Abstract
Cancer stem cells are responsible for tumor initiation and chemo-resistance. In ovarian cancer, the CD44+/MyD88+ ovarian cancer stem cells (OCSCs) are also able to repair the tumor and serve as tumor vascular progenitors. Targeting these cells is therefore necessary to improve treatment outcome and patient survival. The previous demonstration that the OCSCs are resistant to apoptotic cell death induced by conventional chemotherapy agents suggests that other forms of targeted therapy should be explored. We show in this study that targeting mitochondrial bioenergetics is a potent stimulus to induce caspase-independent cell death in a panel of OCSCs. Treatment of these cells with the novel isoflavone derivative, NV-128, significantly depressed mitochondrial function exhibited by decrease in ATP, Cox-I, and Cox-IV levels, and increase in mitochondrial superoxide and hydrogen peroxide. This promotes a state of “cellular starvation” that activates two independent pathways: 1) AMPKα1 pathway leading to mTOR inhibition; and 2) mitochondrial MEK/ERK pathway leading to loss of mitochondrial membrane potential. The demonstration that a compound can specifically target the mitochondria to induce cell death in this otherwise chemo-resistant cell population opens a new venue for treating ovarian cancer patients.
Keywords: ovarian cancer stem cells, mitochondria, caspase-independent cell death
Introduction
Epithelial ovarian cancer (EOC) is the most lethal of the gynecologic malignancies and accounts for about 15,000 deaths every year(1). A major obstacle in the successful treatment of ovarian cancer is the development of chemoresistance. Chemoresistant cancer cells typically have a high threshold for activation of the caspase-dependent apoptotic cascade, mostly brought about by overexpression of anti-apoptotic genes (2, 3) . Several approaches have been used to overcome resistance to apoptosis. These include the use of small molecules directed against anti-apoptotic proteins such as X-linked inhibitor of Apoptosis protein (XIAP), or the use of chemosensitizers, such as Phenoxodiol (4-7). Pre-treatment of chemoresistant EOC cells with Phenoxodiol lowers the GI50 for carboplatin, paclitaxel, topotecan, and gemcitabine and allows these agents to activate caspases and induce cell death (6, 8). Although the use of chemosensitizers was found to be successful in vitro and in mouse in vivo studies, it did not prove to be effective in clinical trials. Following an initial response, tumors recurred and became unresponsive to the addition of chemosensitizers (9) . This suggests that certain cancer cell populations possess a mechanism of chemoresistance, which is not associated with the inhibition of apoptosis. Therefore, in these patients, there is a need for other forms of targeted therapy.
Cancer stem cells are defined as cells that possess the capacity to self-renew and generate the heterogeneous lineage of cancer cells that comprise the whole tumor (10, 11). Current evidence suggests that cancer stem cells are the putative mediators of chemoresistance and tumor progression 8. It is thought that they are able to survive conventional chemotherapeutic treatments, which usually target fast dividing cells, and give rise to recurrent tumors that are even more chemoresistant and more aggressive.
In ovarian cancer, the CD44+/My88+ cells possess attributes described for cancer stem cells. These cells have tumor initiating properties, express stem cell markers, and are extremely chemoresistant (12). Addition of Phenoxodiol does not alter their resistance to carboplatin nor paclitaxel. On the other hand, CD44−/MyD88− EOC cells can not form tumors in mice in limiting dilution assays, do not express stem cell markers, and can respond to carboplatin and paclitaxel when pre-treated with Phenoxodiol. Additionally, the capacity to serve as tumor vascular progenitors and the capability of tissue repair is limited to the CD44+/MyD88+ ovarian cancer stem cells (OCSCs) (13) . Taken together, these data suggest that targeting the OCSCs is necessary to improve treatment outcome and patient survival. The demonstration that OCSCs do not respond to sensitizers of the apoptotic pathway suggest that other forms of cell death should be explored.
Recently we reported that the phenyl-substituted isoflavone compound, NV-128, can induce caspase-independent cell death in all EOC cells tested (14). NV-128-induced cell death was promoted by two apparently independent events - mitochondrial depolarization and mTOR inhibition. The mitochondrial effect was characterized by loss of mitochondrial membrane potential (MMP) as early as 1h post-treatment. Loss of MMP however, was not associated with caspase activation but instead resulted in nuclear translocation of Endonuclease G leading to caspase-independent DNA fragmentation (14). NV-128 also inhibited both complexes of the mammalian target of rapamycin protein (mTOR). Cells treated with NV-128 had significantly lower levels phosphorylated-p70 ribosomal S6 kinase (pS6K) (target of mTOR complex 1), and phosphorylated-AktSer473 (pAkt) (target of mTOR complex2). As a result of mTOR inhibition, NV-128 treated cells acquired autophagic vacuoles and showed increase levels of the autophagic marker, LC3-II (14). Thus, the combined loss of MMP and inhibition of the mTOR pathway potently lead to caspase-independent cell death.
The objectives of this study are two-fold: first is to determine the efficacy of NV-128 in the OCSCs, and the second is to elucidate a link between the observed mitochondrial changes and the inhibition of the mTOR pathway. We demonstrate that by targeting mitochondrial energetic function, NV-128 is able to create a state of cellular starvation and activate two independent, non-canonical pathways to induce death in the OCSCs. NV-128 is able to activate the 5′-AMP kinase (AMPK)-mTOR pathway, and the extracellular signal-regulated kinase (ERK)-Bax pathway to potently induce death in the apoptosis-resistant OCSCs.
