Abstract
Poor functional recovery found after peripheral nerve injury has been attributed to the misdirection of regenerating axons to reinnervate functionally inappropriate muscles. We applied brief electrical stimulation (ES) to the common fibular (CF) but not the tibial (Tib) nerve just prior to transection and repair of the entire rat sciatic nerve, to attempt to influence the misdirection of its regenerating axons. The specificity with which regenerating axons reinnervated appropriate targets was evaluated physiologically using compound muscle action potentials (M responses) evoked from stimulation of the two nerve branches above the injury site. Functional recovery was assayed using the timing of electromyography (EMG) activity recorded from the tibialis anterior (TA) and soleus (Sol) muscles during treadmill locomotion and kinematic analysis of hindlimb locomotor movements. Selective ES of the CF nerve resulted in restored M-responses at earlier times than in unstimulated controls in both TA and Sol muscles. Stimulated CF axons reinnervated inappropriate targets to a greater extent than unstimulated Tib axons. During locomotion, functional antagonist muscles, TA and Sol, were coactivated both in stimulated rats and in unstimulated but injured rats. Hindlimb kinematics in stimulated rats were comparable to untreated rats, but significantly different from intact controls. Selective ES promotes enhanced axon regeneration but does so with decreased fidelity of muscle reinnervation. Functional recovery is neither improved nor degraded, suggesting that compensatory changes in the outputs of the spinal circuits driving locomotion may occur irrespective of the extent of misdirection of regenerating axons in the periphery.
INDEXING TERMS: peripheral nerve, regeneration, electrical stimulation, neuromuscular specificity, electromyography, locomotion
Despite the capacity of axons in peripheral nerves to regenerate, functional recovery from peripheral nerve injuries is very poor (Frostick et al., 1998; Scholz et al., 2009). Incomplete motor recovery is common after moderate to severe peripheral nerve injuries (Burnett and Zager, 2004). Functional recovery depends on successful axon regeneration, as well as on the reinnervation of appropriate target muscles (Valero-Cabre and Navarro, 2002). A number of laboratories have reported that following peripheral nerve transections, significant numbers of regenerating axons are misdirected to reinnervate functionally inappropriate muscles (Brushart et al., 2002; English, 2005; de Ruiter et al., 2008; Robinson and Madison, 2009). This misdirection results in reinnervation of muscles by different motoneurons than had innervated them before the injury and may contribute to poor functional recovery.
After cutting the sciatic nerves of rats and subsequent regeneration of cut axons through gaps (Meek et al., 2003), through bridgeable polymers (Varejao et al., 2003) or 12-mm long autografts (Gramsbergen et al., 2000), the activity patterns of the reinnervated functional antagonistic gastrocnemius and tibialis anterior (TA) muscles during locomotion was abnormal. Although, no systematic pattern of abnormal activity was reported, coactivation of these antagonists was a common finding. Following simple trans-ection and end-to-end repair of the rat sciatic nerve, we found a more consistent coactivation of the TA and soleus (Sol) muscles during treadmill locomotion (Sabatier and English, 2007). This pattern of activation was present from the earliest times of muscle reinnervation and persisted for as long as 25 weeks. One interpretation of these findings is that the outputs of the spinal circuits that drive locomotion did not change and that the abnormal timing of activation of these two muscles is a reflection of the amount of misdirection of regenerating axons.
This interpretation is consistent with the results of several studies of cross-reinnervation of skeletal muscle. When nerves to two different muscles are exchanged, the resulting reinnervation can be viewed as an extreme of misdirection—all of the motoneurons to that muscle are from inappropriate sources. The common finding from functional analyses, as summarized by Gordon et al. (1986), is that the timing of activity of the reinnervated muscle is defined by the reinnervating nerve. For example, Sperry (1941) found that if the tibial (Tib) and common fibular (CF) nerves of rats were crossed, “no adaptive functional adjustment of the nervous system took place” (p. 16). A most striking example of this same conclusion is found in the study by O’Donovan et al. (1985), who cross-reinnervated the Sol and flexor digitorum longus muscles of cats. A change in the very distinct timings of locomotor electromyography (EMG) activity was observed in these reinnervated muscles that matched the expected pattern of activation of the innervating nerve. This was interpreted to mean that there is little or no adaptive change in the outputs of spinal circuits regulating locomotion to accommodate the altered sources of innervation of the two muscle targets.
Despite these findings, some evidence does exist for changes in spinal circuitry in response to changes in muscle innervation. Reinnervation of the Sol by the CF nerve in young animals results in an initial inappropriate timing (swing phase) of activity of this antigravity muscle followed by gradual return of appropriate (stance) activation (Slawinska and Kasicki, 2002). In adult monkeys (Sperry, 1947), cats (Yumiya et al., 1979; Fujito et al., 1989), and rats (Cohen, 1978) some motor readjustments have been reported at long durations after cross-reinnervation. These results are interpreted to mean that at least some capacity exists for adaptive plasticity of the neural circuits controlling movement.
Al-Majed et al. (2000) have shown that as little as 1 hour of continuous electrical stimulation (ES) at the time of repair of a cut peripheral nerve results in an enhancement of axon regeneration. The effect of ES was to increase the number of early regenerating axons but it did not change their rate of growth (Brushart et al., 2002). Following transection and repair of the rat sciatic nerve, the amount of misdirection of Tib motor axons to reinnervate the TA muscle is greater than the amount of inappropriate reinnervation of Sol by axons that had previously innervated targets of the CF nerve (Brushart et al., 1983; Sabatier and English, 2007). Since the Tib branch of the sciatic nerve in rats and mice contains many more axons than the CF branch (McHanwell and Biscoe, 1981; Swett et al., 1986), we hypothesized that application of ES selectively to the CF branch but not the Tib branch of the sciatic nerve might give those axons a competitive advantage during regeneration and result in less misdirection of axons to inappropriate targets. One might also hypothesize that the timing of muscle activity and movements of the hindlimb would be altered less than rats that were injured but not treated with ES (untreated rats).
We applied this selective ES (sES) to the CF nerve prior to whole nerve transection. Rather than reducing the amount of misdirection of regenerating axons, this treatment resulted in a marked increase in the amount of reinnervation of Tib muscle targets by axons derived from the stimulated CF nerve, relative to untreated controls. Despite this marked loss of fidelity of muscle reinnervation, the timing of EMG activity in Sol and TA and the movements of the hindlimb during treadmill locomotion were not different in untreated and sES-treated rats. Thus, similar compensatory changes in the outputs of spinal circuits following sciatic nerve injury must occur, irrespective of the amount of misdirection of regenerating axons in the periphery. A preliminary report of these findings has been made (English et al., 2009).
MATERIAL AND METHODS
The study is a pretest–posttest control trial to investigate the effects of sES. A total of 24 rats were used for the experiments in this study. Eight rats were studied as unoperated intact controls. Twelve rats were studied after transection and surgical repair of the sciatic nerve. Six of these rats were treated with sES and six were untreated. An additional four rats were used as intact controls for terminal electrophysiology experiments. Some of the data from intact and untreated rats have been described previously (Sabatier and English, 2007). Rats receiving sES at the time of the imposed peripheral nerve injury are termed Treated. Rats whose nerves were cut and repaired but who received no sES at the time of the imposed peripheral nerve injury are termed Untreated. All protocols used in this study were approved by the Institutional Animal Care and Use Committee of Emory University and conformed to the Guidelines for the Use of Animals in Research of the Society for Neuroscience.
Surgical procedures
Female Sprague-Dawley rats weighing between 300–350 g before surgery were anesthetized with ketamine and xylazine to perform all implantations. Fine-wire EMG electrodes were implanted into the right Sol and TA muscles. The EMG electrodes used were constructed from pairs of Teflon-coated stranded stainless steel wire (50 µm dia.) (AS-631, Cooner Sales, Chatsworth, CA). The insulation was removed from ≈2 mm of the tips of the wires and these tips were staggered and separated by ≈1 mm. The exposed tips were bent into a barb shape and inserted into the respective muscles using an attached 6-0 nylon suture. Once securely in place in the muscle, the wires were secured using the attached suture. A small bipolar cuff electrode was implanted around the sciatic nerve proximal to its bifurcation into the Tib and CF branches. This cuff electrode was constructed from a short length of silastic tubing and the same type of wire used for EMG electrodes. The tubing was slit longitudinally and the wires sewn into it at a spacing of 2–3 mm. These cuff electrodes have been described in detail by others (Sweeney et al., 1990). Wires from EMG electrodes and the nerve cuff were led subcutaneously to a six-conductor plug mounted onto the skull of the rat using stainless steel bone screws and dental acrylic. All materials needed to formulate this percutaneous interface were obtained from Plastics One (Roanoke, VA).
