Abstract
Purpose:
The aim of this study was to report a comparison between the zinc oxide eugenol dressing and plasma rich in growth factor (PRGF) with gelatin sponge in the treatment of dry socket.
Materials and Methods:
This study comprised of 45 patients of dry socket in the span of one year. The patients were randomly divided into three groups on the basis of treatments: Group A (PRGF with gelatin sponge), group B (zinc oxide eugenol group), and group C (irrigation with sterile saline only). The clinical progress was noted at 1st, 2nd, 3rd, 7th, and 15th day after the treatment.
Results:
Patient's healing was better in group A than in group B but symptomatic pain relief was faster in group B. Group C fared worst in both aspects.
Conclusion:
We conclude that PRGF with gelatin sponge might be a treatment of choice in the management of dry socket.
Keywords: Alveolitis, gelatin sponge, plasma rich in growth factor
Introduction
Management of dry socket is of great concern for all the dental clinicians due to severe pain along with frequent visits of patient to the hospital.[1] It is generally well-localized to the socket but may radiate to the ear or other parts of the face and neck.[2] The incidence is 3-5% in non-surgical extraction[3] and up to 15% in impacted third molar extraction socket.[4,5] Dry socket generally presents with either totally empty or partly covered with grayish-yellow membrane of necrotic tissue. Predisposing factors are pre-existing systemic diseases, female, oral contraceptives, bacterial infections, smoking. Its etiology is still not very clear so it is also known as alveolar osteitis, localized osteitis alveolgia, alveolar sicca dolorosa, localized osteomyelitis, septic socket, necrotizing socket and fibrinolytic alveolitis. Birns hypothesis[6] is the most accepted explanation of dry socket till date. He stated that trauma and inflammation causes release of stable tissue activator from the adjacent bony socket and soft-tissues. Tissue activator converts plasminogen (present in the blood clot) to the plasmin. Plasmin causes lysis of blood clot and pain (kininogen-kinins). Dry socket is unlikely to be present within first 2 days of extraction due to presence of antiplasmin, which gets consumed later. Most of the studies suggest the treatment with intra-alveolar dressing with different medicaments and in some cases antibiotics and analgesics if required. Despite all the research and development it is still posing a challenge in terms of delayed healing and frequent visits along with mental agony of the patients.
Plasma rich in growth factor (PRGF) obtained from autologous blood is used to deliver growth factors in high concentrations to the site of bone defect. So, we have used PRGF[7] along with gelatin sponge to promote healing and compared it with the traditional treatment of zinc oxide eugenol dressing.[8,9] Guided bone regeneration has emerged as accepted surgical procedure used to increase the quantity and quality of host bone in localized alveolar defects.[10] An autologous bone graft requires a second surgical site, postoperative discomfort and time and cost expenditure. In 1990, Gibble and Ness introduced the fibrin gel to act as to improve availability of hemostatic agents. Our research is aimed to promote the socket healing and reduce the visits of the patients.
Materials and Methods
This study comprised of 45 patients of dry socket in the span of 1 year. Exclusion criteria were smokers, steroid therapy, diabetes, pregnant, and lactating women. Stratified random sampling was chosen. The patients reporting with complain suggestive of dry socket were diagnosed for the presence of disease. After confirmation they were subjected to exclusion criteria as mentioned. After the inclusion of patient into study they were attributed into any one group out of three, one by one, i.e., 1st patient goes into group A then 2nd patient goes into group B and 3rd patient goes to group C and repeating the same until each group contains 15 patients each. Selection was exclusive of consideration of sex and age.
A well-informed consent was taken in patient's language. A clearance was taken from the institutional ethical committee. Single surgeon treated all cases in order to standardize the patient care and asepsis.
Group A patients were operated under local anesthesia and PRGF along with gelatin sponge was placed.