Materials and Methods
Cell lines, culture conditions, and reagents
CD44+/MyD88+ OCSCs were isolated from either tumor tissue or ascites obtained from patients diagnosed with stage III/IV serous ovarian carcinoma as previously described (12)(15, 16). Sample collection was performed with patient consent and approved by the Human Investigations Committee of Yale University School of Medicine. Purity of the cultures were tested before each experiment by measuring the levels of CD44 and MyD88 by flow cytometry. The epithelial nature of the isolated cells was determined by staining for Ck18. OCSCs were propagated as previously described (12, 13). The EOC cell lines A2780 and CP70 were obtained from Dr. T.C. Hamilton in 2002 and propagated as previously described (15, 16) . The epithelial nature of the cell lines was determined by immunostaining with Ck7 and authenticated on a regular basis (at least every two months) based on their differential response to carboplatin.
Cell viability assay and determination of cell morphology
Cell viability was determined as previously reported (16). Briefly, cells (5 × 103) were plated in triplicate wells in a 100 μl volume per well of a 96-well microtiter plate (BD Biosciences/Pharmingen, San Diego, CA). The cells were grown to 70% confluence and then incubated in reduced-serum phenol red-depleted Opti-MEM medium (Invitrogen-GIBCO, Carlsbad, CA) for 4h prior to treatment. NV-128 (Novogen, Ltd) and rapamycin (Sigma Aldrich, St. louis, MO) were added to the medium from 10 mg/ml and 10 mM stock, respectively, to give the final concentrations described in the results section. Following treatment for 24h, cell viability was evaluated using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI) according to the manufacturer’s instructions. Optical densities of the samples were measured at 490 nm using an automatic microplate reader (Model 550, Bio-Rad, Hurcules, CA). The values from the treated cells were compared with the values generated from the untreated control and reported as percent viability. Each experiment was done in triplicate. For studies with inhibitors, U0126 (Sigma Aldrich, St. Louis, MO) and MnTBAP (Alexis Biochemicals, Lausen, Germany) were added 1h prior to NV-128 treatment. Cellular morphology was assessed using Incucyte (Essen Instruments, Ann Arbour, MI).
Analysis of mitochondrial function
Levels of ADP and ATP were measured using ApoSENSOR ADP/ATP ratio assay kit (Biovision Inc., Mountain View, CA). Accumulation of mitochondrial superoxide and cellular hydrogen peroxide were measured using MitoSOX Red and CM-H2DCFDA, respectively (Invitrogen, Carlsbad, CA). Mitochondrial membrane potential was determined using Mitocapture apoptosis detection kit based on JC1 dye staining (Biovision Inc., Mountain View, CA) and Mitotracker Red CMXRos (Molecular Probes). Mitochondrial mass was quantified using Mitotracker Green FM (Invitrogen, Carlsbad, CA). Flow cytometry data were acquired using BD FACSCalibur and analyzed using CellQuest (BD Biosciences, San Jose, CA).
Protein preparation and subcellular fractionation
Protein was extracted and measured as previously described (16). For separation of the cytoplasmic and mitochondrial fractions, cell pellets were processed using the ApoAlertTM Cell Fractionation Kit (BD Biosciences, San Jose, CA) according to the manufacturer’s instructions.
SDS-PAGE and Western blots
SDS-PAGE and Western blots were performed as previously described (16). Antibodies used were: mouse anti-phospho-ERK1/2 T204 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), rabbit anti-ERK1/2 (Millipore, Billerica, MA), rabbit anti-phospho-Akt Ser473 (Cell Signaling Technology, Danvers, MA), rabbit anti-Akt (Cell Signaling Technology), rabbit anti-phospho-p70 S6 kinase Thr412/Thr389 (Millipore), rabbit anti-phospho-AMPKα1/2 T174/T172 (Cell Signaling), rabbit anti-Cox-IV (Cell Signaling Technology), mouse anti-Cox-I (MitoSciences Inc., Eugene, Or), mouse anti-Cox-III (MitoSciences Inc.), mouse anti-Bax (BD Biosciences), and rabbit anti-actin (Sigma Aldrich).
Statistical analysis
Data are expressed as mean ± standard error. Statistical significance (p<0.05) was determined using either two tailed unpaired t-tests or Mann-Whitney U test for non - parametric data. Unless stated otherwise, all experiments were performed in triplicate and repeated at least three times.