Once these electrodes were in place a cuff electrode similar to the one implanted around the sciatic nerve was placed about the CF nerve. In animals in the sES Treated group, short (0.1 ms) constant voltage pulses were delivered through this cuff at an intensity twice that required to just evoke a visible twitch and compound muscle action potential in the TA muscle. No response, either visual twitching in the gastrocnemius or evoked EMG potential in the Sol muscle, was observed at this intensity (Fig. 1B). The CF nerve was then stimulated for 1 hour at 20 Hz just prior to transection and repair of the entire sciatic nerve. The efficacy of the stimulation protocol was evaluated every 10 minutes during this time and the stimulus strength adjusted as needed to maintain selective stimulation of the TA muscle. At the end of the hour of continuous stimulation the cuff electrode on the CF nerve was removed. In animals in the Untreated groups, no application of the cuff was applied. It was assumed that since such application without stimulation did not influence subsequent axon regeneration, both in experiments by our group (English et al., 2007; and unpubl.) or others (Al-Majed et al., 2000), that the physical effect of application of the cuff to the nerve did not influence the outcome of the experiments.
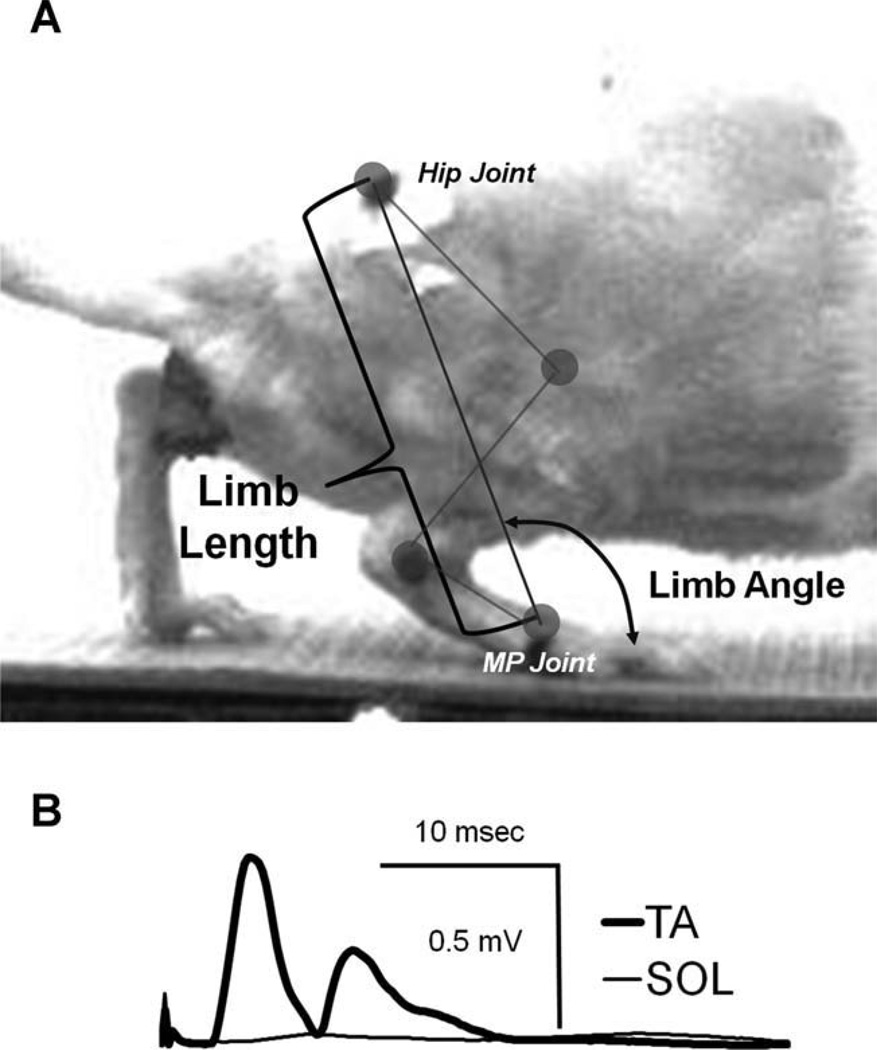
Figure 1.
A: An image from an actual single video frame is shown to demonstrate the measurements for limb length and limb angle based on markers over the hip and MP joints that were used to evaluate the length of the extensible hindlimb and its angle relative to the treadmill belt. B: Representative evoked EMG traces recorded from electrodes on the soleus (SOL, thin line) and tibialis anterior (TA, thick line) muscles during selective electrical stimulation of the common fibular nerve conducted prior to transection and repair of the sciatic nerve.
The sciatic nerve was then exposed in the mid-thigh, between the implanted cuff and its bifurcation into the Tib and CF branches, and severed with sharp scissors. The proximal and distal stumps were approximated and aligned and then secured in place using fibrin glue (Dagum, 1998; de Vries et al., 2002; Suri et al., 2002). This glue was assembled from fibrinogen, fibronectin, and thrombin at the time of use, applied to the nerve with a pipette, and allowed to form a clot. All wounds were sutured closed and the animals were allowed to recover from anesthesia. All animals were observed daily to ensure their health remained in good standing until they had stabilized, which tended to be at least 1 week post-surgery. Additionally, to eliminate the potential for self-mutilation and wound nonclosure posttransection, some rats wore a modified Elizabethan collar (e-collar) 4 inches in diameter.
Evoked potential recordings
Beginning 7 days following implantation, EMG activity evoked by stimulation of the cuff on the sciatic nerve above the lesion was monitored on a daily basis to determine whether muscle reinnervation had begun. Electrical activity in the Sol and TA muscles was amplified and recorded using a laboratory computer system and custom software. Animals were allowed to move about freely in a small enclosure while tethered to the recording equipment. Single stimulus pulses were delivered to the cuff electrode under computer control and recordings of evoked activity in the Sol and TA were saved to hard disk. Stimulus intensity was increased gradually from a predetermined level to record a large range of muscle responses. Once a direct muscle or M-response could be recorded, it was assumed that muscle reinnervation had begun. For each rat studied we determined the earliest time at which a restored M-response was noted. Significance of differences in this timing between Untreated and Treated rats was evaluated using an unpaired t-test (P < 0.05 considered significant).
Assays of functional recovery
Once an M-response reappeared, animals were placed on a motor-driven treadmill designed for rats (Columbus Instruments, Columbus, OH), and EMG activity from the Sol and TA was recorded at modest speeds of 11, 16, and 22 m/min during level walking. Data were collected at weeks 2, 4, and 10 after injury from each animal. During these trials animals were videotaped from the right side at 120 fps using a Dragonfly Express high-speed digital camera (Point-Grey Research, Vancouver, BC, Canada). The camera was placed orthogonal to the rat and magnification was calibrated to cover the entire length of the treadmill belt. Video was streamed through an IEEE-1394 port and recorded to the computer’s hard drive at 620 × 480 pixels and codified in 256 gray levels. Three dark spots were placed on the closely shaved hindlimbs at the metatarsophalangeal (MP) joint, at the lateral malleolus, and over the greater trochanter (Fig. 1). These spots were used to assist in visualization of the specific joint segments during locomotion. Video records were synchronized with EMG records using a custom fabricated device triggered by the video synch output of the video camera. Steady state step cycles, defined by successive paw contact times when the animals were moving at the treadmill belt speed, were selected from the acquired video records. Video segments in which the rat was crouching, hyperextending the neck, or riding the treadmill were not studied.
In individual frames of step cycles selected for analysis, the marked points over the MP, ankle, and hip joints were assigned Cartesian coordinates using a semiautomatic protocol (MaxTraq, Innovision Systems, Columbia-ville, MI). From these digitized records, in each video frame, we calculated the coordinates of the position of the knee joint by determining the point of intersection of two circles whose centers were the positions of the hip and ankle joints and whose radii were the length of the femur and tibia, respectively. The assigned coordinates in each video frame were then used to calculate the enclosed joint angles at the knee and ankle, and the angles made by the foot and femur to the treadmill belt. The latter angle is referred to here as the hip angle. We also determined the magnitude and direction of a whole limb vector (Daley et al., 2007; Auyang et al., 2009; Chang et al., 2009). This position vector is a line between coordinates at the hip and MP joints. The magnitude of this vector defines the length of the extensible hindlimb, irrespective of the angles at the hip, knee, and ankle joints. The direction of this vector is the orientation of the extensible hindlimb to the treadmill belt, measured anteriorly (Fig. 1A). To account for changes in animal size during the study period, we expressed the magnitude of this vector as adjusted limb length, the ratio of limb length to femur length. All of these data were then subjected to a cubic spline interpolation algorithm, resulting in a normalization of the values in each step cycle to 100 bins. Thus, all kinematic data in this article were studied as a percentage of the step cycle duration. Limb angles, limb lengths, joint angles, and foot angles were averaged from the selected step cycles and means and standard error of the mean (SEM) were found per rat, per speed, and per week postoperative (2, 4, and 10) throughout the time normalized step cycle.
