Knee Kinematics and In-Situ Forces in the ACL,” J. Biomech., 32(4), pp. 395–400. 10.1016/S0021-9290(98)00181-X [DOI] [PubMed] [Google Scholar]
- [116]. Li, G. , Zayontz, S. , Most, E. , DeFrate, L. E. , Suggs, J. F. , and Rubash, H. E. , 2004, “ In Situ Forces of the Anterior and Posterior Cruciate Ligaments in High Knee Flexion: An in vitro Investigation,” J. Orthop. Res., 22(2), pp. 293–297. 10.1016/S0736-0266(03)00179-7 [DOI] [PubMed] [Google Scholar]
- [117]. Kanamori, A. , Woo, S. L. , Ma, C. B. , Zeminski, J. , Rudy, T. W. , Li, G. , and Livesay, G. A. , 2000, “The Forces in the Anterior Cruciate Ligament and Knee Kinematics During a Simulated Pivot Shift Test: A Human Cadaveric Study Using Robotic Technology,” Arthroscopy, 16(6), pp. 633–639. 10.1053/jars.2000.7682 [DOI] [PubMed] [Google Scholar]
- [118]. Shin, C. S. , Chaudhari, A. M. , and Andriacchi, T. P. , 2009, “The Effect of Isolated Valgus Moments on ACL Strain During Single-Leg Landing: A Simulation Study,” J. Biomech., 42(3), pp. 280–285. 10.1016/j.jbiomech.2008.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119]. Kinney, A. L. , Besier, T. F. , Silder, A. , Delp, S. L. , D'Lima, D. D. , and Fregly, B. J. , “Changes in in vivo Knee Contact Forces Through Gait Modification,” J. Orthop. Res., (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120]. Kutzner, I. , Heinlein, B. , Graichen, F. , Bender, A. , Rohlmann, A. , Halder, A. , Beier, A. , and Bergmann, G. , 2010, “Loading of the Knee Joint During Activities of Daily Living Measured in vivo in Five Subjects,” J. Biomech., 43(11), pp. 2164–2173. 10.1016/j.jbiomech.2010.03.046 [DOI] [PubMed] [Google Scholar]
- [121]. Georgoulis, A. D. , Papadonikolakis, A. , Papageorgiou, C. D. , Mitsou, A. , and Stergiou, N. , 2003, “Three-Dimensional Tibiofemoral Kinematics of the Anterior Cruciate Ligament-Deficient and Reconstructed Knee During Walking,” Am. J. Sports Med., 31(1), pp. 75–79. [DOI] [PubMed] [Google Scholar]
- [122]. Lafortune, M. A. , Cavanagh, P. R. , Sommer, H. J., III , and Kalenak, A. , 1992, “Three-Dimensional Kinematics of the Human Knee During Walking,” J. Biomech., 25(4), pp. 347–357. 10.1016/0021-9290(92)90254-X [DOI] [PubMed] [Google Scholar]
- [123]. Benoit, D. L. , Ramsey, D. K. , Lamontagne, M. , Xu, L. , Wretenberg, P. , and Renstrom, P. , 2006, “Effect of Skin Movement Artifact on Knee Kinematics During Gait and Cutting Motions Measured in vivo ,” Gait and Posture, 24(2), pp. 152–164. 10.1016/j.gaitpost.2005.04.012 [DOI] [PubMed] [Google Scholar]