Results
NV-128 induces degradation of Cox-I and Cox-IV, loss of ATP, and upregulation of mitochondrial superoxide
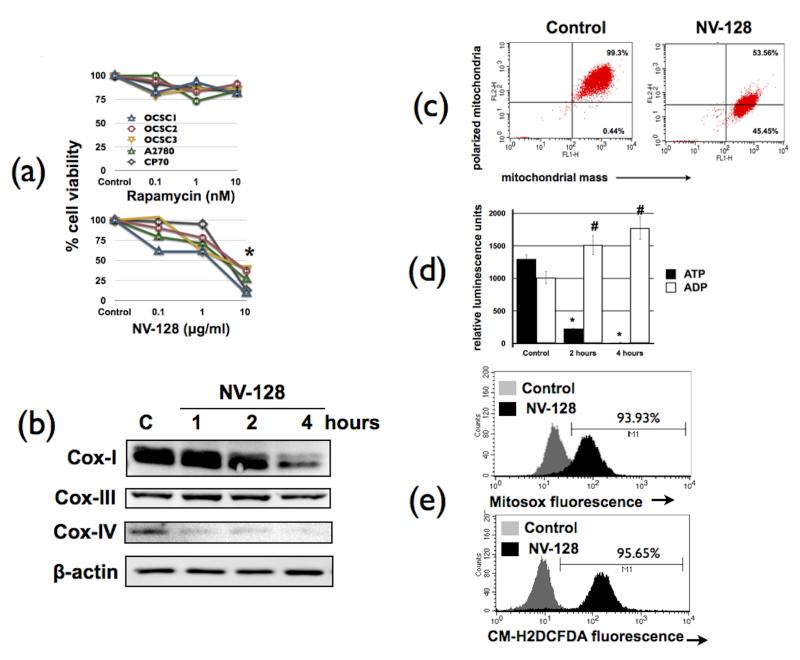
We previously demonstrated the cytotoxic activity of NV-128 on EOC cells and demonstrated that its effect was partly associated with inhibition of the mTOR pathway (14). We then determined if the cytotoxic effect of NV-128 is maintained in the OCSCs. Figure 1a shows that NV-128 is able to significantly reduce the viability of all OCSCs tested. Moreover, NV-128 had a very potent cytotoxic effect such that the addition of carboplatin or paclitaxel did not have any additive effect (Supplementary Fig.1). Interestingly, all EOC cells tested are resistant to rapamycin (a specific mTOR complex 1 inhibitor) (Fig. 1a). The observation that NV-128 can induce cell death in rapamycin resistant cells and the previous demonstration that it can inhibit both mTOR 1 and 2 complexes (14) suggest that NV-128 does not act like a rapalogue.
Figure 1.
NV-128 depresses mitochondrial function in rapamycin-resistant ovarian cancer stem cells: (a) ovarian cancer cells were treated with increasing concentrations of Rapamycin or NV-128 for 24h and percentage of viable cells quantified using Celltiter96 assay; OCSCs were treated with NV-128 (10 μg/ml) at time points shown and (b) status of Cox-I, III, and IV determined by Western blot analysis; (c) effect on mitochondrial membrane potential and mitochondrial mass quantified using MitoTracker red and Mitotracker green, respectively; (d) levels of ATP and ADP quantified as described in the Materials and Methods section; (e) levels of mitochondrial superoxide and cellular hydrogen peroxide quantified using MitoSox and CM-H2DCFDA dyes, respectively; b-e shows results for OCSC1, similar results were observed with other cells tested. C, control. *, # p < 0.001 compared to control. n = 3.
In addition to mTOR inhibition, another event observed during NV-128-induced cell death is the loss of mitochondrial membrane potential (MMP) (14). It is thus possible that this effect on the mitochondria, coupled with mTOR inhibition, act in concert to induce death in the rapamycin-resistant OCSCs. To test this hypothesis, we first performed further characterization of NV-128’s effect on mitochondrial function. Evaluation of the components of the electron transport chain (ETC) such as Cox-I, Cox-III, and Cox-IV showed a significant and time-dependent decrease in the levels of Cox-I and Cox-IV, but not Cox-III, after NV-128 treatment (Fig. 1b). To further show the specific effect on the members of the ETC, we stained NV-128-treated OCSCs with MitoTracker Red and MitoTracker Green. MitoTracker Red quantifies polarized mitochondria while MitoTracker green staining is equivalent to mitochondrial mass. Flow cytometry results showed that NV-128-induced loss of MMP was not associated with loss of mitochondrial mass (Fig. 1c), suggesting that the decrease in the ETC complexes is not due to mitophagy.
Due to the presence of Cox-I and -IV degradation, we next determined the impact of NV-128 on energy production by measuring ADP and ATP levels. Figure 1d shows that OCSCs treated with NV-128 had significantly lower levels of ATP and accumulated ADP after 2 and 4h of treatment. Since an accompanying event in the uncoupling of oxidative phosphorylation is the production of reactive oxygen species (ROS), we next measured the levels of mitochondrial superoxide and cellular hydrogen peroxide. Flow cytometry results using the MitoSox and CM-H2CFDA showed a significant increase in the fluorescence intensity of both dyes in NV-128-treated cells (Fig. 1e). Thus, NV-128 is able to significantly inhibit mitochondrial function in the OCSCs.
Our next objective was to further delineate the molecular signaling cascade and associations between the observed mitochondrial effect and mTOR inhibition. Specifically, we determined whether the increase in mitochondrial ROS and the decrease in ATP have a role in NV-128-induced loss of MMP and NV-128-induced mTOR inhibition.
