Abstract
Diffusion tensor imaging (DTI) is an MRI technique that can measure the macroscopic structural organization in brain tissues. DTI has been shown to provide information complementary to relaxation-based MRI about the changes in the brain's microstructure. In the pediatric population, DTI enables quantitative observation of the maturation process of white matter structures. Its ability to delineate various brain structures during developmental stages makes it an effective tool with which to characterize both the normal and abnormal anatomy of the developing brain. This review will highlight the advantages, as well as the common technical pitfalls of pediatric DTI. In addition, image quantification strategies for various DTI-derived parameters and the normal brain developmental changes associated with these parameters are discussed.
Keywords: Diffusion tensor imaging (DTI), MRI, Normal development
The advantage of pediatric DTI
MRI plays a crucial role in the diagnostic workup of many pediatric brain pathologies. Using MRI, anatomical changes can be characterized in vivo on a larger sample size. However, anatomical evaluation of the brain in the early phases of development is challenging, Previous studies have described a biphasic development of the brain—rapid growth in the tirst 2 years of life, followed by slower and more subtle developmental changes [1–4]. During the first 2 years of life, histological studies have described the temporo-spatial gradients of myelinization, which can cause drastic changes in MRI contrasts. At birth, the contrast of gray and white matter in T1- and T2-weighted images is the opposite of that seen in adult brains. During the contrast transition period when the gray-white matter contrasts are reversed, MRI may even fail to differentiate gray and white matter. For example, in T1-weighted images, the white matter appears brighter than the gray matter in adult brains, but the white matter of the neonate brain appears darker than the gray matter [5–8]. Inevitably, there is a time when the intensity difference between the gray and the white matter disappears.
The rapid changes in T1/T2 contrast during the neonatal phase suggests that these changes are sensitive markers of brain development, believed to be due mainly to the myelination process, but the highly time-dependent nature of these contrasts often interferes with clear structural definitions and limits their value for clinical research into brain maturation [9–17]. DTI has been shown to provide information complementary to relaxation-based MRI about the changes in the brain's microstructure with maturation [14–16, 18–33], Diffusion parameters that describe the brain's microstructure include the three diffusion tensor eigenvalues (λ1, λ2, λ3), which represent diffusion along the three tensor principal axes, mean diffusivity, and indices of anisotropy. These parameters are calculated in each voxel of the image (sec the articles about DTI application to pediatric populations by Miller et al. [34] and Huppi and Dubois [29]. As reported by many authors [14–16, 27, 34, 35], DTI can provide more stable contrasts between white and gray matter throughout the development process (Fig. 1), although both gray and white matter have characteristic contrast changes during that period. At birth, most of the major white matter tracts at the core regions of the white matter are already appreciable by DTI. The DTI information can be displayed quantitatively, or as images, which allows quantitative study of the maturation process of individual white matter structures. These diffusivity and microstructural measures appear to predict long-term developmental outcome [36]. In this respect, DTI is an effective tool with which to characterize both the normal and abnormal pediatric anatomy of developing white matter structures [14–16, 37, 38].
Fig. 1.
The time course of normal maturation detected on T1 (upper row)/T2 (middle row) DTI (FA map, lower row). The maturation (predominantly the myelination pattern) has a profound impact on T1- and T2-weighted imaging. The extent of the contrast change is so large that the gray/white matter contrast inverts in the first 12 months. During this time period, FA remains more stable, especially in the deep brain regions, while the peripheral white matter has a noticeble FA increase
Issues in pediatric DTI
There are several issues in DTI that are noteworthy, In this section, two major issues are discussed. First, DTI is prone to various sources of artifacts, which can cause practical issues in the pediatric population. Second, there is an inherent limitation of DTI caused by the tensor model.
In a recent review by Tournier et al. [39], the types and mechanisms of typical artifacts are described in detail. One type of artifact is caused by image registration quality. As is the case for all quantitative MRI based on multiple raw MR images, image mis-registration (image misalignment of more than one pixel) due to subject motion or hardware instability, would lead to degradation of the images. The second type of artifact is specific to DTI, By sensitizing the MR signal to a small amount of water diffusion, DTI is also sensitive to finite, sub-pixel, tissue motion, such as cardiac pulsation. This would lead to unwanted signal loss when a specific diffusion-encoding direction and a specific cardiac cycle coincide.
Pediatric DTI is challenging because these two sources of artifacts are pronounced. Young children, i.e., under the age of 4 years, cannot be still, and can only be expected to be able to cooperate after the age of 5 years. The pulsation-caused artifacts are more abundant in this population, especially around the third and fourth ventricles. Therefore, careful quality control of the data would be more important than in adult populations.
The inherent limitation of DTI is caused by the fact that the tensor model, in which underlying neuroanatomy is modeled by a six-parameter tensor, suffers from oversimplification. For example, from the tensor model, we can calculate the orientation of the longest axis of the diffusion ellipsoid. We usually assume that the orientation aligns to axonal bundles, but this interpretation has the inherent assumption that all axons have the same orientation within a pixel, which is not a valid assumption for many regions of the brain; with the current image resolution (2–3 mm), each pixel usually contains axons with multiple orientations. There are two potential ways to reduce this limitation, but they are practically mutually exclusive. First, the image resolution could be increased, and thus, with a small pixel size, there would be less of a mixture of axonal populations with different orientations. Please note that if the pixel size is maintained, there would be a lesser number of pixels within a smaller pediatric brain, which would lead to a lower anatomical resolution. However, pediatric brains tend to have a longer T2. and, thus, we can obtain a higher signal-to-noise ratio (SNR) with the same echo time. We can use this signal gain to reduce the pixel size. Typically, a 1.6- to 2.0-m isotropie resolution is feasible, depending on the size and age of the patient. DTI with a higher resolution is, thus, definitely a viable option for pediatric DTI.
The second way to ameliorate the DTI tensor limitation is to employ a family of diffusion-based methods that do not rely on the tensor model, and, thus, are not bounded by the oversimplification to the six-parameter tensor model. These methods include diffusion spectral imaging (DSI), q-ball imaging (OBI), and high-angular resolution diffusion imaging (HARDI) [40–43], all of which aim to increase the amount of information that can be obtained from each pixel. Namely, while high-resolution DTI attempts to increase anatomical information by placing more pixels within a brain, the non-tensor approaches increase information per pixel. Using these approaches, we can estimate the number of axonal populations and their orientations in each pixel. Applications of these methods to pediatric populations are still limited [44], and could constitute an important future research effort. These methods, however, require a longer scan time and higher diffusion-weighting, both of which result in a poorer SNR-to-scan time relationship and higher sensitivity to motion-related artifacts. The practicality for routine clinical uses, therefore, should be carefully evaluated.