Plasma rich in growth factor (PRGF) preparation
Ten milliliter of blood was obtained from cephalic or basilic veins (due to their accessibility and sufficient diameter) using 19-gauge needle to avoid platelet rupture or activation in the lumen. Sampled blood was immediately transferred to a syringe containing anticoagulant (1 ml of 3.8% sodium citrate for 10 ml blood) and centrifuged in PRGF system set for 460 G in 8 min, to sediment platelets. Three layers were isolated after centrifugation: Red blood cells (RBCs) at the bottom, white blood cells in the middle, and plasma on the surface. The surface plasma itself contained three distinct layers:
Platelet poor in growth factors (PPGF) on the top (500 μl).
Plasma growth factors (PGF) in the middle and PRGF at the bottom that contains the highest concentrations of platelets and growth factors accumulated right above the RBCs [Figure 1].
Figure 1.

Test tube containing plasma rich in growth factors
PPGF and PGF were separately retrieved using two 500 μl pipettes. CaCl2 was then added to PRGF (0.05 ml/ml) and coagulation was obtained within 5-8 min. To decrease coagulation time, PRGF was placed on a thermal block prior to activation to reach body temperature (37°C). Finally the formed gel was placed in the socket with help of gelatin sponge [Figures 2 and 3].[11]
Figure 2.
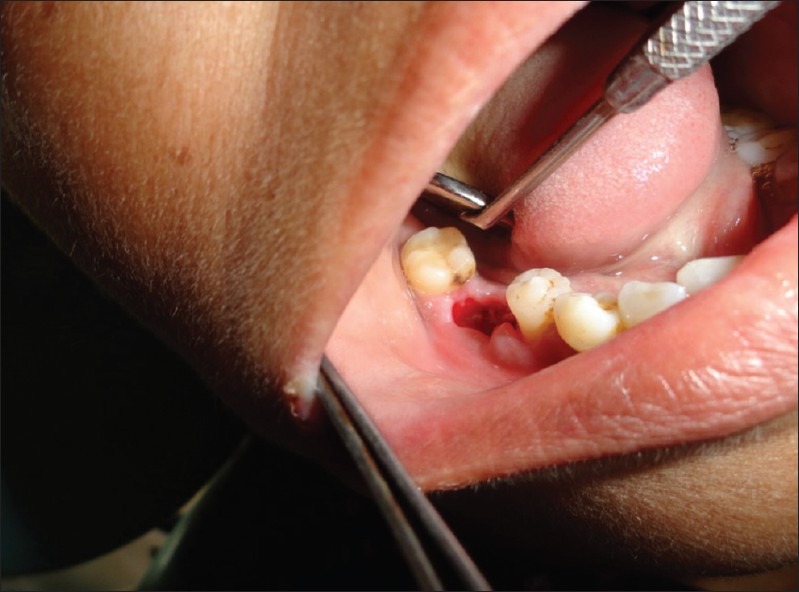
Pre-operative status on day 0
Figure 3.
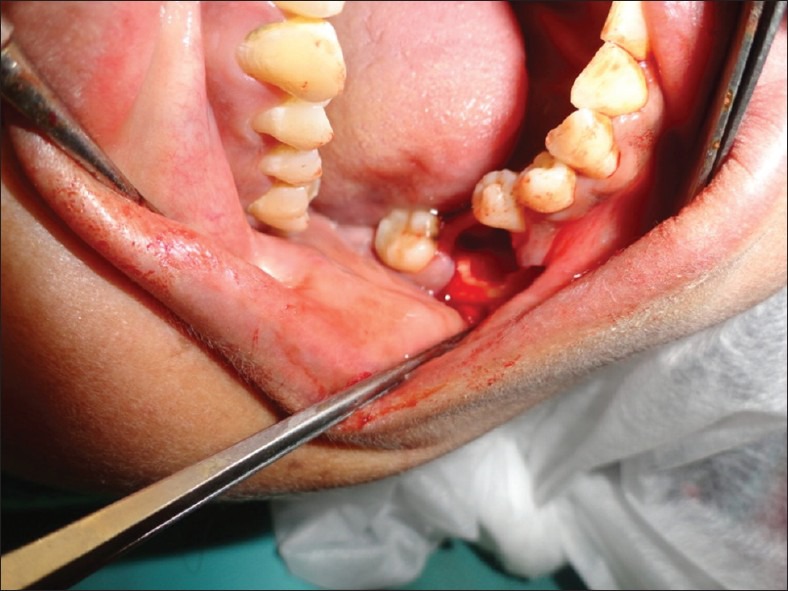
The day 0 status of the dry socket being prepared for the placement of gelatin sponge with PRGF
Patients in group A were treated with this gel placed in the socket. Observation was noted on 1st, 2nd, 3rd, 7th, and 15th days postoperatively for pain and healing [Figure 4].
