Abstract
Aim:
The aim of this study was to evaluate the effects of hyaluronan (HA) and chlorhexidine (CHX) gels as adjunct to scaling and root planing (SRP) in the treatment of chronic periodontitis.
Materials and Methods:
Sixty patients within the age group of 30-65 years recruited to participate in the study were randomly equally divided into three groups. Complete SRP and subgingival debridement were performed within 6 h in all the patients. For control (Group I) patients, SRP was the only treatment modality given; for Group II and III patients, at least 8 teeth with 4-8 mm probing pocket depth (PPD) were selected for subgingival application of HA gel and CHX gel, respectively. Clinical periodontal parameters such as gingival index, PPD, and clinical attachment level (CAL) were recorded at baseline and 3 months, whereas plaque index was recorded at baseline, 1 month, and 3 months. For measuring systemic/hematological parameters, blood samples for laboratory tests for total leucocyte count (TLC), differential leucocyte count (DLC), and C-reactive protein (CRP) were obtained using standard 2-mL syringe from each subject in all the three groups at baseline, 24 h, and on the 1 month and 3 months post-baseline.
Results:
In all the three groups, a significant reduction in PPD and gain in CAL were observed between baseline and 3 months follow-up (P< 0.05); however, at 3 months, change in PPD and CAL was more in Group II than Group III, but the difference was non-significant, and Group I (control) showed less changes in PPD and CAL than both experimental groups. Only one patient revealed positive value for CRP at baseline only, and hence could not be statistically analyzed. In all the three groups, the peak values for TLC count were observed at 24 h. At 1-month and 3-month intervals, a significant improvement in TLC and DLC counts was observed among the experimental (HA gel/SRP and Xan-CHX gel) groups as compared to control group (SRP alone).
Keywords: Chlorhexidine, chronic periodontitis, hyaluronan, total leucocyte counts
Introduction
Scaling and root planing (SRP) is effective means of treating and controlling periodontal disease; however, the ability of the clinician to gain access to deep pockets often results in a substantial variation in its effectiveness, and therefore to compensate this technical limitations, and to prevent early microbial re-colonization, the adjunctive use of antimicrobials or anti-inflammatory agents may be indicated to ensure the best chance for clinical improvements.[1] The recent development of sophisticated, subgingivally placed delivery systems has provided the possibility of maintaining effective, intra-pocket levels of antimicrobial agents for extended periods of time.
Advanced xanthan-based 1.5% chlorhexidine (CHX) gel (CHLO-SITE®, Ghimas, Italy) is supplied in syringe (0.5 mL) with a special needle having a blunt tip and a lateral opening. The rationale for the adjunctive use of xanthan gum in a subgingival gel carrier relates to the increased viscosity of the carrier and in the bioadhesive properties of the polysaccharides, both of which may limit the clearance of CHX (occluding effect) from the periodontal pocket.[2] Hyaluronan (HA) gel (GENGIGEL® Ricerfarma, Milan, Italy) is a novel product that contains high molecular weight fraction of hyaluronic acid that is produced by a non-animal-derived biotechnological process. Hyaluronan is a linear polymer derived from two repeating disaccharide subunits (D-Glucuronic acid and N-acetylglucosamine), and is a natural constituent of the body's glycosaminoglycan (GAG) population.[3] Johannsen et al.[3] reported that the adjunctive use of hyaluronic acid after thorough mechanical debridement potentially has major clinical benefits in terms of improved healing after non-surgical periodontal (NSP) therapy.
Recent years have seen the revival of the concept that periodontitis may have an etiological or modulating role in other systemic diseases; however, there is still much debate regarding the nature and degree to which this may happen.[4] Evidence has indicated that patients with severe periodontitis have increased level of inflammatory and hematological markers such as C-reactive protein (CRP),[5] white blood cells (WBC) counts, and neutrophil counts,[6] and it has also been shown by many[7,8,9] that SRP lowers systemic inflammation. The aim of this study was to evaluate clinical and systemic effects of CHX and hyaluronan gels as adjunct to SRP in the treatment of chronic periodontitis.
Materials and Methods
This study was conducted in the Department of Periodontology, Saraswati Dental College and Hospital, Lucknow (Uttar Pradesh), India from December 2009 to June 2012. Ethical clearance for the study was obtained from Saraswati Dental College's Human Research Ethical Committee. Motivated, non-smoker, non-alcoholic patients with non-contributory medical history suffering from generalized chronic periodontitis were selected among the patients visiting the Department of Periodontics for this randomized clinical study. An informed consent was obtained after fully explaining about possible risk and procedure.