Difficulty of image analysis in neonate and pediatric brains
A regular DTI consists of more than 1 million voxels. Each voxel of different MR contrast includes different types of information representing the background anatomy. The first hurdle here for image quantification is to establish consistent anatomical criteria to define brain structures throughout the development process. This is especially difficult for the first 24 months of age due to the rapid contrast changes (Fig. 1). One well-established method for image quantification is to manually place regions of interest (ROI) on the anatomical structure and quantify the structural size or pixel values. This approach is usually hypothesis-driven, such that there is a pre-defined set of ROIs to capture a specific disease status [16, 35, 45–57]. However, if there is no clear a priori knowledge, findings from an arbitrarily selected ROI do not guarantee structure specificity because other brain areas are not investigated. The underlying limitation of manual ROI is that it would be too time-consuming to manually define ROIs for hundreds of structures covering the entire brain. If one intends to explore the unknown effects of a particular disease, automated whole-brain analysis is typically a good choice.
There are several types of approaches for whole-brain analysis. Voxel-based analysis (VBA) is probably the most widely used method, in which the properties of all voxels are measured by transforming the brain shape to a pre-selected template. The common template serves as a pre-defined ROI set, in which each voxel becomes an independent ROI. VBM has been widely used in children 2 years of age or older [58–66]. One notable issue with the VBA approach is the choice of the common template for children younger than 2 years old. Because of the rapid contrast changes in the first 24 months of age, it is not clear whether one template can serve populations with different ages. Multiple templates for cross-sectional studies are likely required; a template for each age serves only a narrow age window (Fig. 2). This is still a developing research area and it is uncertain how many templates are needed and the proper age range for each template.
Fig. 2.
Multi-contrast, single-subject atlases for a neonate (with 112 parcellations) at 2 years old and 18 months old and in an adult (with 159 parcellations) (available at http://lbam.med.jhmi.edu/)]
There are other issues with VBA, which are not inherent to pediatric populations. Among these, the sensitivity of the measurement is the most common issue. Because each tiny voxel serves as an ROI, the measurement is noisy. The accuracy of voxel-to-voxel alignment across subjects is also an issue, and mis-registration would lead to a large variability in the measured parameters (e.g., fractional anisotropy) within the control group and a loss of statistical power. For example, it has been pointed out that VBA is not suitable for detecting small but widespread change in the brain [67]. Spatial filtering is often applied to ameliorate the sensitivity issue, in which multiple voxel properties are averaged. Tract-Based Spatial Statistics (TBSS) was also developed to enhance sensitivity by focusing on the improved registration accuracy of the core white matter voxels for the pediatric population [68–70].
An alternative voxel-grouping strategy is the “smart ROI” approach, in which voxels that belong to the same anatomical structure are grouped, rather than relying on isotropic voxel grouping. This approach usually requires a pre-defined structural definition file (parcellation map), which can be transformed to each subject image and can quantify the volume and voxel intensities of each anatomical structure (Fig. 3). Statistical analysis can be performed in a structure-by-structure way, which is called atlas-based analysis (ABA), in contrast to the voxel-by-voxel method of VBA. Compared to VBA, which is based on data from more than 1 million independent voxels, the structure-by-structure analysis of the ABA approach provides a more-manageable amount of anatomical information for subsequent image analysis [71–73]. Since each ROI contains many voxels, information about anatomical localization is less than that provided by VBA. If abnormal voxels compose only a small portion of the defined structure, ABA could be less sensitive than VBA. VBA and ABA visualize the same anatomy from different anatomical granularity levels, and different types of abnormalities can be detected by each method. Thus, these two methods are complementary techniques for whole-brain analysis.
Fig. 3.
Schematic pipeline of the image normalization process. The image on the upper left side is an initial image of children with periventricular leukomalacia, clinically diagnosed as spastic cerebral palsy. The orange arrows show “forward” transformation; the subject image was first linearly normalized (affine transformation), followed by nonlinear normalization (LDDMM). After this procedure, all subject images were transformed to a shape similar to that of the atlas (two images in lower left). For the “backward” transformation (green arrows), the brain parcellation map (BPM) was transformed to the original MRI using the same deformation fields used for the forward transformation. This allows the map to be superimposed onto the original images with parcellation into 159 structures. Please note the level of accuracy of the parcellation for this patient with severe injury
Normal developmental patterns
Observation of normal development by DTI—neonate to 2 years
Contrast changes in pediatric DTI comprise three phases: rapid change in the first 3 lo 6 months, followed by slower change until 24 months, and relative stability after 24 months [6, 15, 27, 74] (Fig. 1). A basic pattern of the maturation process in pediatric DTI is a decrease in mean diffusivity (MD) and an increase in FA as a function of gestational age, and a posterior-to-anterior and a central-to-peripheral direction of maturation [4, 16, 20, 22, 75–82].
White matter: mean diffusivity (MD)
Brain water content decreases with maturation. During this process, structures such as cell and axonal membranes become more densely packed, and the restriction to water motion increases. Due to the larger extracellular space of the unmyelinated white matter in the immature brain, the MD of white matter is almost twice that of the fully myelinated brain [23]. As white matter develops, decreases in water diffusion are observed mainly in λ2 and λ3 (and less in λ 1), which reflects changes in water diffusion perpendicular to white matter fibers, and may indicate changes due to premyelination (change in axonal width) and myelination. Differences in water content could also affect the contrast between white and gray matter in the pediatric brain, although not in a simple fashion. In the adult brain, the water content of the white matter is essentially lower than that of the gray matter (65% versus 85%); however, the MD values for the two regions are virtually identical [83, 84]. This indicates that white matter is less restrictive to water motion than gray matter and may be related to the fact that water motion parallel to axons is relatively unrestricted, compared to motion perpendicular to axons or in gray matter. In the premature brain, the percentage of water content is similar in white and gray matter, although MD values in white matter are higher than those in gray matter. This finding is also consistent with the idea that white matter is less restrictive to water motion than gray matter (see a review of DTI application to neonatal populations by Huppi and Dubois [29]).