Figure 4.
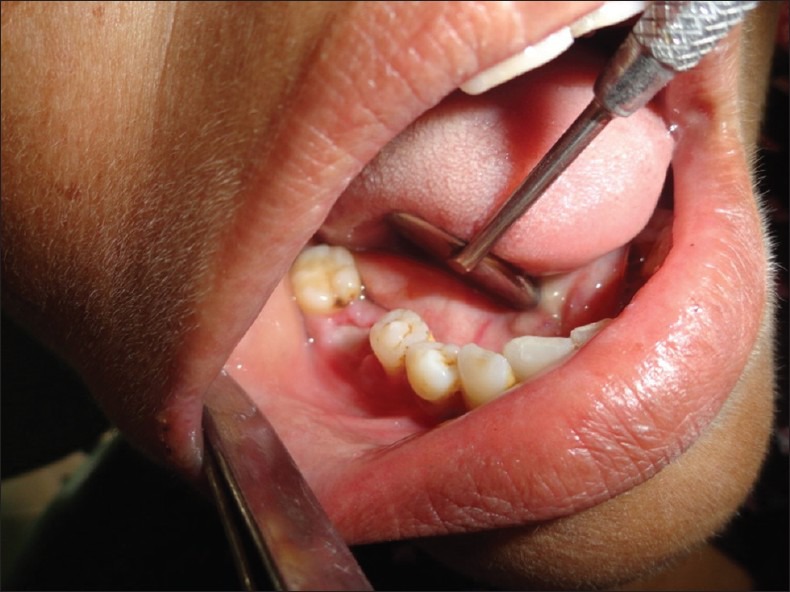
The 7th day status of socket treated with gelatin sponge soaked in PRGF
Zinc oxide eugenol dressing was done in group B cases after proper cleaning and irrigation of the socket without local anesthesia. Observation was noted on 1st, 2nd, 3rd, 7th and 15th days postoperatively for pain and healing.
In group C, i.e., control group, patients were treated only by irrigation with saline without any other manipulation of the socket or any medicament.
The pain was measured on a visual analog scale as follows:
0 – No pain
1 – Slight pain on socket manipulation
2 – Moderate pain on socket manipulation
3 – Severe pain on socket manipulation
4 – Slight continuous pain even in relaxed state
5 – Moderate continuous pain even in relaxed state
6 – Severe continuous pain even in relaxed state
7 – Pt. irritated with pain, not able to relax
8 – Unbearable pain, patient eagerly seeks for relief.
The healing was measured on a scale as follows:
0 – No healing, no clot formation
0.5 – Clot formed/seen
1 – Clot stabilized
1.5 – 1/2 of socket epithelialized and covered
2 – 2/3 of socket epithelialized and covered
2.5 – Epithelialization almost complete, wound closed
3 – Socket appears closed with normal mucosa coverage.
The data were processed with statistical package for social sciences (SPSS) version 15.0 for windows statistical software (SPSS Inc., Chicago, IL, USA). Probability level of P < 0.05 was considered statistically significant.
Results
The total number of patients included in this study was 45, which includes 26 females and 19 males.
Table 1 shows age wise distribution of patients suffering from dry socket. In groups A and B, there was no significant difference of age, the mean being 31.05, 32.57, and 31.98 years respectively.
Table 1.