The rationale for use of the whole limb vector as a kinematic measure is that in cats, humans, and guinea fowl this vector was conserved between subjects and, especially, following peripheral nerve injury (Daley et al., 2007; Auyang et al., 2009; Chang et al., 2009). This means that the intersubject variability in these global measures of hindlimb function was significantly less than that associated with measurements of the angles at individual joints or limb segments. Since the magnitude of such variability is a major determinant of the statistical power of these kinematic measures, we reasoned that whole limb kinematics would be a more sensitive assay of functional recovery following peripheral nerve injury than analysis of movements of individual joints or limb segments. Although this assumption has been supported in studies on other species, it has not been evaluated in rats. Thus, we compared interanimal variability in our global measures of hindlimb movement (limb angle and adjusted limb length) to the interanimal variability in ankle, knee, and hip angle measurements. For each such measure we determined the variance about the means in the different groups in selected step cycles, as described above. To avoid bias in these measures due to differences in means, especially adjusted limb length, this variability was expressed as the coefficient of variation, the ratio of the standard deviation to the mean. Average coefficients of variation were determined for each rat and comparisons were made between treatment groups (Intact, Untreated, and sES Treated) using analysis of variance (ANOVA), with post-hoc paired (Fisher’s least significant difference [LSD]) testing, where appropriate. All statistical analyses in this study were conducted using Statistica software (StatSoft, Tulsa, OK).
Average foot angles at the midpoint of the step cycle, mean limb angles and mean adjusted limb lengths, and average adjusted limb lengths and limb angles at the beginning and end of the stance phase were calculated for each group. Significance of differences between Intact, Untreated, and sES Treated rats was determined using ANOVA, with post-hoc paired (LSD) testing, where appropriate.
All locomotor EMG data were sampled at 1 kHz and stored on a computer. Baseline EMG data already collected from six Intact rats in previous studies (Sabatier and English, 2007) and from two new rats used as a control in order to minimize the interaction and induced stress of multiple surgeries on each rat. Locomotor EMG traces from individual selected step cycles were extracted for different treadmill speeds and postdenervation times (n = 8 for each). The synchronized EMG records were full-wave rectified, low-pass filtered (20 Hz), and normalized to 100 bins using a cubic spline interpolation algorithm. Data from multiple selected step cycles at different treadmill speeds in each EMG experiment were averaged, resulting in average step cycle activity for each rat at each speed and postdenervation survival time.
To determine if the activation patterns in different treatment groups differed from one another, these locomotor EMG data were processed using principal components analysis (PCA). Our dataset consisted of the average Sol and TA EMG activity for each rat at different treadmill speeds and at different times following nerve transection and repair in both Treated and Untreated rats, as well as comparable data from Intact rats. PCA was used to calculate eigenvalues for this dataset. Eigenvalues are new variables that serve to account for the variance in the dataset. Each eigenvalue accounts for progressively smaller portions of this overall variance. The eigenvalues were then varimax normalized to maximize the amount of variance explained. These eigenvalues were plotted as a scree plot (Supporting Fig. 1) to examine the proportion of total variance accounted for by each factor. Since more than 90% of the variance could be accounted for by the first four eigenvalues, only four factors were studied further. From these four factors, factor loadings for each variable in the dataset were calculated. These are the correlation between each of the eigenvalues and the original averages in the dataset. Factor loadings were averaged from each treatment group at each postoperative time and speed and then subjected to ANOVA. Since no significant difference was found between speeds, data for different speeds were pooled. Significance of differences in average factor loadings for each group at each time was determined from ANOVA and post-hoc (LSD) paired testing. Significance was set at P < 0.05.
Terminal electrophysiology experiments
Ten to 30 weeks after the cut and repair of the sciatic nerve, four Intact, four Treated, and four Untreated rats were anesthetized with a ketamine and xylazine mixture and used in terminal experiments. The sciatic nerve, Sol, and TA were exposed surgically. Approximately 10 mm proximal to the lesion site on the sciatic nerve, it was dissected into Tib and CF branches by carefully removing the epineurium (Evans et al., 1991). A fine-wire stimulating cuff was placed around the Tib branch of the dissected nerve and fine-wire patch type EMG electrodes (Loeb and Gans, 1986) were placed on the Sol and TA muscles. The Tib nerve was stimulated at an intensity that produced maximal responses from both Sol and TA. Recorded EMG potentials were averaged over multiple stimulus presentations. A delay of 3 seconds or greater between each stimulus was utilized in order to avoid muscle fatigue. The stimulating cuff was then moved from Tib nerve to CF nerve and the procedure was repeated with EMG electrodes in the same positions on the muscles. At the end of the experiment, rats were euthanized with an overdose of pentobarbital. The proportion of the sum total of the rectified and integrated EMG activity recorded from each muscle as a result of electrically stimulating the CF or Tib nerves was then calculated. Averages of these proportions from recordings made in the injured sides of Treated rats and Untreated rats were compared to each other and to similar results obtained from recordings made from the Intact rats. Significance of differences between these two sets of data were evaluated using ANOVA and appropriate post-hoc paired (LSD) testing.
RESULTS
sES enhances regeneration
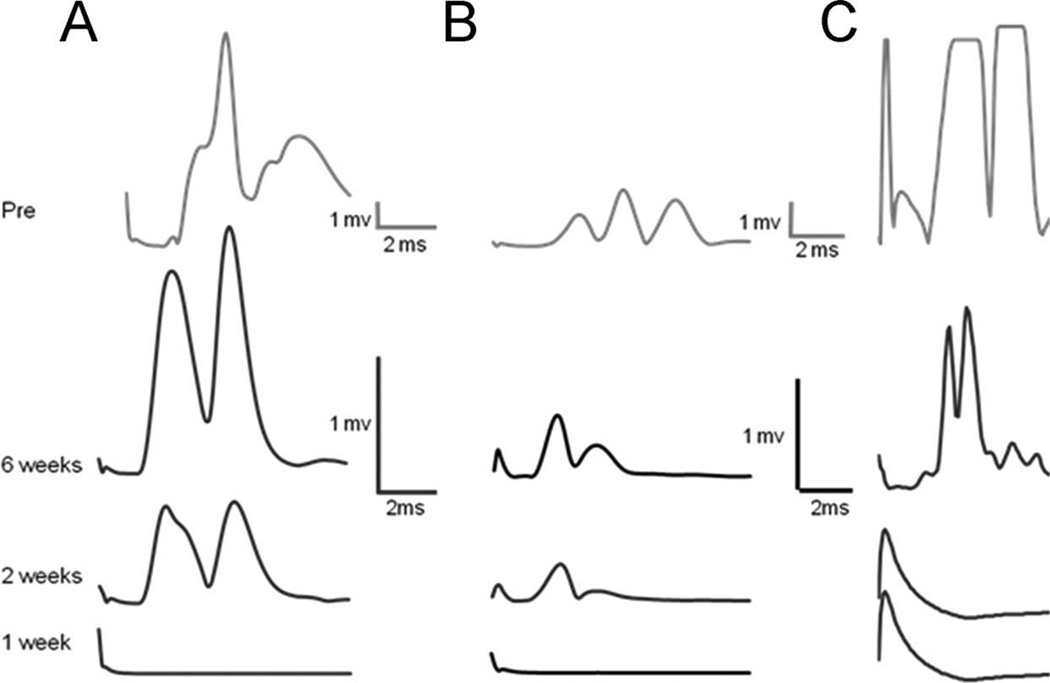
Typical M-response data recorded from Sol and TA muscles at different times after nerve transection and repair in Treated and Untreated rats are shown in Fig. 2. The data are displayed as rectified EMG activity. The amplitude of any evoked activity of the Sol and TA following stimulation to the sciatic nerve above the lesion site was measured as the average rectified activity in a defined time window. An evoked response with amplitude significantly greater than the prestimulus background activity was taken as evidence of a restored evoked M-response. In sES Treated animals an M-response in both the TA and Sol returned at a mean of 15 (±3.82, SEM) days, postoperatively. This is significantly (t-test, P < 0.05) sooner than the first evidence for a restored M-response (23 ± 4 days, SEM) that we observed in the Sol muscle of Untreated animals or than described in the literature (English et al., 2007). Thus, sES results in enhancement of axon regeneration into both the Tib and CF nerves.
Figure 2.