The quantitative changes in MD have a regional variation in white matter maturation, as measured in many studies [14, 15, 22, 27, 28, 34, 74, 76, 78, 82, 85–90]. Partridge et al. [87] reported that the lowest MD values were found in the projection fibers of the internal capsule and the cerebral peduncles, with decreasing values from 30 weeks of gestational age to term age among several deep white matter structures (in commissural tracts, in projection tracts, and in association tracts). Provenzale et al. [82] defined white-matter maturation in the deep (the posterior limb of the internal capsule, the genu, and the splenium of the corpus callosum) and the peripheral (subcortical) white matter structures, using multiple ROIs in neonatal DTI. At term, the MD for the peripheral white matter regions was higher than the MD for the deep white matter structures. In the early period after birth (before day 100), the MD in the peripheral white matter decreased at approximately twice the rate of the MD in the deep white matter, and no differences were observed throughout the late period (after day 100) [82].
White matter anisotropy
For white matter areas, white matter anisotropy is relatively low in neonates and increases steadily with increasing age. While changes in MD and anisotropy for the white matter typically occur together during maturation, with MD values decreasing and anisotropy values increasing, the processes by which the two parameters change are theoretically different [29, 91]. Although little has been reported on the correlation of FA changes and MD changes, they are independent of one another, and a change in one is not always accompanied by an opposite change in the other [37, 71, 90]. The increase in white matter anisotropy values during development appears to occur in three stages: fiber organization into fascicles, proliferation and maturation of glial cell bodies and intracellular compartments, and myelination [76]. The first stage, fiber organization, occurs largely in utero in humans, which is evidenced by the presence of anisotropy in late intrauterine and premature infants [87, 92]. This process would be expected to increase anisotropy predominantly, without affecting MD [90]. The first increase occurs before the histological appearance of myelin [16, 78], and it seems to correlate with the developmental expansion of immature oligodendrocytes during the premyelination period [93]. Notably, premyelination is seen as increase in anisotropy whilst it is not detectable at T1- or T2-weighted imaging. The second stage includes the maturation of glial cell bodies and their processes, as well as the development of the cytoskeleton and various intracellular structures, and a decrease in MD without increasing anisotropy is predominant. The third stage, in which an increase in anisotropy is continuous, is associated with the histological appearance of myelin and its maturation around axons. This three-stage increase in white matter anisotropy is not synchronous for different brain areas, as is brain maturation [76, 90].
In FA measurements of the white matter, large regional differences were observed. These differences typically follow a “high FA in the core and low FA in the peripheral white matter” rule [94]. In a study by Hermoye et al. [27], which used multiple ROIs, several exceptions to this rule were demonstrated. First, the area where the corpus callosum and the anterior limb of the internal capsule meet (“crossing” region) has low FA at birth, despite its relatively deep location, and also lacks an initial steep FA increase. Second, the association fibers, especially the superior longitudinal fasciculus, mature at a relatively later stage of development. However, limbic fibers (the fornix and the cingulum), regardless of their relatively small size, can be well appreciated in the early phase of development, The early appearance of the limbic fibers and other tracts, such as the core regions of projection fibers, commissural fibers, and the uncinate fasciculus, agrees with histology-based fetal brain atlases [95] and DTI studies in premature newborns [87]. In normal-term infants [76], diffusivity and anisotropy along the tracts suggest that the corticospinal tract is the most mature, followed by the spinothalamic tract and fornix, and then the arcuate and inferior longitudinal fasciculus, optic radiations, and the anterior limb of the internal capsule and the cingulum. Autopsy studies have shown that the corticospinal tract, the corpus callosum, and the superior cerebellar peduncles mature early, which is in accordance with MRI studies. The late maturation of the association tracts was also confirmed by histological analyses. Autopsy studies have shown, however, that the fornix, which demonstrates a relatively high FA in newborns, does not reach full myelination until 2 years of age [10, 13]. Regional anisotropy is thought to be influenced not only by myelination, but also by axon packing, the relative membrane permeability to water, the internal axonal structure, and the tissue water content [29].
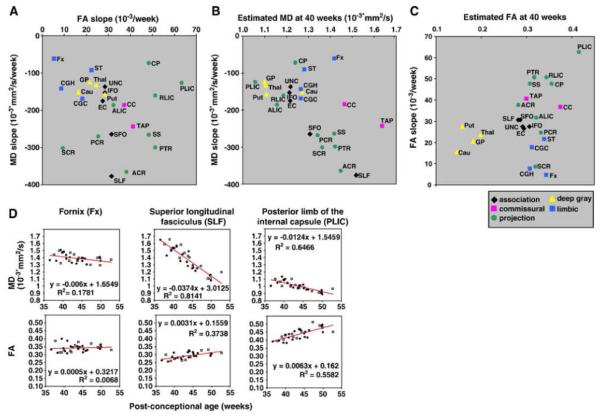
In our atlas-based, whole-brain study, which evaluated DTI parameters during the normal brain developmental process within 3 months after birth, several patterns of the relationship between the MD and the FA at 40 post-conceptional weeks were observed [71]. Namely, in the more superior locations, the MD was higher and decreased more rapidly with age in the corticofugal pathway (i.e. the superior corona radiata, the posterior limb of the internal capsule, the cerebral peduncle). In the more anterior regions, the MD was higher and decreased more rapidly with age in the corona radiata (the anterior portion, the superior portion, the posterior portion) (Fig. 4). These tendencies for MD were not observed in the FA analysis, especially for the structures with rich crossing fibers, such as the corona radiata. This analysis suggests that the time-dependent FA changes may provide more information about the development of the crossing fibers, compared to the time-dependent MD changes.
Fig. 4.