NV-128-induced increase in mitochondrial ROS activates the ERK pathway
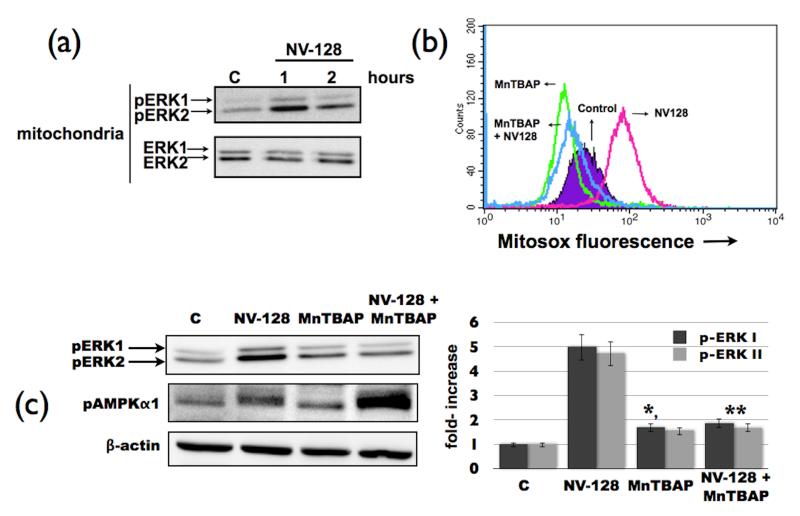
Previous studies have shown that up-regulation of ROS can induce activation of the MEK/ERK pathway (17) . Evaluation of the phosphorylation status of ERK 1/2 in OCSCs treated with NV-128 showed a significant increase in the levels of phosphorylated-ERK1/2 (pERK) in the mitochondria (Fig. 2a). This was however, not associated with an increase in total mitochondrial ERK 1/2 (tERK) suggesting that NV-128 does not induce translocation of pERK into the mitochondria, but instead induces phosphorylation of resident mitochondrial ERK 1/2. Indeed, baseline mitochondrial ERK is mostly in the non-phosphorylated state and only becomes phosphorylated after treatment with NV-128 (Fig. 2a).
Figure 2.
NV-128 activates the ERK pathway through ROS. (a) OCSCs were treated with NV-128 (10 μg/ml) at time points shown and mitochondrial fractions analyzed for levels of phosphorylated and total ERK1/2; (b) OCSCs were pre-teated with the ROS scavenger MnTBAP (500μM) for 1h prior to NV-128 treatment and effect on mitochondrial superoxide determined by flow cytometry; (c) effect of MnTBAP pre-pretreatment on mitochondrial ERK and AMPKα1 activation analyzed by Western blot analysis and a densitometer graph depicting levels of pERK1/2. * p = 0.0001 compared to NV-128 alone, ** p = 0.01 compared to control. Results shown are for OCSC1, similar results were observed with other cells tested. C, control. n = 3.
To determine if the increase in pERK is a direct consequence ROS production, OCSCs were pretreated with the cell permeable superoxide dismutase mimetic, MnTBAP, 1h prior to NV-128 treatment. MnTBAP is able to inhibit NV-128-induced mitochondrial ROS production (Fig. 2b), and more importantly, MnTBAP pre-treatment abrogated NV-128-induced phosphorylation of mitochondrial ERK 1/2 (Fig. 2C). This suggests that NV-128-induced ROS is the cause of mitochondrial ERK activation. Our next objective was to determine the role of NV-128-induced mitochondrial ERK activation on the observed effect on mTOR and MMP.
NV128-induced mTOR inhibition is independent of the MEK/ERK pathway
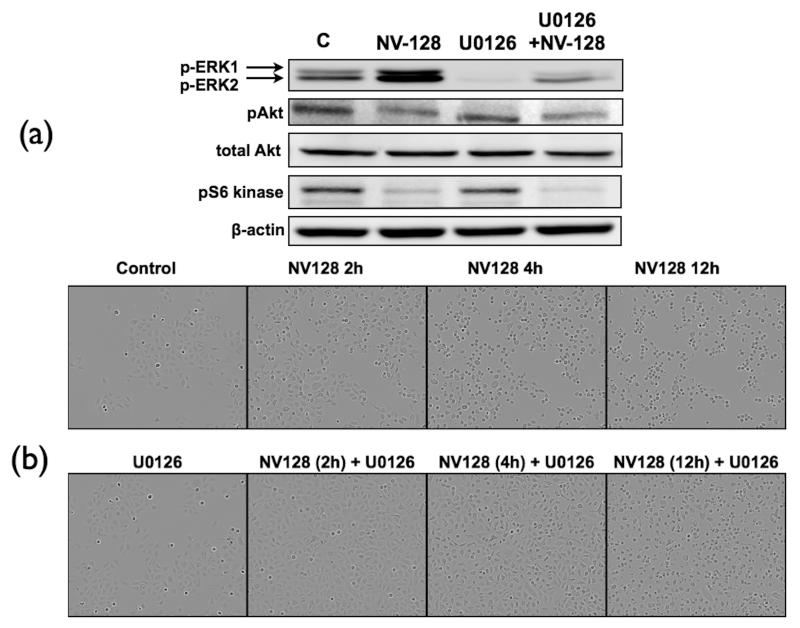
Thus, we used the specific MEK1/2 inhibitor, U0126, which is known to inhibit phosphorylation of ERK. Pre-treatment of OCSCs for 1h with U0126 was able to abolish NV-128-induced up-regulation of mitochondrial pERK (Fig. 3a). However, U0126 was not able to prevent NV-128-induced mTOR inhibition. Levels of pS6K and pAkt remained low in NV-128 treated cells even in the presence of U0126 (Fig. 3a). This suggests that NV128-induced mTOR inhibition is independent of MEK/ERK activation.