Typical direct muscle (M) responses of Sol (A) and TA (B) in sES Treated rats and Sol (C) in Untreated rats, recorded in response to stimulation of the sciatic nerve in Intact rats (top row) and at 1, 2, and 6 weeks postoperative. These M-responses were absent in all muscles at 1 week postoperative and were seen in both muscles at 2 weeks, but only in Treated rats. By 6 weeks postoperative the amplitudes of the M-responses in both muscles had increased, although they were still less than preoperative values.
sES is selective
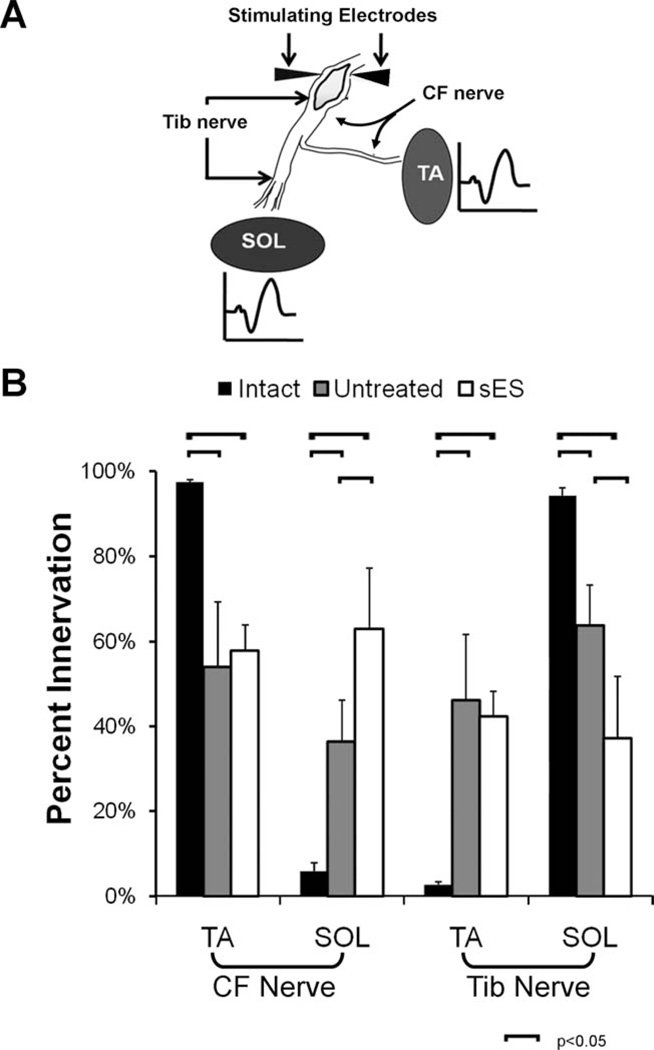
In acute terminal experiments, EMG data were collected from the TA and Sol when the CF and Tib nerves were stimulated separately above the injury site (Evans et al., 1991) (Fig. 3A). Since we stimulated nerves proximal to the sciatic nerve injury, we assumed that the proportion of the M-responses recorded in TA and Sol is that contributed to by axons that had coursed in these two nerves prior to injury. In Intact rats (n = 4), 94% of the EMG activity recorded from the Sol can be attributed to the stimulation of the Tib nerve and only 6% from stimulation of the CF nerve (Fig. 3B, black bars). Unavoidable EMG crosstalk (English and Weeks, 1989) is almost certainly the source of this small amount of evoked activity and it represents the limits of sensitivity of this method. In rats who received sES (n = 4), only 37% of the EMG activity recorded from the Sol can be attributed to the (appropriate) Tib nerve and 63% from stimulation of the (inappropriate) CF nerve (Fig. 3B: white bars). These differences from Intact rats are significant (LSD, P < 0.001). In contrast, in rats that received no treatment following the same injury (n = 4), 63% of the EMG activity recorded from the Sol can be attributed to the Tib nerve and only 37% from stimulation of the CF nerve (Fig. 3B: gray bars). These differences from Intact rats are statistically significant (LSD, P < 0.01). Differences between Untreated rats and sES Treated rats also are statistically significant (LSD, P < 0.005). These data are strong evidence that sES results in an increase in the misdirection of regenerating CF axons into the Tib nerve.
Figure 3.
A: Diagram of the terminal electrophysiology experiment. B: The mean (±SEM) proportion of total recorded EMG activity in the Sol and TA muscles in Intact, Untreated, and sES Treated rats (n = 4 for each) after stimulation to the CF and Tib nerves is shown.
In Intact rats, nearly 98% of the EMG activity recorded from the TA can be attributed to stimulation of the CF nerve and less than 3%, probably representing crosstalk, from stimulation of the Tib nerve (Fig. 3B: black bars). In rats receiving sES, only 58% of the EMG activity recorded from the TA can be attributed to stimulation of the CF nerve and 42% from stimulation of the Tib nerve (Fig. 3B: white bars). These data are significantly different from our findings in Intact rats (LSD, P < 0.05). In rats receiving no treatment following the same injury, the distribution of reinnervation of the TA muscles was also significantly altered from that found in Intact rats but not significantly different from that observed in the sES rats (Fig. 3B: gray bars). Nearly half of all regenerating motor axons reinnervating the TA muscle is from functionally inappropriate (Tib nerve) sources in both groups.
sES does not change locomotor EMG activity
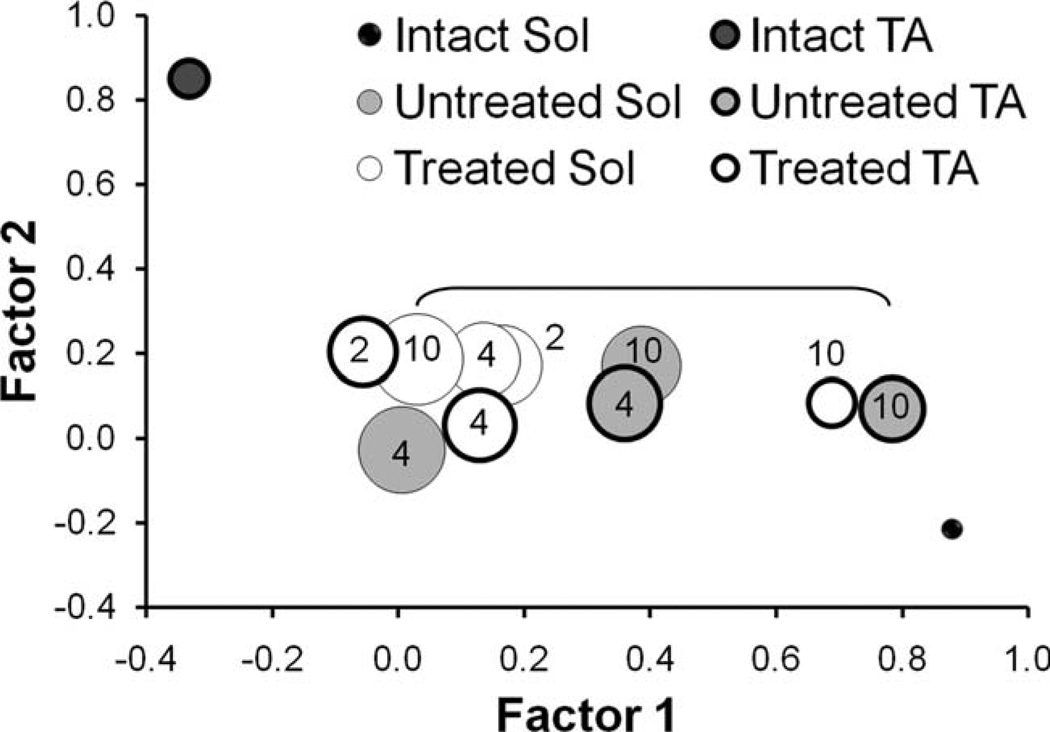
Locomotor EMG data were subjected to factor analysis using PCA. The first four factors accounted for more than 90% of the variance in the dataset (Supporting Fig. 1). The first two factors, which together account for >70% of the variance in the dataset, are plotted against each other in Fig. 4. No points are plotted for Untreated rats at 2 weeks following sciatic nerve repair, since no evidence for muscle reinnervation was found in these animals at that time (see above). Each point on this graph represents the average factor loading of all rats (Intact n = 8, Untreated n = 6, Treated n = 6) in our dataset.
Figure 4.
Results of PCA of locomotor EMG activity. Scatterplot graph of factor 1 vs. factor 2 from Intact, sES Treated, and Untreated rats at different times following transection and surgical repair of the sciatic nerve is shown. Numeric text designations on or next to each point indicate the survival time in weeks. No point is represented for the untreated rats at 2 weeks because innervation had not occurred at that time. The size of the symbol is proportional to the SEM. At all survival times, either factor 1 or factor 2 was significantly different from both the Intact TA and SOL for all injured animals. The bracketed points for the Untreated TA and Treated SOL at the 10-week survival times are the only two paired comparisons that were significantly (P < 0.05) different.
Activity patterns of TA and Sol are widely different in rats prior to sciatic nerve transection and repair. The factor loadings for these two data points are at opposite ends of the portion of factor loading space shown in Fig. 4. This distinction is a reflection of their normal reciprocal pattern of locomotor activity (see also Supporting Fig. 2A). In both Untreated rats and rats exposed to sES, variation between rats in the magnitude of factors 1 and 2 is greater than that observed in Intact rats, as noted by the differences in symbol sizes in Fig. 4. In comparing the factor loadings for all four factors between TA and Sol at both 4 and 10 weeks following transection of the sciatic nerve, a significant difference was observed only once out of eight paired comparisons (Fig. 4: Fig week Untreated TA vs. Treated Sol). This overall similarity between Sol and TA is quite different from the differences noted in Intact rats and it is consistent with the observation of coactivation of TA and Sol during locomotion in both groups of injured rats.