The relationship between the age-dependent mean diffusivity (MD) decreasing slope and the age-dependent fractional anisotropy (FA) increasing slope (a), the estimated MD at 40 post-conceptional weeks and the age-dependent MD decreasing slope (b), and the estimated FA at 40 post-conceptional weeks and the age-dependent FA increasing slope (c). The white matter structures were categorized based on the association fibers (black dots: SLF, SFO, ILF, IFO, and UNC), the commissural fibers (pink dots: CC and TAP), the limbic fibers (blue dots: CGC, CGH, Fx, and ST), and the projection fibers (green dots: ALIC, PLIC, RLIC, CP, PTR, SS, ACR, SCR, and PCR). (d) Linear regression analyses of MD and FA from three representive areas. Open squares indicates data from boys, and black circles indicate data from girls. The MD and FA of each structure show time-dependent changes with markedly different slopes and intercepts [71] (used by permission copyright). SLF superior longitudinal fasciculus, SFO superior fronto-occipital fasciculus, ILF inferior longitudinal fasciculus, IFO inferior fronto-occipital fasciculus, UNC uncinate fasciculus, CC corpus callosum, TAP tapetum, CGC cingulum cingular part, CGH cingulum hippocampal part, Fx fornix, ST stria terminalis, ALIC anterior limb of internal capsule, PLIC posterior limb of internal capsule, RLIC retrorenticular part of internal capsule, CP cerebral peduncle. PTR posterior thalamic radiation, SS sagittal stratum, ACR anterior corona radiata, SCR superior corona radiata, PCR posterior corona radiata
Cortex
Anisotropy values for the cortical gray matter decrease after birth compared to those in the fetuses of many different species, including human, mouse, cat, and pig brains [15, 16, 37, 55, 83, 96–102].
The tensor principal eigenvectors are oriented radially to the cortical surface. A recent study about the human fetal brain has shown that cortical anisotropy increases from 15 weeks' gestation to approximately 27 weeks' gestation, and then shows a gradual decline until 32 weeks' gestation [103]. This anisotropy is believed to result from the radial organization of the immature cerebral cortex [97]. In the immature cortex, the predominant features are radial glial cells and large pyramidal neurons with prominent, radially oriented apical dendrites; these structures cause horizontal water motion to be relatively impaired, resulting in radially oriented anisotropy [1]. With neocortical maturation, the radial glial cells are transformed into the astrocytic neuropil and this architecture is disrupted by the addition of basal dendrites, as well as thalamocortical afferents, which tend to be oriented orthogonally to the apical dendrites [104]. In addition, intracortical association axons navigate horizontally through the developing cortex, which causes impairment of radially directed water motion, resulting in increasing isotropic water motion in the cortex. Unlike the changes observed in the white matter maturation process, the changes in fractional anisotropy observed in the cortex are mainly due to significant decreases in λ1, without changes in λ2 and λ3 [105].
This cortical developmental process is not homogeneous throughout the brain and shows considerable regional differences, with cortical anisotropy decreasing first in the pre-central cortex, followed by the occipital and the frontal cortex [105]. An MR tractography study on postmortem fetal human brains reported that the regional regression of radial organization and regional emergence of fetal brain connectivity proceeds in general from posterodorsal to anteroventral with local variations [106]. In addition, the early cortical lateralization in anisotropy and the asymmetry of early cortical folding are also reported [103, 107].
Normal development in DTI—after 2 years of age
After the age of 2 years, developmental changes become much more subtle. The average brain weight at 2 years of age has already reached approximately 80% of that of the adult brain weight, and at 5 years old it is approximately 90% of the adult weight, and there is no real significant difference in weight [108, 109]. In our study [72], the brain volume at 2 years of age was already approximately 78% of an adult's volume, and after 5 years of age, as expected, there were almost no significant time-dependent changes. In terms of MR contrasts, T1-weighted imaging studies found changes in a confined area of the brain [32, 110, 111], DTI is sensitive enough to show a pattern of maturation with considerable regional variation, generally characterized by an increase in FA and a decrease in MD through childhood and adolescence [20, 25, 26, 32, 76, 112–115].
Previous data also consistently demonstrate strong, positive, linear correlations between age and white matter and CSF volumes [72, 110, 116, 117], Other studies have already shown that the white matter volume does not begin to decrease until the fourth decade [110, 118, 119]. With regard to the CSF volumes, the recognition of its normal increase with age is an important consideration when interpreting reports of increased ventricular volumes in several neuropsychiatric conditions [120–122].
In the gray matter, the correlation between age and volume was not monotonic. Cortical growth is known to obey more complex curves, usually following an “inverted U” developmental course, with volumes peaking at different times in different lobes, most of which peak between 10 and 17 years of age [72, 118, 123].
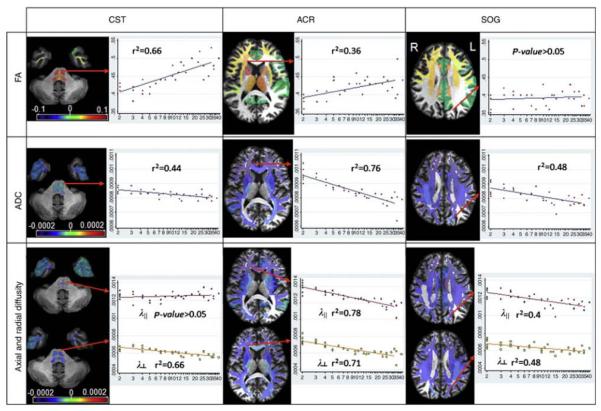
With regard to the regional volume and diffusivity indices of white matter in our study [72], the entire white matter undergoes a similar time course—an increase in the volume and a decrease in diffusion constants, while there was a small tendency toward a steeper volume increase in the white matter regions rich with projection fibers. The regional differences in the amount of decrease in axial and radial diffusivity led to significant FA increases in a confined number of brain regions, which included the corticospinal tract (CST), the frontal white matter, and the thalamus. These changes may be related to the changes in axon diameters and the amount of myelination in these regions. It has been suggested that the diameter of the thickest fibers in the CST increases linearly as a function of body height [124]. To maintain passive cable conduction, dendrites need to increase four times in diameter when they double in length [125]. This expansion requires an increase in myelination, and, as a result, the increased volume of the insulating sheaths surrounding axonal fibers bulks up the volume of the white matter compartment. Moreover, the significant shortening of the central conduction time during childhood and adolescence, observed in the motor pathway [126–128]. functionally supports the myelinization and organization of CST fibers that occur in this phase, and is a plausible explanation for both the increase in volume and FA due to the decrease in radial diffusivity (Fig. 5). A positive relationship between FA and age was also present in the peripheral white matter of the frontal and parietal lobes, as well as in the superior temporal gyrus of the right hemisphere. In these areas, both axial and radial diffusivity decrease, although radial diffusivity, compared to axial diffusivity, consistently had a steeper decline, explaining the FA increase (Fig. 6). In other regions, such as the posterior corona radiata, the centrum semiovale, and the white matter of the superior occipital region, axial and radial diffusivity proportionally decreased while FA remained stable. Decreases in both axial and radial diffusivity might indicate that those regions are under a process of myelination and increasing compactness, but with an additional component of increasingly complex fiber structural design [129].