Figure 3.
NV-128-induced inhibition of mTOR is independent of the MEK/ERK pathway. (a) OCSCs were pre-treated with U0126 (10 μM) for 1h prior to NV-128 treatment and effect on ERK and mTOR activity determined using Western blot analysis; (b) effect on cell morphology analyzed using a real-time video imaging system. Results shown are for OCSC1, similar results were observed with other cells tested. C, control. n = 3.
Interestingly, analysis of cellular morphology showed that inhibition of the MEK/ERK pathway is able to delay NV128-induced cell death (Fig. 3b). This suggests that although NV-128-induced mTOR inhibition is independent of the MEK/ERK pathway, the activation of ERK still has a significant contribution to NV128-induced cell death.
Since another event observed in NV-128 treated cells is the loss of MMP, we next determined the association between MEK/ERK activation and loss of MMP .
NV128-induced ERK activation controls MMP through Bax
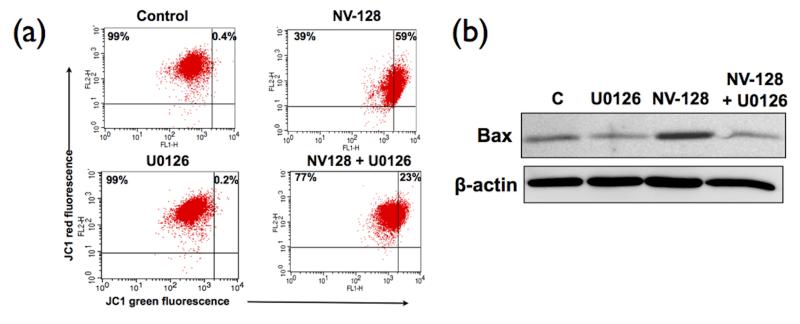
To determine the association between ERK activation and loss of MMP, OCSCs were pre-treated with U0126 prior to NV-128 treatment. Flow cytometry analysis using the JC1 dye showed that inhibition of ERK with U0126 is able to reverse the effect of NV-128 on MMP. As shown in Figure 4a, pre-treatment with U0126, significantly reduced the percentage of cells that lost MMP (59% vs 23% for NV-128 alone and NV128 with U0126, respectively). These results suggest that NV-128-induced loss of MMP is dependent on the activation of the MEK/ERK pathway.
Figure 4.
NV-128-induced loss of MMP was secondary to ERK-induced mitochondrial translocation of Bax. OCSCs were pre-treated with U0126 (10 μM) for 1h prior to NV-128 treatment: (a) effect on mitochondrial membrane potential determined using JC1 dye; (b) effect on mitochondrial Bax determined by Western blot analysis using mitochondrial fractions. Results shown are for OCSC1, similar results were observed with other cells tested. C, control. n = 3
MMP is controlled in part by the Bcl2 family of proteins (18). To determine if NV-128-induced activation of the MEK/ERK pathway affects the status of Bcl2 family members, we analyzed mitochondrial fractions of cells treated with NV-128 in the presence or absence of U0126. Our results showed that NV-128 was able to upregulate the pro-apoptotic Bcl2 family member, Bax (Fig. 4b). More importantly, pre-treatment of OCSCs with U0126 prevented NV-128-induced upregulation of mitochondrial Bax. Thus, ERK activation after NV-128 treatment contributes to cellular loss of MMP through Bax.
NV-128-induced loss of ATP leads to mTOR inhibition
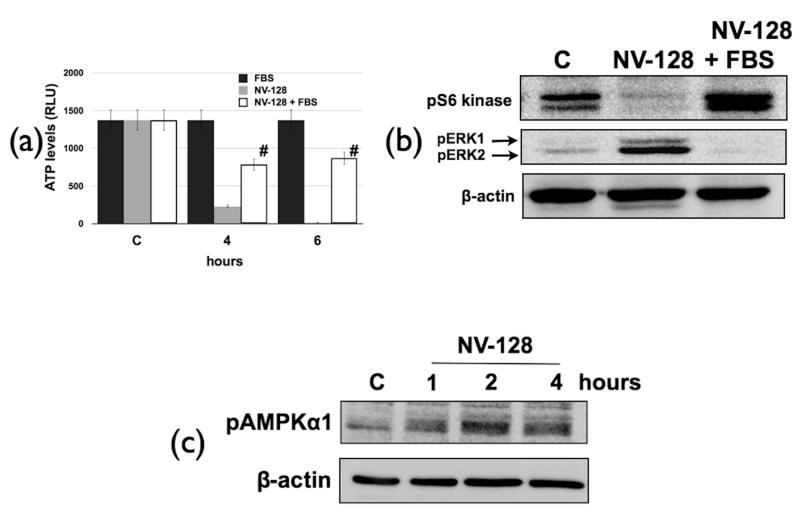
Our data so far showed that NV-128-induced ROS production activates the MEK/ERK/Bax axis leading to loss of MMP. This was however, not associated with NV-128-induced mTOR inhibition. As there is significant loss of ATP in NV-128-treated OCSCs, our next objective was to determine the association between ATP loss and mTOR. We hypothesized that loss of ATP may create a state of cellular starvation that can potentially inhibit mTOR. To test this hypothesis we compensated for reduced ATP levels in NV-128-treated cells by adding 20% FBS. Figure 5a shows that NV-128-induced ATP loss was partially inhibited upon the addition of FBS. More importantly, abrogation of ATP loss is able to rescue the mTOR pathway. Treatment of OCSCs with NV-128 in the presence of FBS was able to prevent NV-128-induced decrease in pS6k (Fig. 5b). This suggests that inhibition of ATP production by NV-128 triggers mTOR inhibition.