Age distribution in groups A, B, and C
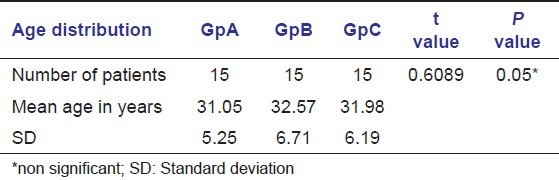
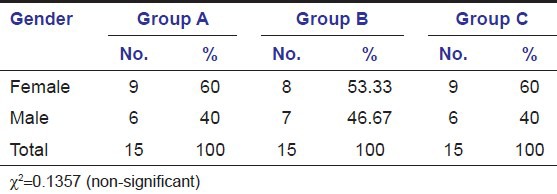
Table 2 shows sex wise distribution of patients suffering from dry socket. In groups A, B, and C; it was found that there was equal distribution of sex in both the groups.
Table 2.
Gender distribution in groups A, B, and C
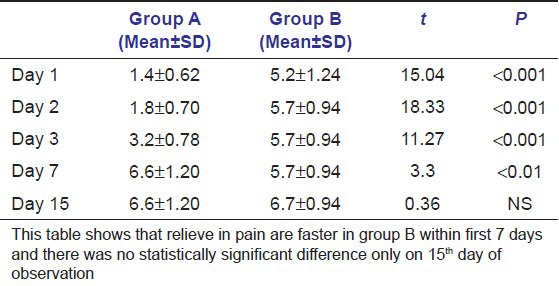
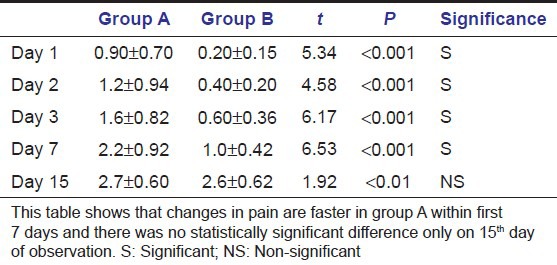
Tables 3a and b show that probability is less than 0.0001. This suggests that there were significant differences in treatment outcomes in different groups. Table 4 shows pain reduction is more rapid in group B than in groups A and C from day 1 to day 7 but the change is non-significant at day 15 in all the groups.
Table 3a.
Comparison of pain among different groups. The results of an analysis of variance statistical test
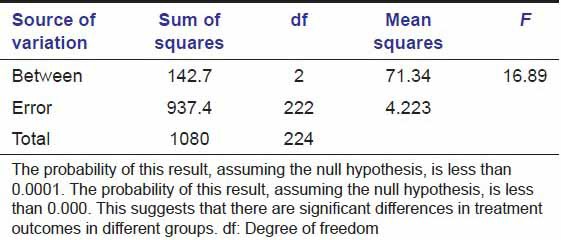
Table 3b.
Comparison of pain among different groups
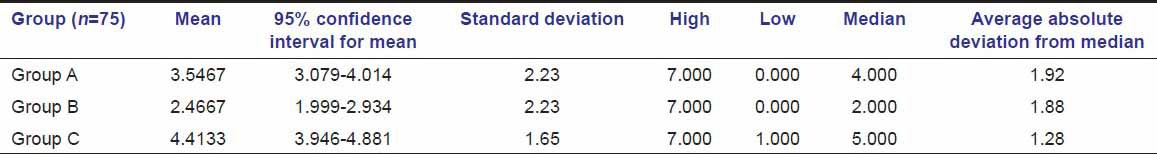
Table 4.
Comparison of change in pain in groups A and B
Tables 5a and b show that probability is less than 0.0001. This suggests that there were significant differences in treatment outcomes in different groups [Figures 2–4]. Table 6 shows healing is faster in group A than in group B at day 1, day 2, day 3, day 7, and it was significant; whereas at day 15, change is non-significant in both the groups. Group C lagged behind in complete healing still after 15 days.
Table 5a.
Healing comparison between groups. The results of an analysis of variance statistical test
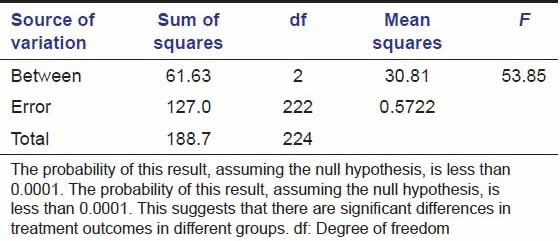
Table 5b.