Significant differences between Untreated and sES Treated rats were found only for Factor 1 and even there no significant differences were found between Untreated and Treated rats at the same survival time. For example, the Factor 1 loadings for TA at 10 weeks for both Untreated and sES Treated rats are significantly greater than many of the Factor 1 loadings for other muscles at other times but they are not significantly different from each other. Thus, we believe that following transection and surgical repair of the sciatic nerve, the activation of TA and Sol during treadmill locomotion changes from a reciprocal pattern to coactivation in both Untreated and sES Treated rats. Only subtle distinctions between these two groups are present.
sES does not affect whole limb kinematics
Markers on the rats’ hindlimbs were tracked during selected step cycles to determine the angle of the foot with respect to the treadmill belt, the angles at the hip, knee, and ankle joints, and also a global whole limb variable. The global measure of hindlimb movements has two components: limb length, measured as the distance between the hip and MP joints; and limb angle, the orientation of the limb to the treadmill belt (see Fig. 1). Limb length is reported here as a function of femur length, termed adjusted limb length, to control for size differences between rats and differences in sizes of individual rats over the time course of the study.
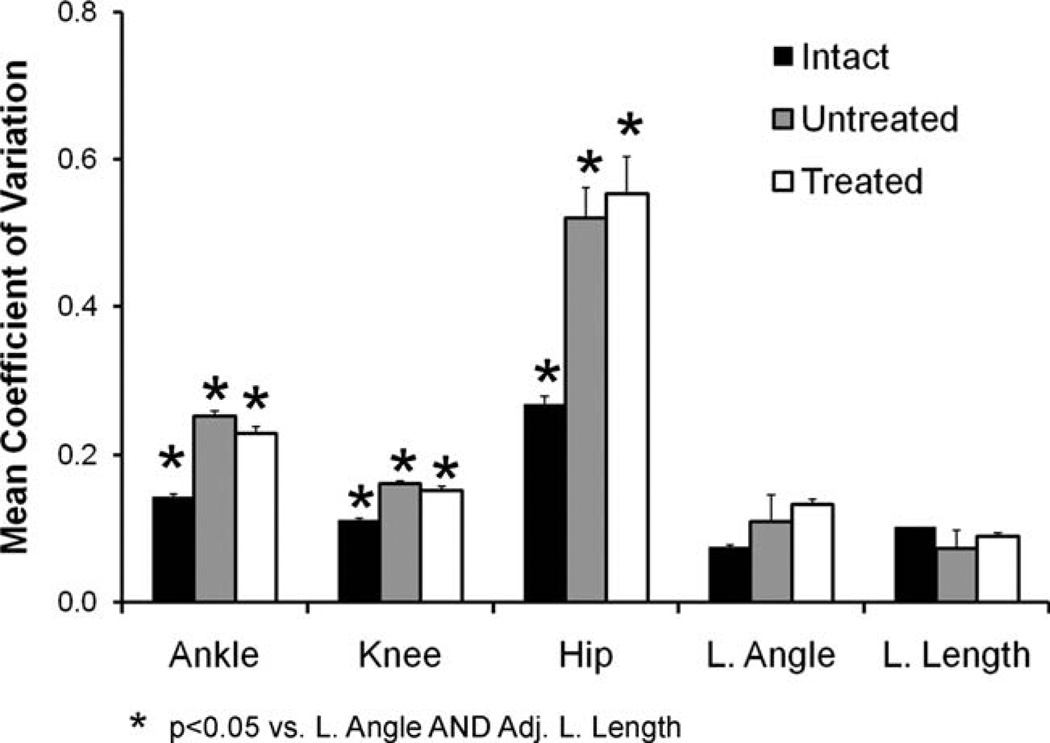
The global measure used in this study has been favored in studies of cat, human, and guinea fowl locomotion (Daley et al., 2007; Auyang et al., 2009; Chang et al., 2009) but its use in rats has not yet been reported. We compared the inter-rat variability in limb angle and adjusted limb length to the variability in ankle, knee, and hip angles in our three treatment groups using the coefficient of variation (ratio of standard deviation to mean). Average (±SEM) coefficients of variation of variation are shown in Fig. 5 for Intact rats (n = 8) and rats in the Untreated (n = 6) and sES Treated groups (n = 6). Data are shown for the 10-week survival time. In Intact rats, mean coefficient of variation of the ankle, knee, and hip joints all are significantly (LSD, P < 0.05) larger than either of the components of the global limb variable, limb angle, and adjusted limb length (Fig. 5, black bars). These differences are even more striking when the data from Untreated and sES Treated rats are considered (Fig. 5, gray and white bars, respectively). Thus, the conserved nature of the global limb measurements found in other species was observed in rats.
Figure 5.
Results of comparison of coefficients of variation of included joint angles to whole limb kinematic measures are shown. For each percentile of each step cycle studied, interanimal variability was determined in each treatment group and this was expressed as the coefficient of variation (standard deviation/mean). Each bar represents the average (±SEM) coefficient of variation in a step cycle for Intact rats and Untreated and sES Treated rats 10 weeks following sciatic nerve transection and repair.
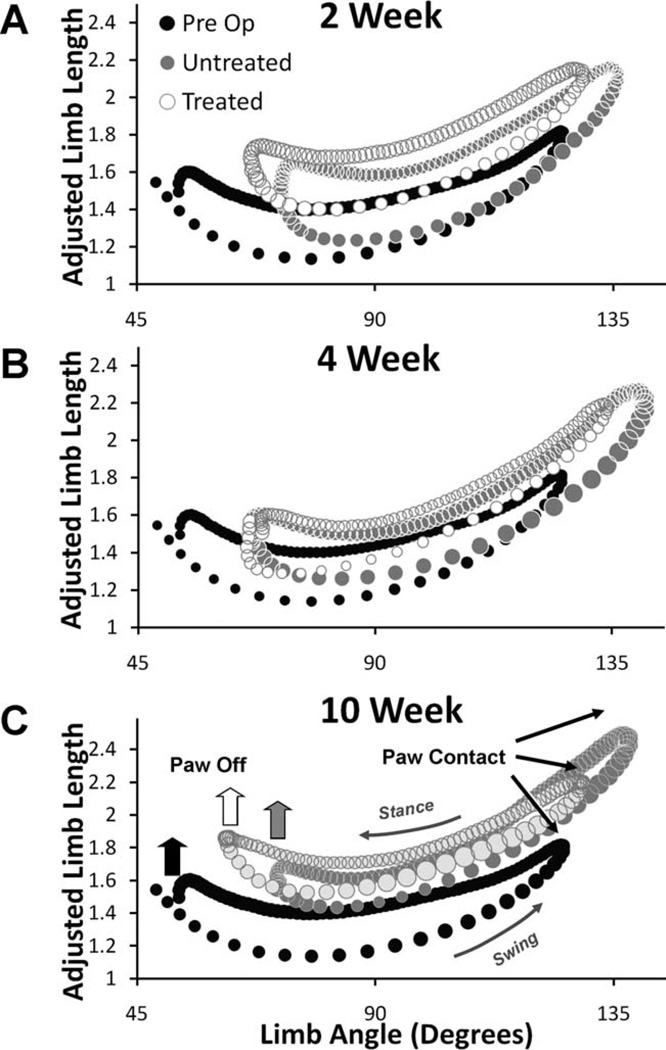
Graphs were generated plotting the limb angle versus adjusted limb length for Intact rats, sES Treated rats, and Untreated rats at 2, 4, and 10 weeks postoperative (Fig. 6A – C). Limb angle/adjusted limb length trajectories are a similar overall shape for all rats at all treadmill speeds studied. In these graphs, paw contact occurs at the largest limb angle and longest adjusted limb length, and paw off occurs at the smallest limb angle (Fig. 6C). The step cycle projects in a counterclockwise direction in these graphs. The bottom side of the polygons so described contains values from the swing phase and the top of each polygon contains data from the stance phase.
Figure 6.
In each panel, overall limb angle (abscissa) is plotted against adjusted limb length (ordinate). Data from Untreated (n = 4) and sES Treated (n = 6) rats are overlaid onto data from Intact rats (n = 8). Data points represent averages between rats of selected step cycles. Symbol size is proportional to the SEM. Data are from 2 weeks (A), 4 weeks (B), and 10 weeks (C) postoperative survival times.
At all three times studied following sciatic nerve transection, the shapes of the polygons defined by limb angle and adjusted limb length in both Untreated and sES Treated rats are roughly similar to that of Intact rats. In the injured animals the positions of the polygons in this space are altered, relative to preoperative controls. The significance of these changes was determined by comparing the components of the limb position vector–adjusted limb length and limb angle–at the onset of stance (Paw Contact, Fig. 7A) and the end of stance (Paw Off, Fig. 7B), and also the average means of the two components (Fig. 7C), between the three groups.