Figure 5.
NV-128-induced loss of ATP inhibits mTOR. OCSCs were treated with NV-128 in the presence of 20% FBS: (a) effect on ATP levels determined as described in the Methods section; (b) effect on pERK and mTOR target, pS6k determined by Western blot; (c) NV-128 activates the AMPK pathway. OCSCs were treated with NV-128 (10 μg/ml) at time points shown and phosphorylation status of AMPKα1 determined by Western blot analysis. Results shown are for OCSC1, similar results were observed with other cells tested. C, control (without FBS). # p < 0.001 compared to NV-128 alone. n = 3.
The addition of FBS also abrogated NV-128-induced increase in pERK. This further shows that the mitochondrial effects are upstream of ERK activation.
NV-128 activates AMPKα1
AMPKα1 is an energy sensor, which is responsive to levels of cellular AMP (19) and is therefore activated when ATP production is low. Previous studies have linked ATP loss and mTOR inhibition through AMPKα1 (20). AMPKα1 can inhibit mTOR by phosphorylating TSC2 resulting in the activation of the TSC1/TSC2 complex and subsequent inhibition of mTOR (21). Therefore we hypothesized that inhibition of ATP production by NV-128 may activate AMPKα1. Indeed, analysis of phosphorylation status of AMPKα1 showed that NV-128 is able to activate the AMPK pathway in a time dependent manner (Fig. 5c). Interestingly, activation of AMPKα1 is not abrogated with the ROS scavenger, MnTBAP (Fig. 2c).
Discussion
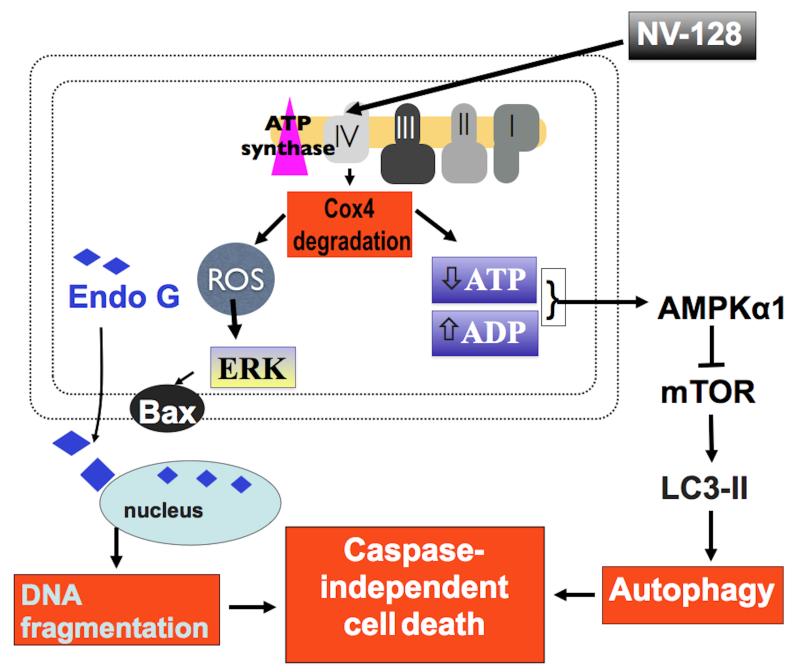
We show in this study that by disrupting oxidative phosphorylation, NV-128 can induce cell death in the OCSCs through two independent non-canonical pathways. NV-128-induced degradation of Cox-I and IV can lead to accumulation of ROS and decrease ATP production. Elevated ROS activates the MEK/ERK/Bax axis leading to loss of MMP, and the resulting decrease in ATP levels creates a low energy state that leads to inhibition of the mTOR pathway through AMPKα1. The combination of these two signaling events lead to OCSC cell death and correlates with the previous observation that NV-128 can induce autophagic cell death and caspase-independent DNA fragmentation (Fig. 6) (14).
Figure 6.
Proposed model for NV-128-induced casapase-independent cell death in OCSCs. By targeting the mitochondria, NV-128 activates two independent cell death pathways. Degradation of Cox-IV leads to ATP loss and increase mitochondrial ROS. ATP loss leads to inhibition of mTOR pathway and autophagic cell death. ROS activates the ERK/Bax axis leading to loss of mitochondrial membrane potential and EndoG-dependent DNA fragmentation.