Healing comparison between groups
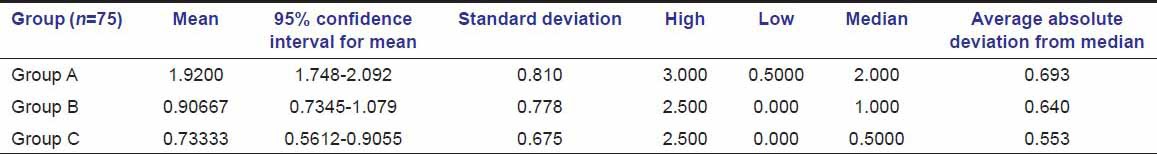
Table 6.
Comparison of healing in groups A and B
Discussion
Dry socket is a complication of extraction. In this study, the disease had female predilection, the cause may be the use of oral contraceptives.[5,12] The mean age was reported to be 31.05 years in group A, 32.57 years in group B, and 31.98 years in group C, which are close to the age group reported in the literature.[13]
The rationale for using PRGF along with gelatin sponge was based on previous studies, which showed the potential of PRGF in the process of bone healing.[10,14] PRGF contains platelets, growth factors and fibrinogen.[15] Alpha granules of platelets include a high concentration of growth factors such as plateled derived growth factor (PDGF), tissue growth factor (TGF), platelet derived endothelial growth factor (PDEGF), platelet derived angiogenesis factor (PDAF), interstitial growth factor IGF-1, and platelet factor 4 (PF-4). These factors increase tissue vascularity through increased angiogenesis, chemotaxis of macrophages and fibroblasts, increased granulation tissue production and epithelialization, enhanced osteogenesis. These might also act antimicrobial effect and provide with an immediate surgical chemostatic agent that is biocompatible, effective and safe. Recent reports have suggested that more rapid epithelialization, denser and mature bone with better-organized trabeculae and greater bone regeneration occurs with platelet rich plasma. Gelatin sponge may act as a scaffold and as a good carrier of osteoblasts due to its flexibility, biocompatibility, and biodegradability. Different studies[16,17] had reported the use of Platelet-rich Plasma PRP in extraction socket. We had reported the contributing effect of PRGF along with gelatin sponge in the socket healing. In our study, healing is faster in group A as compared to group B. These findings of better healing in group A may be supported by the previous studies of PRGF.[18,19] Resolution of pain is earlier in group B as compared to group A, which may be due to the obtundant nature of eugenol.[8] The use of PRGF along with gelatin sponge significantly enhances the healing of dry socket as compared to zinc oxide eugenol or irrigation only; but in terms of pain relief, zinc oxide eugenol dressing was more effective than others. In this study, disease was observed to have female predilection and the mean age was found to be 31.5 years.
Our study data indicated that with the use of PRGF along with gelatin sponge frequent visits and time may be saved.
Conclusion
The combination of gelatin sponge along with PRGF seems to accelerate socket healing due to the growth factors incorporated in the PRGF and scaffold forming capability of gelatin sponge.