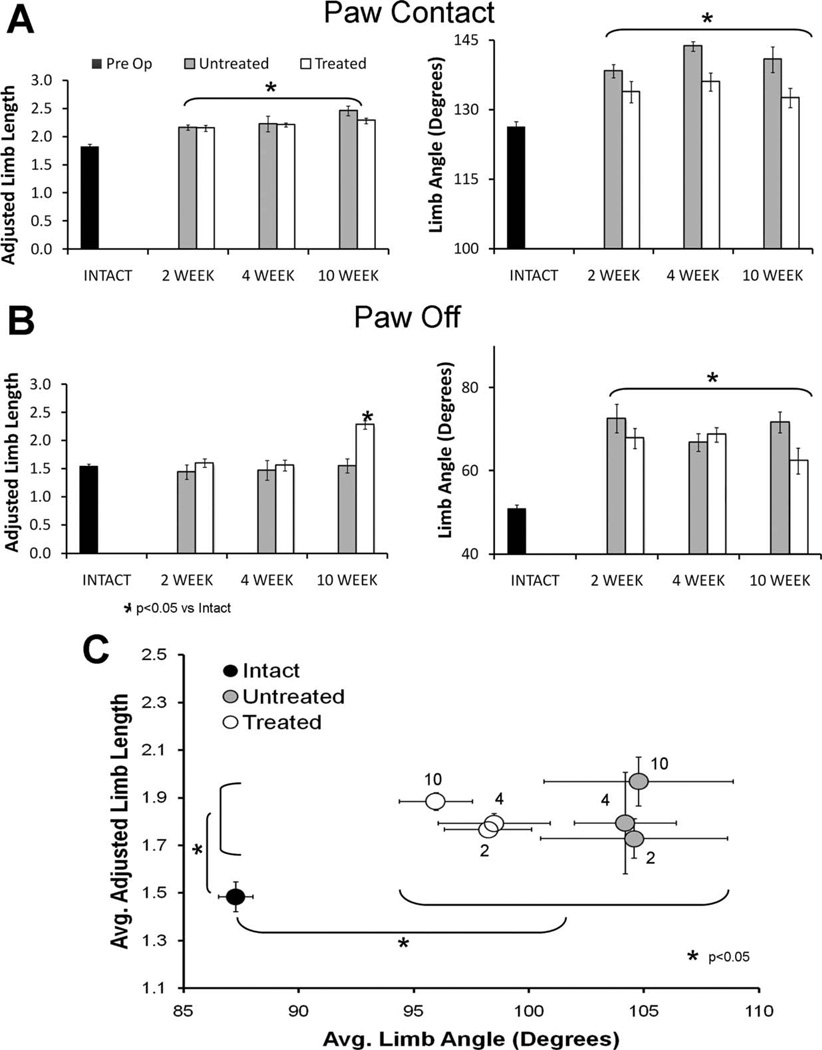
Figure 7.
A: Bar graph representation of adjusted limb length (left) and limb angle (right) at paw contact for Intact rats and sES Treated and Untreated rats studied at 2 weeks, 4 weeks, and 10 weeks postoperative survival times. B: Bar graph representation of adjusted limb length (left) and limb angle (right) at paw off (the end of stance) for Intact rats and sES Treated and Untreated rats studied at 2 weeks, 4 weeks, and 10 weeks postoperative survival times. C: Average median limb angle (6SEM) is plotted against average median adjusted limb length (±SEM) for Intact rats and sES Treated and Untreated rats studied at 2 weeks, 4 weeks, and 10 weeks postoperative survival times. Numbers next to the symbols indicate the survival time in weeks. Brackets indicate paired (Fisher’s least significant difference) comparisons that are significantly different (P < 0.05).
Two weeks following transection and surgical repair of the sciatic nerve, when muscles are completely denervated in Untreated rats and when only the earliest signs of rein-nervation are found in the sES Treated rats, limb angle is larger throughout the step cycle in both groups of injured rats than Intact controls (Fig. 7A). Mean limb angle, limb angle at the end of stance (Paw Off), and limb angle at paw contact are all significantly (LSD, P < 0.05) larger than in Intact rats (Fig. 7). This means that the hindlimb is held at a more acute angle to the treadmill belt throughout the step cycle than in Intact rats (see Fig. 1). Significant (LSD, P < 0.05) increases in mean adjusted limb length and adjusted limb length at paw contact were found between Intact rats and both Untreated and Treated rats. The differences in adjusted limb length are interpreted to mean that the extensible hindlimb is held in a longer position throughout the step cycle in injured rats than in Intact rats. To the extent that the walking kinematics of the rats at this time might reflect a compensation for the paralysis or near paralysis of the ankle musculature, both Untreated and Treated rats step with their limbs held in more lengthened positions and with the limb placed on and removed from the treadmill belt at a more acute angle than in intact rats. No significant differences in any of the measurements of limb angle or of adjusted limb length were found between Untreated and sES Treated rats at this time (n = 6 for both).
By 4 weeks after sciatic nerve transection and repair, muscle reinnervation is found in both Untreated and sES Treated rats. Relative to preoperative controls, all three measures of limb angle remain significantly increased in both groups of injured animals (Fig. 7). Both mean adjusted limb length and adjusted limb length at paw contact are significantly (LSD, P < 0.005) increased in both groups of injured animals, relative to Intact controls, but adjusted limb length at paw off is not (Fig. 7B). The polygons described in the graphs in Fig. 6 are shifted vertically. No significant differences in any of the measurements of limb angle or of adjusted limb length were found between Untreated and sES Treated rats at this time. Despite the greater extent of muscle reinnervation found at this time, global measures of limb kinematics remain the same as observed at 2 weeks posttransection.
By 10 weeks after transection and surgical repair of the sciatic nerve, muscle reinnervation is nearly complete (Brushart et al., 1983). As noted at the 2- and 4-week survival times, limb angle is larger throughout the step cycle in both groups of injured rats than preoperative controls. Limb angle at both paw contact and paw off and median limb angle remain significantly increased in both groups of injured animals. The polygons defined by adjusted limb length and limb angle are shifted significantly toward longer adjusted limb lengths (Fig. 7C). Differences in mean adjusted limb length and adjusted limb length at paw contact are significant for both Untreated and sES Treated rats, relative to Untreated controls. Adjusted limb length at paw off is significantly (LSD, P < 0.001) larger in Treated rats than in either Intact rats or Untreated rats. Thus, over the 10-week study period, patterns of hindlimb movements change little in rats following transection and surgical repair of the sciatic nerve. There is very little difference between Untreated rats and rats exposed to sES prior to transection and these do not change as muscles become reinnervated.