Fig. 5.
Actual fitting results for the fractional anisotropy (FA) (first row), apparent diffusion coefficient (ADC) (second row, in mm2/s), and axial and radial diffusivity (third row, in mm2/s), by age (in years, logarithmic scale), at representative locations. In the corticospinal tract (CST, first column), the FA increase can be explained by the radial diiffusivity decrease. In the anterior corona radiata (ACR, second column), the age-related changes in the axial diffusivity cause a weaker time-dependent FA change. In the white matter of the superior occipital gyrus (SOG, third column), the parallel decreases in both axial and radial diffusivity lead to no significant changes in FA. The orientation of the slices follows the radiologic convention (L left, R right) (printed with permission [72])
Fig. 6.
Map of slopes measured by the voxel- (a) and atlas-based analyses (b) for the volume, fractional anisotropy (FA), and diffusivity values. Note the overall agreement between the two methods. The orientation of the slices follows the radiologic convention (L left, R right. Th thalamus) (printed with permission [72])
Among the subcortical areas, the frontal lobe presented bigger slopes (in absolute value) and R2 in both volume and diffusivity analyses [72]. It is possible that the different trends represent distinct maturation patterns, in which higher-order association areas mature after the lower-order sensorimotor regions they integrate, This heterogeneous comportment has been previously described for the cortex [117, 130] and for the white matter of older adults [131], but not for the white matter of younger subjects. However, since the gray and white matter have inseparable connections and share lifelong reciprocal relationships [132–134], it is not surprising that we detected the same maturation pattern in the white matter.
Previous studies have consistently reported brain maturation during adolescence in the internal capsule, the arcuate fasciculus, superior longitudinal fasciculus, and the CST [72, 81, 91, 135–137]. Some of these recent studies have described not linear but mono-exponential equations that modulate the components of the white matter diffusivity over time [114, 136], Nevertheless, those studies covered a different age range, some including people as old as 80 years of age. But, in fact, they are unanimous in concluding that white matter FA does not begin to decrease (and mean diffusivity does not begin to increase) until the fourth decade.
Conclusion
DTI is a promising modality for the study and analysis of brain development and abnormalities. Currently, pediatric DTI is the subject of very active research. The knowledge that will be obtained by these research endeavors will build the foundation that will both improve our understanding of the normal brain development and also enable us to explore the pathophysiological basis of developmental diseases.
Acknowledgments
The authors thank Ms. Mary McAllister for help with manuscript editing. This publication was made possible by NIH grants RO1AG20012, and P41EB015909 from NCRR/NIBIB (SM), R01HD065955 from NICHD (KO) and R03EB014357 from NIBIB/NIH (AF). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of any of these institutes.
References
- 1.Barkovich AJ, Raybaud C. Pediatric neuroimaging. 5th edn. Lippincott Williams & Wilkins; Philadelphia: 2012. [Google Scholar]
- 2.Keene MFL, Hewer EE. Some observations on myelination in the human nervous system. J Anat. 1931;6:1–13. [PMC free article] [PubMed] [Google Scholar]
- 3.van der Knaap MS, Valk J. Magnetic resonance of myelination and myelin disorders. 3rd edn. Springer; Berlin: 2005. [Google Scholar]
- 4.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Blackwell; Oxford: 1967. [Google Scholar]
- 5.Ballesteros MC, Hansen PE, Solla K. MR imaging of the developing human brain. Part 2. Postnatal development. Radiographics. 1993;13:611–622. doi: 10.1148/radiographics.13.3.8316668. [DOI] [PubMed] [Google Scholar]
- 6.Barkovich AJ, Kios BO, Jackson DE, Jr, et al. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166:173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- 7.Konishi Y, Hayakawa K, Kuriyama M, et al. Developmental features of the brain in preterm and fullterm infants on MR imaging. Early Hum Dev. 1993;34:155–162. doi: 10.1016/0378-3782(93)90050-5. [DOI] [PubMed] [Google Scholar]
- 8.van der Knaap MS, Valk J. MR imaging of various stages of normal myelination during the first year of life. Neuroradiology. 1990;31:459–470. doi: 10.1007/BF00340123. [DOI] [PubMed] [Google Scholar]
- 9.Barkovich AJ. Concepts of myelin and myelination in neuroradiology. AJNR. 2000;21:1099–1109. [PMC free article] [PubMed] [Google Scholar]
- 10.Brody BA, Kinney HC, Kloman AS, et al. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neural. 1987;46:283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Huppi PS, Warfield S, Kikinis R, et al. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- 12.Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structures is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 13.Kinney HC, Brody BA, Kloman AS, et al. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee P, Miller JH, Shimony JS, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee P, Miller JH, Shimony JS, et al. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR. 2002;23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 16.Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology. 1998;209:57–66. doi: 10.1148/radiology.209.1.9769812. [DOI] [PubMed] [Google Scholar]
- 17.Petanjek Z, Judas M, Kostovic I, et al. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: a layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- 18.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartha AI, Yap KR, Miller SP, et al. The normal neonatal brain: MR imaging, diffusion tensor imaging, and 3D MR spectroscopy in healthy term neonates. AJNR. 2007;28:1015–1021. doi: 10.3174/ajnr.A0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cascio CJ, Gerig G, Piven J. Diffusion tensor imaging: application to the study of the developmental brain. J Am Acad Child Adolesc Psychiatry. 2007;46:213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- 21.Ding XQ, Sun Y, Braass H, et al. Evidence of rapid ongoing brain development beyond 2 years of age detected by fiber tracking. AJNR. 2008;29:1261–1265. doi: 10.3174/ajnr.A1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, et al. Assessment of the early organization and maturation of infants' cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30:1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Engelbrecht V, Scherer A, Rassek M, et al. Diffusion-weighted MR imaging in the brain in children: findings in the normal brain and in the brain with white matter diseases. Radiology. 2002;222:410–418. doi: 10.1148/radiol.2222010492. [DOI] [PubMed] [Google Scholar]