However, a long-term randomized study with bigger sample size and control is required to determine the effectiveness of PRGF along with gelatin sponge. This is a first endeavor of this kind and is expected to stimulate further exploration into the deeper aspects of advantages of PRGF along with gelatin sponge in the healing of dry socket.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Kolokythas A, Olech E, Miloro M. Alveolar osteitis: Acomprehensive review of concepts and controversies. Int J Dent. 2010 doi: 10.1155/2010/249073. 249073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanson AE. A double-blind study on the effectiveness of tetracycline in reducing the incidence of fibrinolytic alveolitis. J Oral Maxillofac Surg. 1989;47:165–7. doi: 10.1016/s0278-2391(89)80110-7. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso CL, Rodrigues MT, Ferreira Júnior O, Garlet GP, de Carvalho PS. Clinical concepts of dry socket. J Oral Maxillofac Surg. 2010;68:1922–32. doi: 10.1016/j.joms.2009.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Nusair YM, Younis MH. Prevalence, clinical picture, and risk factors of dry socket in a Jordanian dental teaching center. J Contemp Dent Pract. 2007;8:53–63. [PubMed] [Google Scholar]
- 5.Parthasarathi K, Smith A, Chandu A. Factors affecting incidence of dry socket: A prospective community-based study. J Oral Maxillofac Surg. 2011;69:1880–4. doi: 10.1016/j.joms.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Birn H. Etiology and pathogenesis of fibrinolytic alveolitis (“dry socket”) Int J Oral Surg. 1973;2:211. [Google Scholar]
- 7.Anitua E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35. [PubMed] [Google Scholar]
- 8.Schofield ID, Warren BA, Rozanis J. Review of localized osteitis. J Can Dent Assoc. 1980;46:189–94. [PubMed] [Google Scholar]
- 9.MacGregor AJ. Treatment of dry socket by general dental practitioners. Br Dent J. 1967;122:63–5. [PubMed] [Google Scholar]
- 10.Martínez-Zapata MJ, Martí-Carvajal A, Solà I, Bolibar I, Angel Expósito J, Rodriguez L, et al. Efficacy and safety of the use of autologous plasma rich in platelets for tissue regeneration: A systematic review. Transfusion. 2009;49:44–56. doi: 10.1111/j.1537-2995.2008.01945.x. [DOI] [PubMed] [Google Scholar]
- 11.Anitua E, Orive G. Use of PRGF to accelerate bone and soft tissue regeneration in postextraction sites. Implant Dialogue. 2003;36:3–14. [Google Scholar]
- 12.Alexander RE. Dental extraction wound management: A case against medicating postextraction sockets. J Oral Maxillofac Surg. 2000;58:538–51. doi: 10.1016/s0278-2391(00)90017-x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Safar MT, Al-Sandook TA, Suleiman MS. Protective effect of topical ibuprofen against dry socket. Al-Rafiadain. Dent J. 2008;8:136–43. [Google Scholar]
- 14.Plachokova AS, Nikolidakis D, Mulder J, Jansen JA, Creugers NH. Effect of platelet-rich plasma on bone regeneration in dentistry: A systematic review. Clin Oral Implants Res. 2008;19:539–45. doi: 10.1111/j.1600-0501.2008.01525.x. [DOI] [PubMed] [Google Scholar]
- 15.Aldecoa EA, Ortiz Andia Esabel. Vitoria S.L., Spain: Puesta al dia publication; 2001. Mar, A New Approach to Bone Regeneration: Plasma Rich in Growth Factors. Ch. 9; p. 172. Ch 9. [Google Scholar]
- 16.Sammartino G, Tia M, Marenzi G, di Lauro AE, D’Agostino E, Claudio PP. Use of autologous platelet-rich plasma (PRP) in periodontal defect treatment after extraction of impacted mandibular third molars. J Oral Maxillofac Surg. 2005;63:766–70. doi: 10.1016/j.joms.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Simon D, Manuel S, Geetha V, Naik BR. Potential for osseous regeneration of platelet-rich plasma: A comparative study in mandibular third molar sockets. Indian J Dent Res. 2004;15:133–6. [PubMed] [Google Scholar]
- 18.Mozzati M, Martinasso G, Pol R, Polastri C, Cristiano A, Muzio G, et al. The impact of plasma rich in growth factors on clinical and biological factors involved in healing processes after third molar extraction. J Biomed Mater Res A. 2010;95:741–6. doi: 10.1002/jbm.a.32882. [DOI] [PubMed] [Google Scholar]
- 19.Rutkowski JL, Johnson DA, Radio NM, Fennell JW. Platelet rich plasma to facilitate wound healing following tooth extraction. J Oral Implantol. 2010;36:11–23. doi: 10.1563/AAID-JOI-09-00063. [DOI] [PubMed] [Google Scholar]