Cells have evolved broad mechanisms to sense growth conditions in the forms of nutrients, growth factors, as well as cellular energy levels. Specific signaling pathways then relay the status of these conditions to key regulators, which include mTOR and MEK/ERK pathways. The mTOR pathway maintains homeostatic control over the anabolic processes that drive cell growth and proliferation and the MEK/ERK pathway connects the diverse extracellular signals to the nucleus (22-24). A property of cancer cells is their ability to disconnect growth promoting processes from the perception of growth signals. Therefore it is not surprising that elevated mTOR activity is a characteristic of many human cancers, rendering mTOR active even in conditions of growth factor withdrawal (25, 26). As aberrant mTOR activation is a common molecular event in cancer, there is much interest in developing and testing mTOR inhibitors as anti-tumor agents. To date, there are completed and ongoing clinical trials testing the efficacy of rapamycin or its analogs against all major forms of cancer (27). However, recent studies have showed that these compounds only block a subset of mTOR functions and upon direct inhibition of mTOR, alternative pathways are activated (28, 29). Therefore, it is necessary to elucidate alternative approaches that can inhibit all the signals originated and related to the mTOR pathway. In this study, we demonstrate that by inducing energy stress, through the inhibition of mitochondrial energy production, we were able to trigger an inhibitory cascade, mediated by AMPKα1, which can potently suppress mTOR activity.
Under conditions of energy depletion, the highly conserved energy sensing protein kinase AMPK (5′ AMP-activated protein kinase) is activated and phosphorylates TSC2, which by still unclear mechanisms, enhances the ability of the TSC1-TSC2 complex to turn off the mTOR pathway (21). In this study, we show that the NV-128-induced decrease in ATP is able to create a depleted energy status, which activates the energy sensing AMPK leading to inhibition of mTOR. This inhibitory effect is sufficiently potent to induce autophagic cell death in the OCSCs.
Another characteristic of cancer cells is their dependence on the glycolytic pathway to fulfill their energy needs (30). This observation leads to the hypothesis that cancer cells have inefficient or sub-optimal oxidative phosphorylation capacity or that their anti-oxidant system is suppressed (31, 32) . Both were later shown true with the demonstration that under basal conditions, several human carcinoma cell lines produce high levels of hydrogen peroxide (33). In addition, it has been shown that tumor cells have lower levels of catalase and dismutase activity to counteract ROS levels (34, 35). Taken together, this suggests that the highly energy-dependent cancer cells are under persistent oxidative stress. Interestingly, the elevated level of ROS in cancer cells is insufficient to induce cell death suggesting that cancer cells are able to withstand oxidative stress. Instead, elevated ROS have been shown to be favorable for tumor growth. Persistently high ROS levels in cancer cells can contribute to oxidative DNA damage and the induction of pro-growth transcription factors such as NFκB, the activation of oncogenes, and suppression of tumor suppressor genes (36, 37) .
In hematopoietic cancer stem cells, it was previously reported that low basal ROS is a requirement for maintaining self-renewal potential (38). Minute changes in ROS levels induce either senescence or apoptosis in these cells. The redox state in OCSCs remains to be elucidated. However, the demonstration that a compound like NV-128, which can disrupt oxidative phosphorylation and increase mitochondrial ROS, can induce cell death in OCSCs suggests that the OCSCs probably share this characteristic.
Given the highly oxidative status of cancer cells compared to normal cells, one can argue that cancer cells are “primed” for cell death induced by agents that can target the ETC. Minute perturbation in oxidative phosphorylation can possibly induce cell death in the “primed” cancer cells without harming the surrounding normal stroma. Indeed, we show in this study that NV-128, by disrupting the ETC can cause increase in mitochondrial ROS leading to the activation of mitochondrial ERK.
ERK1/2 are conserved serine/threonine protein kinases, which are classically known as mediators of growth and survival (39). Ligation of receptor tyrosine kinases with growth factors results in the activation of MEK/ERK pathway and nuclear translocation of ERK1/2, where it activates multiple transcription factors leading to cell proliferation, differentiation, and survival (40). On the other hand, although not as well characterized as nuclear ERK, mitochondrial ERK has been described to be involved in the regulation of mitochondrial function, as well as cell death decisions. Activation of ERK by the redox cycling dopamine analog, 6-hydroxydopamine (6-OHDA) results in mitochondrial localization of pERK, mitophagy, and neuronal cell death (17). Moreover, activation of ERK in response to cisplatin is required for cisplatin-induced apoptosis in renal cells (41). In this study, we show that in OCSCs treated with NV-128, the ROS-dependent activation of ERK is able to disrupt mitochondrial function through Bax-mediated loss of MMP. The ERK-related mitochondrial changes observed however, did not involve mitophagy as seen in the neuronal model.
In summary, we show that disruption of mitochondrial function by NV-128, activates two independent cell death pathways in the OCSCs. Our previous data, using an ovarian cancer xenograft model, showed that NV-128 is able to inhibit tumor growth without inducing toxicity in mice (14). This suggests that a sufficient therapeutic window exist that will allow NV-128 to be safely administered to patients. Therefore, the studies described here provide sufficient proof of concept study to initiate clinical trial in ovarian cancer patients.
Supplementary Material
Acknowledgements
This study was supported by the NIH grants RO1CA118678 and RO1CA127913 to GM. The authors would like to thank Dr. Gerald S. Shadel for assistance with the analysis of mitochondrial function.
Financial support: This study was supported by the NIH grants RO1CA118678 and RO1CA127913 to GM.
