Abstract
A recent study in the journal Science offer insights into the mechanism behind feto-maternal tolerance, as evidenced by changes in the immunological environment of the uterus and decidua, they also provide a rich area of research for the understanding of the regulation of the immune system in other complicated medical conditions, including cancer, and pregnancies affected by infection or autoimmunity.
Establishment and maintenance of proper maternal-fetal interface is essential for the success of pregnancy. A critical aspect in the establishment of a proper maternal-fetal interaction is the regulation of the maternal immune system present at the implantation site. Our understanding of this interaction has significantly increased since the original observation by Sir Peter Medawar, over fiftyyears ago proposing the theory of the fetus as a semi-allograft that is not rejected by the maternal immune system1, 2. The presence of a high number of innate immune cells (macrophages, dendritic cells, Natural Killer cells) was presented as evidence for the recognition of the maternal immune system to the paternal antigens present in the trophoblast3.
We know, today, that the innate immune system is present at the implantation site as a supportive element for the process of implantation, trophoblast invasion and spiral arteries transformation4-7. There is strong supporting evidence that the early presence of innate immune cells is not related to antigens from the father, but rather helps tissue renewal and establishment of the pregnancy 8, 9. That is not the case for the regulation of the adaptive immune system, T and B cells. Many of the studies associated with the uterine regulation of T cells have focused on characterizing the presence and the role of Treg10, 11. However the function of Treg could not explain the control of T cell distribution in the pregnant uterus. New findings by Nancy, et al,12may provide some insights into this process.
The June 2012 issue of Science published an article entitled “Chemokine Gene Silencing in Decidual Stromal Cells Limits T Cell Access to the Maternal-Fetal Interface” by Nancy, et al. 12. The authors presented data to support their hypothesis that decreased chemoattraction of T cells to the decidua occurs in order to support fetomaternal tolerance. They used a mouse model to study the effects of pre-pregnancy antigen exposure with subsequent re-exposure during pregnancy on the inflammatory cascade.
C57BL/6 female non-pregnant mice were immunized with soluble OVA prior to mating with a male mouse hemizygous for Act-mOVA transgene. Then, on E5.5, the pregnant mice were rechallenged with both OVA and the combination of CD40 antibodies+poly(I:C). Using a variety of immunostaining techniques the authors were able to show a significant lack of decidual response to the inflammatory stimulus as evidenced by a decreased level of CD3+ Tcell infiltration in the decidua compared to the myometrium overlying the implantation site and both the myometrium and endometrium of the interimplantation sites. Parallel to these findings the levels of key Th1/Tc1-attracting chemokines were decreased in the decidua compared to the other sites. Specifically, gene expression of Cxcl9 and Ccl5 were not increased in the decidua as they were in the myometrium. (Cxcl10 expression was only minimally increased in the decidua, but not above the basal level of that seen in the myometrium.)
These expression differences were then shown functionally with transwell migration assays. Interestingly, this differential expression appeared to be occurring at the level of the individual gene regulation and not as a result of an inefficient inflammatory response of the cell. To support this finding, chromatin immunoprecipitation assays showed that the expression of the chemoattractants increased in non-pregnant endometrial stromal cells as well as in the myometrium and interimplantation sites of pregnant uteri but not in the decidua. This suggested a change in gene expression during the cellular transformation of endometrial stromal cells to decidual stromal cells. Ex vivo investigation of the promoter region of Cxcl9 and Cxcl10 revealed elevated levels of the repressive histone mark H3K27me3 in decidual versus myometrial stromal cells, which was confirmed in vivo. Furthermore, in response to inflammation, myometrial stromal cells showed upregulation of the marker of active gene transcriptionH4Ac in the promotion of chemoattract genes Cxcl9/10, whereas decidual stromal cells did not.
These findings provide a new interpretation of the regulation of the maternal immune system by the pregnant uterus. Contrary to previous studies focused on mechanisms by the placenta (trophoblast cells) inducing either cell death of T cells (e.g. Fas-FasL hypothesis 13) or deletion of T cells, this study suggests an active role of the decidua controlling the migration of maternal T cells through the implantation site.
The fact that the inhibition of chemokine production in the decidua is associated with methylation of these genes suggests that epigenetic regulatorscontrol the capacity of the decidua to attract T cells. Although this study does not provide an answer to this question, it begs the question as to what the source is and what the factors are, that are controlling the expression of chemokines by decidual cells. A potential source could be the trophoblast (Figure 1A). We and others have shown that trophoblasts secrete cytokines that regulate the function and differention of decidual immune cells. It is plausible that the same factors could induce epigenetic changes in stromal decidual cells, consequently inhibiting their capacity to produce chemokines responsible for T cell recruitment. However; in pathologic conditions, such as infection, the inhibitory status can be broken and the same stromal decidual cells would become actively involved in the recruitment and activation of T cells to the implantation site 14. These changes may have detrimental consequences for pregnancy.