Changes in foot posture during locomotion
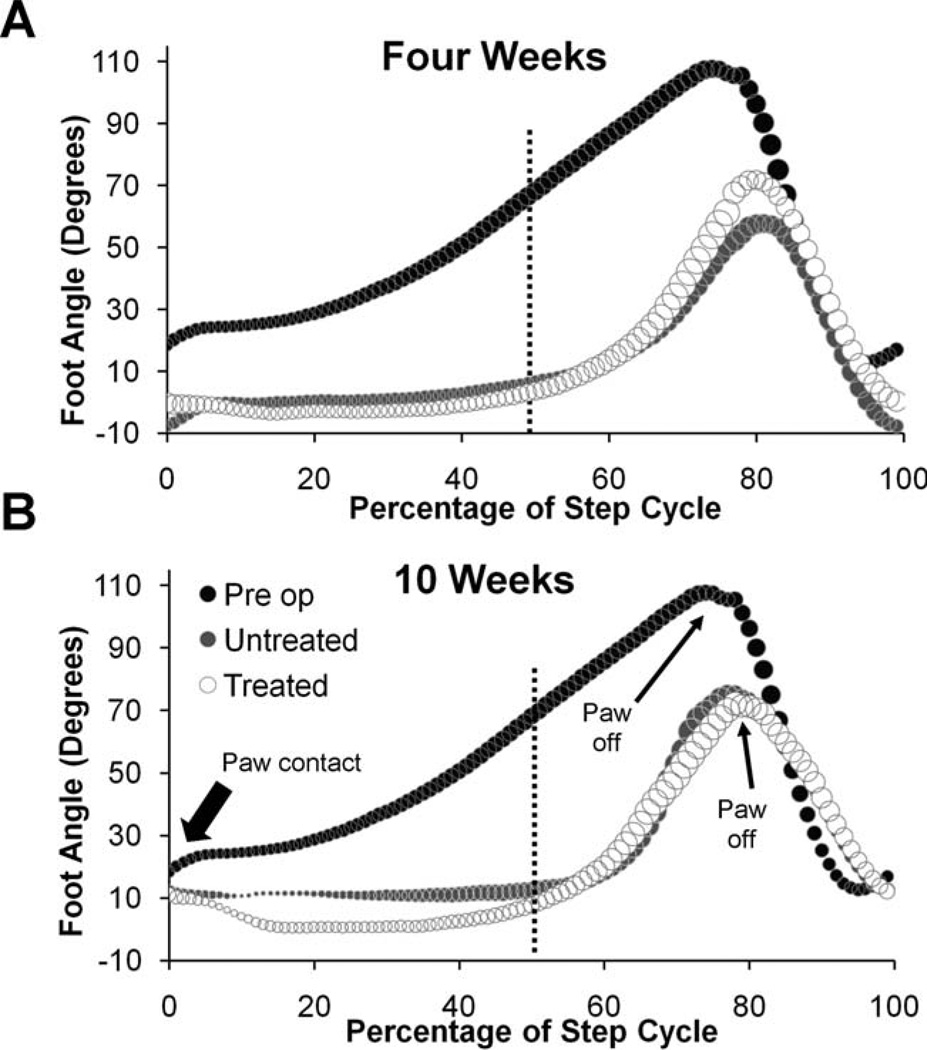
Graphs of foot angle changes during the step cycle are shown in Fig. 8. In Intact rats foot angles are maintained at about 20° during the early stance phase and then increase steadily until the paw pushes off. Following sciatic nerve transection, foot angles from both Untreated and sES Treated rats are nearly zero at paw contact and remain so until late in the stance phase when the foot is lifted from the treadmill belt. In some injured rats, the toes are curved ventrally in a plantar flexed position, and this leads to foot angles during stance that are less than zero (see Supporting Fig. 3A). The difference in foot angle between Intact and both groups of injured rats is especially marked at mid-step, at the 50th percentile of the step cycle (Fig. 8, vertical dashed line). Mean foot angles at 50% of the step cycle are significantly smaller (LSD, P ≤ 0.0001) for both sES Treated and Untreated animals when compared to Intact animals at all times studied. No significant differences were found between sES Treated and Untreated rats at any time studied (see also Supporting Fig. 3B). Foot posture is changed in injured rats and, despite extensive reinnervation of ankle muscles over time, it does not change in either group of animals.
Figure 8.
Foot angle changes during treadmill locomotion do not change in sES rats. Each graph is a plot of the changes in foot angle (in degrees) during a single step cycle. Data from rats 4 (A) and 10 (B) weeks following transection and surgical repair of the sciatic nerve are shown. In each graph the time of paw contact is shown to the left and the time of paw off (end of stance phase) is indicated by small arrows. The vertical dashed line is drawn at the mid-step time (50th percentile) when average values were compared between groups (see Supporting Fig. 3). In each graph, different shadings on symbols are used to show changes in Intact rats (n = 8), Untreated rats (n = 4), and sES Treated rats (n = 6). The sizes of the symbols are proportional to the SEM in each case. In injured animals, foot angle sometimes can be less than zero if the MP joints are higher than the ankle joint.
DISCUSSION
Following application of sES to the CF nerve just prior to sciatic nerve injury, enhanced axon regeneration was found in both the CF and Tib nerves. A return of M-responses to sciatic nerve stimulation was found in both the TA and Sol at earlier times in sES rats than in Untreated rats. Faster reinnervation of the TA was consistent with the results of previous experiments using ES as a means to enhance axon regeneration (Al-Majed et al., 2000). The faster than normal return of the M-response in the Sol, even though the Tib nerve was not directly stimulated, was not anticipated. Such a response in the muscle innervated by the unstimulated nerve could be explained either by a misdirection of CF axons into the distal segment of the sciatic nerve leading to the Tib nerve (Kobbert and Thanos, 2000) or by a widespread effect of the sES to affect regeneration of unstimulated axons from the Tib branch.
Our results favor the notion that sES resulted in greater misdirection of the stimulated axons from the CF nerve. In terminal electrophysiology experiments we found that most axons reinnervating the Sol muscle of sES Treated animals were derived from motoneurons whose axons originated in the CF nerve. In contrast, the relative contributions of the CF and Tib nerves to reinnervation of the TA muscle were not affected by sES. We believe that the simplest explanation of these findings is that sES applied to the CF nerve enhanced the regeneration of axons from the CF portion of the cut sciatic nerve selectively and that this exuberant outgrowth caused many of those axons to enter the distal segment of the Tib portion of the cut nerve. We think that sES did not have a similar effect on regenerating axons from the Tib portion of the cut nerve so that a disproportionate number of misdirected CF axons reinnervated the Sol muscle.
One of the main reasons given for poor functional recovery following peripheral nerve injury is the misdirection of regenerating axons to reinnervate functionally inappropriate muscle targets (Brushart et al., 2002; English, 2005; de Ruiter et al., 2008; Robinson and Madison, 2009). Our original goal in investigating sES was to try to increase the rein-nervation of the TA muscle by functionally appropriate motoneurons whose axons originate in the CF nerve and thus reduce the amount of this misdirection. This goal was not achieved. Not only did regenerating CF axons not rein-nervate the TA muscle with any more fidelity than untreated animals, but the misdirection of CF motor axons to reinnervate muscles formerly innervated by the Tib nerve was increased. The reason that sES caused proportionally more CF axons to reinnervate Tib nerve targets, such as Sol, than CF nerve targets, such as TA, is not clear. There are many more axons in the Tib nerve than the CF nerve (Swett et al., 1986). As suggested by others (Brushart et al., 1998; Robinson and Madison, 2009), the larger number of available endoneurial tubes in the distal segment of the cut Tib nerve could form a more hospitable pathway for regenerating axons than tubes available in the distal segment of the cut CF nerve.
Given this increased misdirection of regenerating motor axons with sES, one might have expected that the activation of TA and Sol during treadmill locomotion and functional recovery would be even more disturbed in sES rats than observed in untreated rats. This was not the case. The pattern of locomotor EMG activity in the TA and Sol was significantly different from intact animals in both untreated and sES treated rats. In intact rats these functionally antagonist muscles are reciprocally activated. In both untreated and sES treated rats this pattern changes to one of coactivation. Based on our results from PCA, we have no reason to believe that the nature of the coactivation patterns in these two treatment groups is different. This finding is in stark contrast to the results described above. Despite a frank difference in accuracy of reinnervation, we were unable to detect a difference in the patterns of activation of TA and Sol during locomotion in these two groups.
Similarly, based on our global measures of hindlimb kinematics during treadmill locomotion, we found that hind-limb movements during locomotion in both untreated and sES treated rats were significantly different from those observed in intact animals, but only slightly different from each other. Rats in both groups seemed to develop compensatory limb movement strategies prior to muscle rein-nervation, and these changed little as muscles gained full reinnervation. None of these compensatory strategies involve extensive use of the foot, either in accepting body weight during early stance to exploit the springiness of the calf muscles or in plantar flexion toward the end of stance to propel the animal forward. Instead, they involved changes in the movements of the knee and hip that increased overall limb length and rotated the limb through more acute angles than intact rats.
We chose to study whole limb kinematics largely because others (Daley et al., 2007; Auyang et al., 2009; Chang et al., 2009) had shown that intersubject variability in these global measures of hindlimb movement was less than the more traditional measurement of angular movements at the individual joints. Such decreased intersubject variability leads to increased statistical power and discriminative ability in our assays. Indeed, we found that inter-rat variability in these global measures of limb movement was significantly smaller than that of ankle, knee, and hip joint angles. The reduced variability was found in intact rats, suggesting that subtle differences in movements of hindlimb joints during locomotion in different rats are dampened if whole limb movements are considered. In animals walking following transection and repair of the sciatic nerve, the differences in intersubject variability between global measures and those of individual joint angles becomes more marked, suggesting that the compensation for the injury in different rats may differ considerably at the level of the movements of the hip, knee, and ankle joints, but at the level of whole limb length and limb angle they differ less. Our observations are entirely in keeping with the conclusions of Chang et al. (2009) on this point.
Thus, given the similar locomotor activation patterns of TA and Sol and the similarity in the parameters of limb movements observed, we conclude that no significant difference in functional recovery was observed between untreated rats and rats treated using sES. We interpret these findings to mean that compensatory changes in the outputs of the spinal circuits driving locomotion occur in these two groups of animals and that these result in similar abnormal patterns of locomotion, irrespective of the extent of misdirection of regenerating axons in the periphery. We do not believe that the similarity of functional outcome measures in these two groups means that misdirection of regenerating axons is not an important determinant of functional recovery following peripheral nerve injury. Instead, we believe that the strengths of the compensatory strategies developed in response to sciatic nerve transection are much more potent than any effect of innervation patterns. In order to make a significant impact on functional recovery following peripheral nerve injury, we feel that any effective treatments will need to impact on these compensatory strategies as well as enhance axon regeneration.