Abbreviations
- OCSCs
ovarian cancer stem cells
- EOC
epithelial ovarian cancer cells
- MMP
mitochondrial membrane potential
- mTOR
mammalian target of rapamycin
- AMPK
AMP-kinase
- ERK
extracellular signal-regulated kinase
- Cox-IV
complex IV
Footnotes
Conflict of Interest: David Brown is an employee of Marshall Edwards Inc. All other authors have nothing to disclose.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Mor G, Montagna MK, Alvero AB. Modulation of apoptosis to reverse chemoresistance. Methods Mol Biol. 2008;414:1–12. doi: 10.1007/978-1-59745-339-4_1. [DOI] [PubMed] [Google Scholar]
- 3.Fraser M, Leung B, Jahani-Asl A, Yan X, Thompson WE, Tsang BK. Chemoresistance in human ovarian cancer: the role of apoptotic regulators. Reprod Biol Endocrinol. 2003;1:66. doi: 10.1186/1477-7827-1-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vellanki SH, Grabrucker A, Liebau S, Proepper C, Eramo A, Braun V, et al. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia. 2009;11:743–52. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, et al. Small molecule XIAP inhibitors enhance TRAIL-induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res. 2009;69:2425–34. doi: 10.1158/0008-5472.CAN-08-2436. [DOI] [PubMed] [Google Scholar]
- 6.Alvero AB, O’Malley D, Brown D, Kelly G, Garg M, Chen W, et al. Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer. 2006;106:599–608. doi: 10.1002/cncr.21633. [DOI] [PubMed] [Google Scholar]
- 7.Rutherford TOM D, Makkenchery A, Mor G. Phenoxodiol phase Ib/II study in patients with recurrent ovarian cancer that resistant to second line chemotherapy. Journal of the Society of Gynecologic Investigation. 2004;11:254. [Google Scholar]
- 8.Alvero AB, Brown D, Montagna M, Matthews M, Mor G. Phenoxodiol-Topotecan Co-Administration Exhibit Significant Anti-Tumor Activity Without Major Adverse Side Effects. Cancer Biol Ther. 2007;6 doi: 10.4161/cbt.6.4.3891. [DOI] [PubMed] [Google Scholar]
- 9.Alvero AB, Rossi P, Brown D, Leizer A, Kelly M, Rutherford T, Husband A, Mor G. Phenoxodiol—A Chemosensitizer in the Midst of Cancer Chemoresistance. US Oncology. 2008;4:39–41. [Google Scholar]
- 10.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 11.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 12.Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–66. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvero AB, Fu HH, Holmberg J, Visintin I, Mor L, Marquina CC, et al. Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells. 2009;27:2405–13. doi: 10.1002/stem.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvero AB, Montagna MK, Chen R, Kim KH, Kyungjin K, Visintin I, et al. NV-128, a novel isoflavone derivative, induces caspase-independent cell death through the Akt/mammalian target of rapamycin pathway. Cancer. 2009 doi: 10.1002/cncr.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flick MB, O’Malley D, Rutherford T, Rodov S, Kamsteeg M, Hao XY, et al. Apoptosis-based evaluation of chemosensitivity in ovarian cancer patients. J Soc Gynecol Investig. 2004;11:252–9. doi: 10.1016/j.jsgi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Kamsteeg M, Rutherford T, Sapi E, Hanczaruk B, Shahabi S, Flick M, et al. Phenoxodiol--an isoflavone analog--induces apoptosis in chemoresistant ovarian cancer cells. Oncogene. 2003;22:2611–20. doi: 10.1038/sj.onc.1206422. [DOI] [PubMed] [Google Scholar]
- 17.Kulich SM, Horbinski C, Patel M, Chu CT. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic Biol Med. 2007;43:372–83. doi: 10.1016/j.freeradbiomed.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 19.Sanz P. AMP-activated protein kinase: structure and regulation. Curr Protein Pept Sci. 2008;9:478–92. doi: 10.2174/138920308785915254. [DOI] [PubMed] [Google Scholar]
- 20.Kimball SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc. 2006;38:1958–64. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- 21.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 22.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–60. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 23.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–16. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 25.Ciuffreda L, Di Sanza C, Incani UC, Milella M. The mTOR pathway: a new target in cancer therapy. Curr Cancer Drug Targets; 10:484–95. doi: 10.2174/156800910791517172. [DOI] [PubMed] [Google Scholar]
- 26.Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 40:323–32. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–88. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 28.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 39:171–83. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(Suppl 2):S43–51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–7. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Dias N, Bailly C. Drugs targeting mitochondrial functions to control tumor cell growth. Biochem Pharmacol. 2005;70:1–12. doi: 10.1016/j.bcp.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–73. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–8. [PubMed] [Google Scholar]
- 34.Mates JM, Sanchez-Jimenez F. Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci. 1999;4:D339–45. doi: 10.2741/mates. [DOI] [PubMed] [Google Scholar]
- 35.Oberley TD, Oberley LW. Antioxidant enzyme levels in cancer. Histol Histopathol. 1997;12:525–35. [PubMed] [Google Scholar]
- 36.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 37.Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–11. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 38.Naka K, Muraguchi T, Hoshii T, Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal. 2008;10:1883–94. doi: 10.1089/ars.2008.2114. [DOI] [PubMed] [Google Scholar]
- 39.Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–11. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- 40.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. [PubMed] [Google Scholar]
- 41.Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS, Jung JS, et al. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J Appl Toxicol. 2005;25:374–82. doi: 10.1002/jat.1081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.