- 24.Gilmore JH, Lin W, Corouge I, et al. Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. AJNR. 2007;28:1789–1795. doi: 10.3174/ajnr.A0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasan KM, Halphen C, Sankar A, et al. Diffusion tensor imaging-based tissue segmentation: validation and application to the developing child and adolescent brain. Neuroimage. 2007;34:1497–1505. doi: 10.1016/j.neuroimage.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasan KM, Sankar A, Halphen C, et al. Development and organization of the human brain tissue compartments across the lifespan using diffusion tensor imaging. Neuroreport. 2007;18:1735–1739. doi: 10.1097/WNR.0b013e3282f0d40c. [DOI] [PubMed] [Google Scholar]
- 27.Hermoye L, Saint-Martin C, Cosnard G, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Huang H, Zhang J, Wakana S, et al. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 31.Moseley M. Diffusion tensor imaging and aging a review. NMR Biomed. 2002;15:553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- 32.Snook L, Paulson LA, Roy D, et al. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Stegemann T, Heimann M, Dusterhus P, et al. Diffusion tensor imaging (DTI) and its importance for exploration of normal or pathological brain development. Fortschr Neurol Psychiatr. 2006;74:136–148. doi: 10.1055/s-2005-870948. [DOI] [PubMed] [Google Scholar]
- 34.Miller JH, McKinstry RC, Philip JV, et al. Diffusion-tensor MR imaging of normal brain maturation: a guide to structural development and myelination. AJR. 2003;180:851–859. doi: 10.2214/ajr.180.3.1800851. [DOI] [PubMed] [Google Scholar]
- 35.Huppi PS, Inder TE. Magnetic resonance techniques in the evaluation of the perinatal brain: recent advances and future directions. Semin Neonatol. 2001;6:195–210. doi: 10.1053/siny.2001.0039. [DOI] [PubMed] [Google Scholar]
- 36.Limperopoulos C. Advanced neuroimaging techniques: their role in the development of future fetal and neonatal neuroprotection. Semin Perinatol. 2010;34:93–101. doi: 10.1053/j.semperi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Neil JJ, Miller J, Mukherjee P, et al. Diffusion tensor imaging of normal and injured developing human brain a technical review. NMR Biomed. 2002;15:543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- 38.Lee SK, Kim DI, Kim J, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in development CNS abnormalities. Radio-Graphics. 2005;25:53–65. doi: 10.1148/rg.251045085. [DOI] [PubMed] [Google Scholar]
- 39.Tournier JD, Mori S, Leemails A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65:1532–1556. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wedeen VJ, Hagmann P, Tseng WY, et al. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- 41.Frank LR. Characterization of anisotropy in high angular resolution diffusion-weighted MRI. Magn Reson Med. 2002;47:1083–1099. doi: 10.1002/mrm.10156. [DOI] [PubMed] [Google Scholar]
- 42.Tournier JD, Calamante F, Gadian DG, et al. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage. 2004;23:1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 43.Tuch DS, Reese TG, Wiegell MR, et al. Diffusion MRI of complex neural architecture. Neuron. 2003;40:885–895. doi: 10.1016/s0896-6273(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee P, Hess CP, Xu D, et al. Development and initial evaluation of 7-T q-ball imaging of the human brain. Magn Reson Imaging. 2008;26:171–180. doi: 10.1016/j.mri.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoon AH, Jr, L WT, Jr, Melhem ER, et al. Diffusion tensor imaging of periventricular leukomalacia shows affected sensory cortex white matter pathways. Neurology. 2002;59:752–756. doi: 10.1212/wnl.59.5.752. [DOI] [PubMed] [Google Scholar]
- 46.Arzoumanian Y, Mirmiran M, Barnes PD, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR. 2003;24:1646–1653. [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas B, Elyssen M, Peelers R, et al. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular while matter injury. Brain. 2005;128:2562–2577. doi: 10.1093/brain/awh600. [DOI] [PubMed] [Google Scholar]
- 48.Nagae LM, Hoon AH, Jr, Stashinko E, et al. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR. 2007;28:1213–1222. doi: 10.3174/ajnr.A0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glenn OA, Ludeman NA, Berman JI, et al. Diffusion tensor MR imaging tractography of the pyramidal tracts correlates with clinical motor function in children with congenital hemiparesis. AJNR. 2007;28:1796–1802. doi: 10.3174/ajnr.A0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakami A, Morimoto M, Yamada K, et al. Fiber-tracking techniques can predict the degree of neurologic impairment for periventricular leukomalacia. Pediatrics. 2008;122:500–506. doi: 10.1542/peds.2007-2816. [DOI] [PubMed] [Google Scholar]
- 51.Ludeman NA, Berman JI, Wu YW, et al. Diffusion tensor imaging of the pyramidal tracts in infants with motor dysfunction. Neurology. 2008;71:1676–1682. doi: 10.1212/01.wnl.0000304084.59964.e2. [DOI] [PubMed] [Google Scholar]
- 52.Hoon AH, Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlates with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;52:697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida S, Hayakawa K, Yamamoto A, et al. Quantitative diffusion tensor tractography of the motor and sensory tract in children with cerebral palsy. Dev Med Child Neurol. 2010;52:935–940. doi: 10.1111/j.1469-8749.2010.03669.x. [DOI] [PubMed] [Google Scholar]
- 54.Frye RE, Hasan K, Malmberg B, et al. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Dev Med Child Neurol. 2010;52:760–766. doi: 10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koerte I, Pelavin P, Kirmess B, et al. Anisotropy of transcallosal motor fibres indicates functional impairment in children with periventriculat leukomalacia. Dev Med Child Neural. 2010;53:179–186. doi: 10.1111/j.1469-8749.2010.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida S, Hayakawa K, Oishi K, et al. Athetotic and spastic cerebral palsy: anatomic characterization in diffusion tensor imaging. Radiology. 2011;260:511–520. doi: 10.1148/radiol.11101783. [DOI] [PubMed] [Google Scholar]
- 57.Holmstrom L, Lennartsson F, Eliasson AC, et al. Diffusion MRI in corticofugal fibers correlates with hand function in unilateral cerebral palsy. Neurology. 2011;77:775–783. doi: 10.1212/WNL.0b013e31822b0040. [DOI] [PubMed] [Google Scholar]
- 58.Hulshoff Pol HE, Schnack HG, Mandl RCW, et al. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58:1118–1125. doi: 10.1001/archpsyc.58.12.1118. [DOI] [PubMed] [Google Scholar]