We used the rat sciatic nerve model because the effect of misdirection of regenerating axons is substantial. Study of the functional consequences of misdirection is relatively simplified in this model. In animal models that incorporate smaller nerves, which some might find more clinically relevant and where misdirection of regenerating axons might give rise to inappropriate reinnervation of synergist, rather than antagonist muscles, more subtle differences in the effects of misdirection of regenerating axons might be anticipated. Whether the methods employed in this study have the power to detect such differences is not known. However, we would speculate that the overall conclusion of this article, that such effects must be interpreted in the context of the compensatory efforts of the animals, would remain unchanged.
Supplementary Material
Acknowledgments
Grant sponsor: United States Public Health Service (USPHS); Grant number: HD032571.
Footnotes
Additional supporting information may be found in the online version of this article.
LITERATURE CITED
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12:4381–4390. [PubMed] [Google Scholar]
- Auyang AG, Yen JT, Chang YH. Neuromechanical stabilization of leg length and orientation through interjoint compensation during human hopping. Exp Brain Res. 2009;192:253–264. doi: 10.1007/s00221-008-1582-7. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Tarlov EC, Mesulam MM. Specificity of muscle reinnervation after epineurial and individual fascicular suture of the rat sciatic nerve. J Hand Surg [Am] 1983;8:248–253. doi: 10.1016/s0363-5023(83)80152-x. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Gerber J, Kessens P, Chen YG, Royall RM. Contributions of pathway and neuron to preferential motor reinnervation. J Neurosci. 1998;18:8674–8681. doi: 10.1523/JNEUROSCI.18-21-08674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett M, Zager E. Pathophysiology of PNI: a brief review. Neurosurg Focus. 2004;16:1–7. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- Chang YH, Auyang AG, Scholz JP, Nichols TR. Whole limb kinematics are preferentially conserved over individual joint kinematics after peripheral nerve injury. J Exp Biol. 2009;212:3511–3521. doi: 10.1242/jeb.033886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AH. Functional recovery following cross-reinnervation of antagonistic forelimb muscles in rats. Acta Physiol Scand. 1978;103:331–333. doi: 10.1111/j.1748-1716.1978.tb06220.x. [DOI] [PubMed] [Google Scholar]
- Dagum AB. Peripheral nerve regeneration, repair, and grafting. J Hand Ther. 1998;11:111–117. doi: 10.1016/s0894-1130(98)80007-0. [DOI] [PubMed] [Google Scholar]
- Daley MA, Felix G, Biewener AA. Running stability is enhanced by a proximo-distal gradient in joint neuromechanical control. J Exp Biol. 2007;210:383–394. doi: 10.1242/jeb.02668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter GC, Malessy MJ, Alaid AO, Spinner RJ, Engelstad JK, Sorenson EJ, Kaufman KR, Dyck PJ, Windebank AJ. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211:339–350. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J, Menovsky T, van Gulik S, Wesseling P. Histological effects of fibrin glue on nervous tissue: a safety study in rats. Surg Neurol. 2002;57:415–422. doi: 10.1016/s0090-3019(02)00736-x. [DOI] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J Comp Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- English AW, Weeks OI. Electromyographic cross-talk within a compartmentalized muscle of the cat. J Physiol. 1989;416:327–336. doi: 10.1113/jphysiol.1989.sp017763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Chen Y, Carp JS, Wolpaw JR, Chen XY. Recovery of electromyographic activity after transection and surgical repair of the rat sciatic nerve. J Neurophysiol. 2007;97:1127–1134. doi: 10.1152/jn.01035.2006. [DOI] [PubMed] [Google Scholar]
- English AW, Hamilton SK, Hinkle ML, Kaufman M, Nicolini J, Rambo LN, Rexwinkle AM, Rose SJ, Backus D, Sabatier M. Misdirection of regenerating motor axons has little effect on functional recovery following peripheral nerve injury. Soc Neurosci. 2009 Abstr 510. 522. [Google Scholar]
- Evans PJ, Bain JR, Mackinnon SE, Makino AP, Hunter DA. Selective rinnervation: a comparison of recovery following microsuture and conduit nerve repair. Brain Res. 1991;559:315–321. doi: 10.1016/0006-8993(91)90018-q. [DOI] [PubMed] [Google Scholar]
- Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18:397–405. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Fujito Y, Kawasaki H, Aoki M. Motor recovery after cross-reinnervation of a forelimb nerve by the phrenic nerve in cats. Brain Res. 1989;492:36–44. doi: 10.1016/0006-8993(89)90886-x. [DOI] [PubMed] [Google Scholar]
- Gordon T, Stein RB, Thomas CK. Innervation and function of hind-limb muscles in the cat after cross-union of the tibial and peroneal nerves. J Physiol. 1986;374:429–441. doi: 10.1113/jphysiol.1986.sp016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen A, IJkema-Paassen J, Meek MF. Sciatic nerve transection in the adult rat: abnormal EMG patterns during locomotion by aberrant innervation of hindleg muscles. Exp Neurol. 2000;161:183–193. doi: 10.1006/exnr.1999.7233. [DOI] [PubMed] [Google Scholar]
- Kobbert C, Thanos S. Topographic representation of the sciatic nerve motor neurons in the spinal cord of the adult rat correlates to region-specific activation patterns of microglia. J Neurocytol. 2000;29:271–283. doi: 10.1023/a:1026523821261. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Gans C. Electromyography for experimentalists. Chicago: University of Chicago Press; 1986. [Google Scholar]
- McHanwell S, Biscoe TJ. The localization of motoneur-ons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci. 1981;293:477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- Meek MF, van der Werff JF, Klok F, Robinson PH, Nicolai JP, Gramsbergen A. Functional nerve recovery after bridging a 15 mm gap in rat sciatic nerve with a biodegradable nerve guide. Scand J Plast Reconstr Surg Hand Surg. 2003;37:258–265. doi: 10.1080/02844310310019464. [DOI] [PubMed] [Google Scholar]
- O’Donovan MJ, Pinter MJ, Dum RP, Burke RE. Kinesiological studies of self- and cross-reinnervated FDL and soleus muscles in freely moving cats. J Neurophysiol. 1985;54:852–866. doi: 10.1152/jn.1985.54.4.852. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Influence of terminal nerve branch size on motor neuron regeneration accuracy. Exp Neurol. 2009;215:228–235. doi: 10.1016/j.expneurol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Sabatier MJ, English AW. Soleus and tibialis anterior are co-activated during treadmill locomotion after recovery from sciatic nerve transection. Soc Neurosci. 2007 Abstr: 872. 810. [Google Scholar]
- Scholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR. Peripheral nerve injuries: an international survey of current treatments and future perspectives. J Reconstr Microsurg. 2009;25:339–344. doi: 10.1055/s-0029-1215529. [DOI] [PubMed] [Google Scholar]
- Slawinska U, Kasicki S. Altered electromyographic activity pattern of rat soleus muscle transposed into the bed of antagonist muscle. J Neurosci. 2002;22:5808–5812. doi: 10.1523/JNEUROSCI.22-14-05808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry RW. The effect of crossing nerves to antagonistic muscles in the hind limb of the rat. J Comp Neurol. 1941;75:1–19. [Google Scholar]
- Sperry RW. Effect of crossing nerves to antagonist limb muscles in the monkey. Arch Neurol Psychiatr. 1947;58:452–473. doi: 10.1001/archneurpsyc.1947.02300330064006. [DOI] [PubMed] [Google Scholar]
- Suri A, Mehta VS, Sarkar C. Microneural anastomosis with fibrin glue: an experimental study. Neurol India. 2002;50:23–26. [PubMed] [Google Scholar]
- Sweeney JD, Ksienski DA, Mortimer JT. A nerve cuff technique for selective excitation of peripheral nerve trunk regions. IEEE Trans Biomed Eng. 1990;37:706–715. doi: 10.1109/10.55681. [DOI] [PubMed] [Google Scholar]
- Swett JE, Wikholm RP, Blanks RH, Swett AL, Conley LC. Motoneurons of the rat sciatic nerve. Exp Neurol. 1986;93:227–252. doi: 10.1016/0014-4886(86)90161-5. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Navarro X. Functional impact of axonal misdirection after peripheral nerve injuries followed by graft or tube repair. J Neurotrauma. 2002;19:1475–1485. doi: 10.1089/089771502320914705. [DOI] [PubMed] [Google Scholar]
- Varejao AS, Cabrita AM, Geuna S, Patricio JA, Azevedo HR, Ferreira AJ, Meek MF. Functional assessment of sciatic nerve recovery: biodegradable poly (DLLA-epsilon-CL) nerve guide filled with fresh skeletal muscle. Microsurgery. 2003;23:346–353. doi: 10.1002/micr.10148. [DOI] [PubMed] [Google Scholar]
- Yumiya H, Larsen KD, Asanuma H. Motor readjustment and input-output relationship of motor cortex following cross-connection of forearm muscles in cats. Brain Res. 1979;177:566–570. doi: 10.1016/0006-8993(79)90474-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.