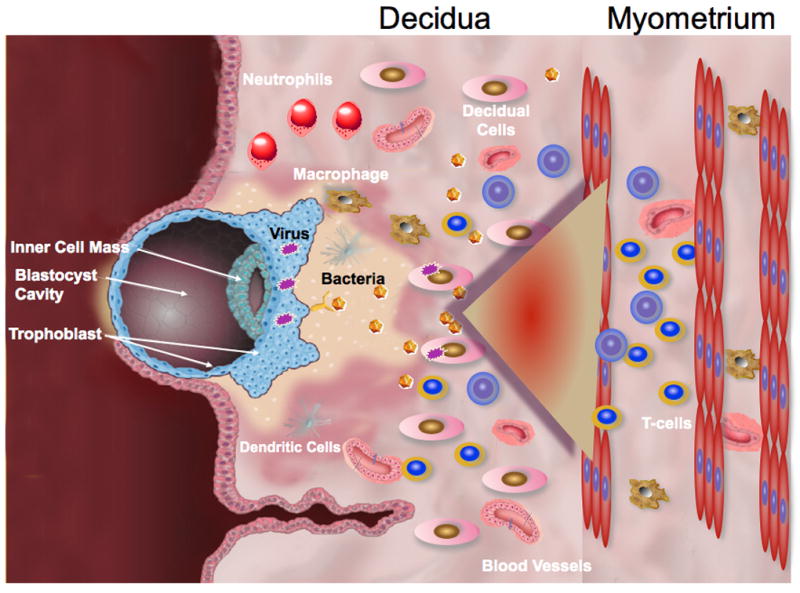
Figure 1. Model of molecular interaction between the trophoblast and decidua.
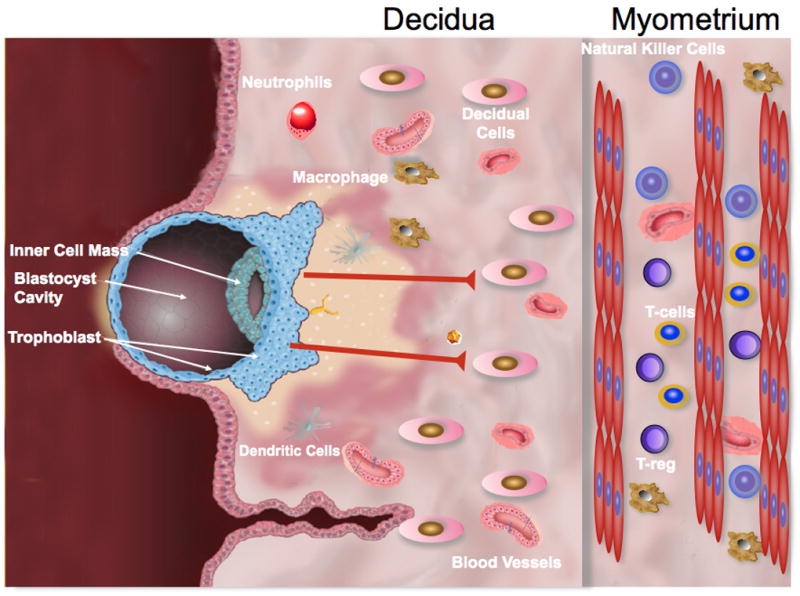
A. Decidual cells are unable to attract T cells to the endometrium as result of lack of chemokine production. Consequently, the implantation site is free of adaptive immune cells. Trophoblast cells may constitute the source of regulatory factors preventing chemokine production by stromal decidua cells.
B. Viral or bacterial infection inhibit the regulatory factors allowing decidual cells to produce chemokines, enhancing the migration of T cells and NK cells towards the decidua
In conclusion, not only do these findings offer insight into the mechanism behind feto-maternal tolerance, as evidenced by changes in the immunological environment of the uterus and decidua, they also provide a rich area of research for understanding the regulation of the immune system in other complicated medical conditions, including cancer, and pregnancies affected by infection or autoimmunity.
Acknowledgments
This study is in part funded by grants from the National Institute of Health, NICDH P01HD054713 and 3N01 HD23342
References
- 1.Medawar P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp SocExp Biol. 1952;7:320–338. [Google Scholar]
- 2.Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. British Journal of Experimental Pathology. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 3.Mor G, Cardenas I. The immune system in pregnancy: A unique complexity. Am J Reprod Immunol. 2010:63. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 5.Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. 2010;63:460–471. doi: 10.1111/j.1600-0897.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- 6.Dekel N, Gnainsky Y, Granot I, Mor G. Inflammation and implantation. Am J Reprod Immunol. 2010;63:17–21. doi: 10.1111/j.1600-0897.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118:3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke SD, Barrette VF, Gravel J, Carter AL, Hatta K, Zhang J, Chen Z, Leno-Duran E, Bianco J, Leonard S, Murrant C, Adams MA, Anne Croy B. Uterine NK cells, spiral artery modification and the regulation of blood pressure during mouse pregnancy. Am J Reprod Immunol. 2010;63:472–481. doi: 10.1111/j.1600-0897.2010.00818.x. [DOI] [PubMed] [Google Scholar]
- 9.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Annals of the New York Academy of Sciences. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramhorst R, Fraccaroli L, Aldo P, Alvero AB, Cardenas I, Leiros CP, Mor G. Modulation and Recruitment of Inducible Regulatory T Cells by First Trimester Trophoblast Cells. Am J Reprod Immunol. 2011 doi: 10.1111/j.1600-0897.2011.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leber A, Teles A, Zenclussen AC. Regulatory T cells and their role in pregnancy. Am J Reprod Immunol. 2010;63:445–459. doi: 10.1111/j.1600-0897.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 12.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 14.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, Means RE. Placental Viral Infection Sensitizes to Endotoxin-Induced Pre-Term Labor: A Double Hit Hypothesis. Am J Reprod Immunol. 2010 doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