- 59.Good CD, Scahill RL, Fox NC, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- 60.Job DE, Whalley HC, McConnell S, et al. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880–889. [PubMed] [Google Scholar]
- 61.Kubicki M, Shenton ME, Salisbury DF, et al. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2001;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Counsell SJ, Edward AD, Chew AT, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children burn preterm. Brain. 2008;131:3201–3208. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- 63.Gimenez M, Miranda MJ, Born AP, et al. Accelerated cerebral white matter development in preterm infants: a voxel-based morphometry study with diffusion tensor MR imaging. Neuroimage. 2008;41:728–734. doi: 10.1016/j.neuroimage.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 64.Tzarouchi LC, Astrakas LG, Xydis V, et al. Age-related gray matter changes in preterm infants: an MRI study. Neuroimage. 2009;47:1148–1153. doi: 10.1016/j.neuroimage.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 65.Sonia-Pastor S, Padilla N, Zubiaurre-Elorza L, et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124:1161–1170. doi: 10.1542/peds.2009-0244. [DOI] [PubMed] [Google Scholar]
- 66.Lee JD, Park H, Park ES, et al. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain. 2011;134:1199–1210. doi: 10.1093/brain/awr021. [DOI] [PubMed] [Google Scholar]
- 67.Davatzikos C. Why voxel-based morphometric analysis should be used with great caution when characterizing group differences. Neuroimage. 2004;23:17–20. doi: 10.1016/j.neuroimage.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Ball G, Counsell SJ, Anjari M, et al. An optimized tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage. 2010;53:94–102. doi: 10.1016/j.neuroimage.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 69.van Kooij BJM, de Vries LS, Ball G, et al. Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR. 2012;33:188–194. doi: 10.3174/ajnr.A2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 71.Oishi K, Mori S, Donohue PK, et al. Multi-contrast human neonatal brain atlas: application to normal neonate developmental analysis. Neuroimage. 2011;56:8–20. doi: 10.1016/j.neuroimage.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faria AV, Zhang J, Oishi K, et al. Atlas-based analysis of neurodevelopment from infancy to adult hood using diffusion tensor imaging and applications for automated abnormality detection. Neuroimage. 2010;52:415–428. doi: 10.1016/j.neuroimage.2010.04.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faria AV, Hoon AH, Jr, Stashinko EE, et al. Quantitative analysis of brain pathology based on MRI and brain atlases-applications for cerebral palsy. Neuroimage. 2011;54:1854–1861. doi: 10.1016/j.neuroimage.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneider JF, Il'yasow KA, Hennig J, et al. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- 75.Berman JI, Mukherjee P, Partridge SC, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27:862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 76.Dubois J, Dehaene-Lambertz G, Perrin M, et al. Asynchrony of the early maturation of while matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp. 2008;29:14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao W, Lin W, Chen Y, et al. Temporal and spatial development of axonal maturation and myelination of while matter in the developing brain. AJNR. 2009;30:290–296. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huppi PS, Maier SE, Peled S, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 79.Lobel U, Sedlacik J, Gullmar D, et al. Diffusion tensor imaging: the normal evolution of ADC, RA, FA and eigenvalues studied in multiple anatomical regions of the brain. Neuroradiology. 2009;51:253–263. doi: 10.1007/s00234-008-0488-1. [DOI] [PubMed] [Google Scholar]
- 80.Partridge SC, Mukherjee P, Berman JI, et al. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging. 2005;22:467–474. doi: 10.1002/jmri.20410. [DOI] [PubMed] [Google Scholar]
- 81.Pans T, Collins DL, Evans AC, et al. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 82.Provcnzale JM, Liang L, DeLong D, et al. Diffusion tensor imaging assessment of brain white matter maturation during the first postnatal year. AJR. 2007;189:476–486. doi: 10.2214/AJR.07.2132. [DOI] [PubMed] [Google Scholar]
- 83.Maas LC, Mukherjee P, Carballido-Gamio J, et al. Early laminar organization of the human cerebrum demonstrated with diffusion tensor imaging in extremely premature infants. Neuroimage. 2004;22:1134–1140. doi: 10.1016/j.neuroimage.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 84.Beauchamp N, Jr, Bryan RN, van Zijl PC. Absolute quantitation of diffusion constants in human stroke. Stroke. 1997;28:483–490. doi: 10.1161/01.str.28.3.483. [DOI] [PubMed] [Google Scholar]
- 85.McGraw P, Liang L, Provenzale JM. Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. AJR. 2002;179:1515–1522. doi: 10.2214/ajr.179.6.1791515. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki Y, Matsukawa H, Kwee IL, et al. Absolute eigenvalue diffusion tensor analysis for human brain maturation. NMR Biomed. 2003;16:257–260. doi: 10.1002/nbm.848. [DOI] [PubMed] [Google Scholar]
- 87.Partridge SC, Mukherjee P, Henry RG, et al. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22:1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 88.Yoo SS, Park HJ, Soul JS, et al. In vivo visualization of white matter fiber tracts of preterm- and term-infant brains with diffusion tensor magnetic resonance imaging. Invest Radiol. 2005;40:110–115. doi: 10.1097/01.rli.0000149491.69201.cb. [DOI] [PubMed] [Google Scholar]
- 89.Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am. 2006;16:19–43. vii. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Provenzale JM, Isaacson J, Chen S, et al. Correlation of apparent diffusion coefficient and fractional anisotropy values in the developing infant brain. AJR. 2010;195:456–462. doi: 10.2214/AJR.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmithorst VJ, Wike M, Dardzinski BJ, et al. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20 45 weeks' gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Drobysehvsky A, Song SK, Gamkrelidze G, et al. Developmental changes in diffusion anisolropy coincide with immature oligodendtocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhai G, Lin W, Wilber KP, et al. Comparisons of regional white matter diffusion in healthy neonates and adults performed with a 3.0-T head-only MR imaging unit. Radiology. 2003;229:673–681. doi: 10.1148/radiol.2293021462. [DOI] [PubMed] [Google Scholar]
- 95.Bayer SA, Altman J. The human brain during the third trimester. CRC Press; Boca Raton: 2004. pp. 1–392. [Google Scholar]
- 96.Baratti C, Bamett A, Pierpaoli C. Comparative MR imaging study of brain maturation in kittens with T1, T2, and the trace of the diffusion tensor. Radiology. 1999;210:133–142. doi: 10.1148/radiology.210.1.r99ja09133. [DOI] [PubMed] [Google Scholar]
- 97.McKinstry RC, Mathur A, Miller JH, et al. Radial organization of developing preterm human cerebral cortex, revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12:1237–1243. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- 98.Thomton JS, Ordidge RJ, Penrice J, et al. Anisotropic water diffusion in white and gray matter of the neonatal piglet brain before and after transient hypoxia-ischaemia. Magn Reson Imaging. 1997;15:433–440. doi: 10.1016/s0730-725x(96)00378-5. [DOI] [PubMed] [Google Scholar]
- 99.Mori S, Itoh R, Zhang J, et al. Diffusion tensor imaging of the developing mouse brain. Magn Reson Med. 2001;46:18–23. doi: 10.1002/mrm.1155. [DOI] [PubMed] [Google Scholar]
- 100.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 101.Zhang J, Richards LJ, Yarowsky P, et al. Three-dimensional anatomical characterization of the developing mouse brain by diffusion tensor microimaging. Neuroimage. 2003;20:1639–1648. doi: 10.1016/s1053-8119(03)00410-5. [DOI] [PubMed] [Google Scholar]
- 102.Kroenke CD, Bretthorst GL, Inder TE, et al. Diffusion MR imaging characteristics of the developing primate brain. Neuroimage. 2005;25:1205–1213. doi: 10.1016/j.neuroimage.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 103.Gupta RK, Hasan KM, Trivedi R, et al. Diffusion tensor imaging of the developing human cerebrum. J Neurosci Res. 2005;81:172–178. doi: 10.1002/jnr.20547. [DOI] [PubMed] [Google Scholar]
- 104.Marin-Padilla M. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitcetonics: a unifying theory. J Comp Neurol. 1992;321:223–240. doi: 10.1002/cne.903210205. [DOI] [PubMed] [Google Scholar]
- 105.Deipolyi AR, Mukherjee P, Gill K, et al. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. Neuroimage. 2005;27:579–586. doi: 10.1016/j.neuroimage.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi E, Folkerth RD, Galaburda AM, et al. Emerging cerebral connectivity in the human fetal brain: an MR tractography study. Cereb Cortex. 2012;22:455–464. doi: 10.1093/cercor/bhr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dubois J, Benders M, Lazeyras F, et al. Structural asymmetries of perisylvian regions in the preterm newborn. Neuroimage. 2010;52:32–42. doi: 10.1016/j.neuroimage.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 108.Dekaban AS. Changes in brain weight during the in brain weight during the span of human life: relation of brain weights lo body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- 109.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 110.Paus T, Zijdenbos A, Worsley K, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 111.Thompson PM, Giedd JN, Woods RP, et al. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 112.Hasan KM, Kamali A, Kramer LA, et al. Diffusion tensor quantification of the human midsagittal corpus callosum subdivisions across the lifespan. Brain Res. 2008;1227:52–67. doi: 10.1016/j.brainres.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klingberg T, Vaidya CJ, Gabrieli JD, et al. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 114.Lebel C, Walker L, Leemans A, et al. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 115.Qiu D, Tan LH, Zhou K, et al. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41:223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 116.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 117.Sowell ER, Thompson PM, Tessner KD, et al. Mapping continued brain growth and gray mailer density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Giedd Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 119.Reiss AL, Abrams MT, Singer HS, et al. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 120.Benedict RH, Bobholz JH. Multiple sclerosis. Semin Neurol. 2007;27:78–85. doi: 10.1055/s-2006-956758. [DOI] [PubMed] [Google Scholar]
- 121.Bigler ED, Kerr B, Victoroff J, et al. White matter lesions, quantitative magnetic resonance imaging, and dementia. Alzheimer Dis Assoc Disord. 2002;16:161–170. doi: 10.1097/00002093-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 122.Bigler ED, Neeley ES, Miller MJ, et al. Cerebral volume loss, cognitive deficit and neuropsychological performance: comparative measures of brain atrophy: I. Dementia. J Int Neuropsychol Soc. 2004;10:442–452. doi: 10.1017/S1355617704103111. [DOI] [PubMed] [Google Scholar]
- 123.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eyre JA, Miller S, Ramesh V. Constancy of central conduction delays during development in man: investigation of motor and somatosensory pathways. J Physiol. 1991;434:441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kandel ER, Schwarz JH, Jessel TM. Principles of neural science. 4th edn. McGraw-Hill Medical; New York: 2000. [Google Scholar]
- 126.Armand J, Olivier E, Edgley SA, et al. Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey. J Neurosci. 1997;17:251–266. doi: 10.1523/JNEUROSCI.17-01-00251.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muller K, Homberg V, Lenard HG. Magnetic stimulation of motor cortex and nerve roots in children. Maturation of cortico-motoneuronal projections. Electroencephalogr Clin Neurophysiol. 1991;81:63–70. doi: 10.1016/0168-5597(91)90105-7. [DOI] [PubMed] [Google Scholar]
- 128.Nezu A, Kimura S, Uehara S, et al. Magnetic stimulation of motor cortex in children: maturity of corticospinal pathway and problem of clinical application. Brain Dev. 1997;19:176–180. doi: 10.1016/s0387-7604(96)00552-9. [DOI] [PubMed] [Google Scholar]
- 129.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 130.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salat DH, Tuch DS, Hevelone ND, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann NY Acad Sci. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- 132.Barres BA, Barde Y. Neuronal and glial cell biology. Curr Opin Neurobiol. 2000;10:642–648. doi: 10.1016/s0959-4388(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 133.Du Y, Dreyfus CF. Oligodendrocytes as providers of growth factors. J Neurosci Res. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- 134.Fields RD, Stevens-Graham B. New insights into neuron glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 136.Ben Bashat D, Ben Sira L, Graif M, et al. Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging. 2005;21:503–511. doi: 10.1002/jmri.20281. [DOI] [PubMed] [Google Scholar]
- 137.Peters BD, Szeszko PR, Radua J, et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophrenia Bull. 2012 May 2; doi: 10.1093/schbul/sbs054. Epub ahead on print. [DOI] [PMC free article] [PubMed] [Google Scholar]