Inclusion criteria
Patients between the age group of 30-65 years, who were diagnosed as moderate to severe generalized chronic periodontitis, having at least 20 teeth, minimum of 8 teeth with probing pocket depth (PPD) of 4-8 mm, and should not have received any antibiotic and periodontal therapy for past 6 months were included for the study.
Exclusion criteria
Patients with known allergy to hyaluronic acid and CHX, pregnant women, and nursing mothers were excluded. Teeth with periapical disease and sites with overhanging restorations were also not included for the study.
Clinical parameters
Clinical parameters, PPD and clinical attachment level (CAL), were measured using University of North Carolina (UNC)-15 probe (Hu-Fridey Instruments, Chicago, IL, USA) in the periodontal pocket parallel to the vertical axis of the tooth to nearest millimeter up to 1 mm. PPD, CAL, and gingival index (GI) (Loe and Silness)[10] were recorded at baseline and 3 month, whereas plaque index (PI) (Silness and Loe)[10] was recorded at baseline, 1 month, and 3 months.
Systemic parameters
Vital signs, temperature (T), pulse (P), respiratory rate (R), and blood pressure (BP) were recorded by digital thermometer and digital BP apparatus. Serum levels of CRP were determined using a latex slide agglutination method with commercially available chair side kit (SPAN-CRP™, Span Diagnostic Ltd.). Total leucocyte/WBC and differential leucocyte count (DLC) were measured by digital machine (CELL-TECH™ Machine). All systemic parameters were recorded at base line, after 24 h, 1 month, and 3 months.
Methodology
Sixty patients within the age group of 30-65 years were recruited to participate in the study. Complete SRP and subgingival debridement were performed within 6 h[11] using magnetostrictive ultrasonic scalers and tips No. TFI 3 and 10 (Cavitron, Dentsply), and Gracey curettes (Hu-Fridey Instruments, Chicago, IL, USA). Local anesthesia in the form of infiltration and spray were used as and when required. After debridement, patients were randomly selected to form control (Group I) and experimental (Group II and Group III) groups, consisting of 20 patients in each group.
For Group I (control) patients, SRP was the only treatment modality given and eight teeth with 4-8 mm PPD were selected for measuring the clinical parameters. For Group II patients, at least eight teeth with 4-8 mm PPD received subgingival application of hyaluronan (HA) gel. Selected teeth were isolated and dried with cotton rolls. Prefilled hyaluronan gel bulb was loaded into applicator. End of the bulb was cut and placed into the selected periodontal pockets up to the gingival margin (till material flowed out of sulcus) and coe-pak was applied over the experimental sites. For Group III patients, at least eight teeth with 4-8 mm PPD received subgingival application of CHX gel. Selected teeth were isolated and dried with cotton rolls. Blunt needle of prefilled syringe was inserted into the pockets in those sites that had been randomly assigned to receive it and coe-pak was applied over test sites. All the participated patients were asked to maintain meticulous oral hygiene. Coe-pak applied was removed after 10 days.
For measuring systemic/hematological parameters, blood samples for CRP, WBC, and DLC laboratory tests were obtained by means of venous puncture from the antecubital vein using a standard 2-mL syringe and analyzed at the general pathology laboratory of Saraswati Dental College and Hospital, Lucknow. Venous blood samples were obtained from each subject in all the three groups at baseline, 24 h, and on the 1 month and 3 month post-baseline. Serum levels of CRP were determined using a latex slide agglutination method. The test specimen (serum) is mixed with latex reagent and allowed to react. If CRP concentration is greater than 6 mg/L, a visible agglutination is observed. If CRP concentration is less than 6 mg/L, then no agglutination is observed.
Statistical analysis
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0 Statistical Analysis Software (SPSS Package Version 15.0, Lead Technology Incorporated, USA). The values were represented in Number (%) and Mean ± SD. Statistical formulas were used: Mean, standard deviation, Kruskal-Wallis H test, The Wilcoxon signed rank statistic, and Mann-Whitney U test. P < 0.05 was considered statistically significant.
Results
All recruited 60 patients (30 males and 30 females, mean age: 38.17 years ± 8.84 years) completed the study uneventfully. Over the following 3 months, no major changes in the medical history (BP, 132.03 ± 16.74/80.93 ± 10.31 mm Hg; P, 78.6 ± 11.44 beats/mins; T, 37.56°C ± 0.78°C, R, 16.8 ± 1.23 breath/min) were reported at the time of all the study visits.
Clinical periodontal parameters
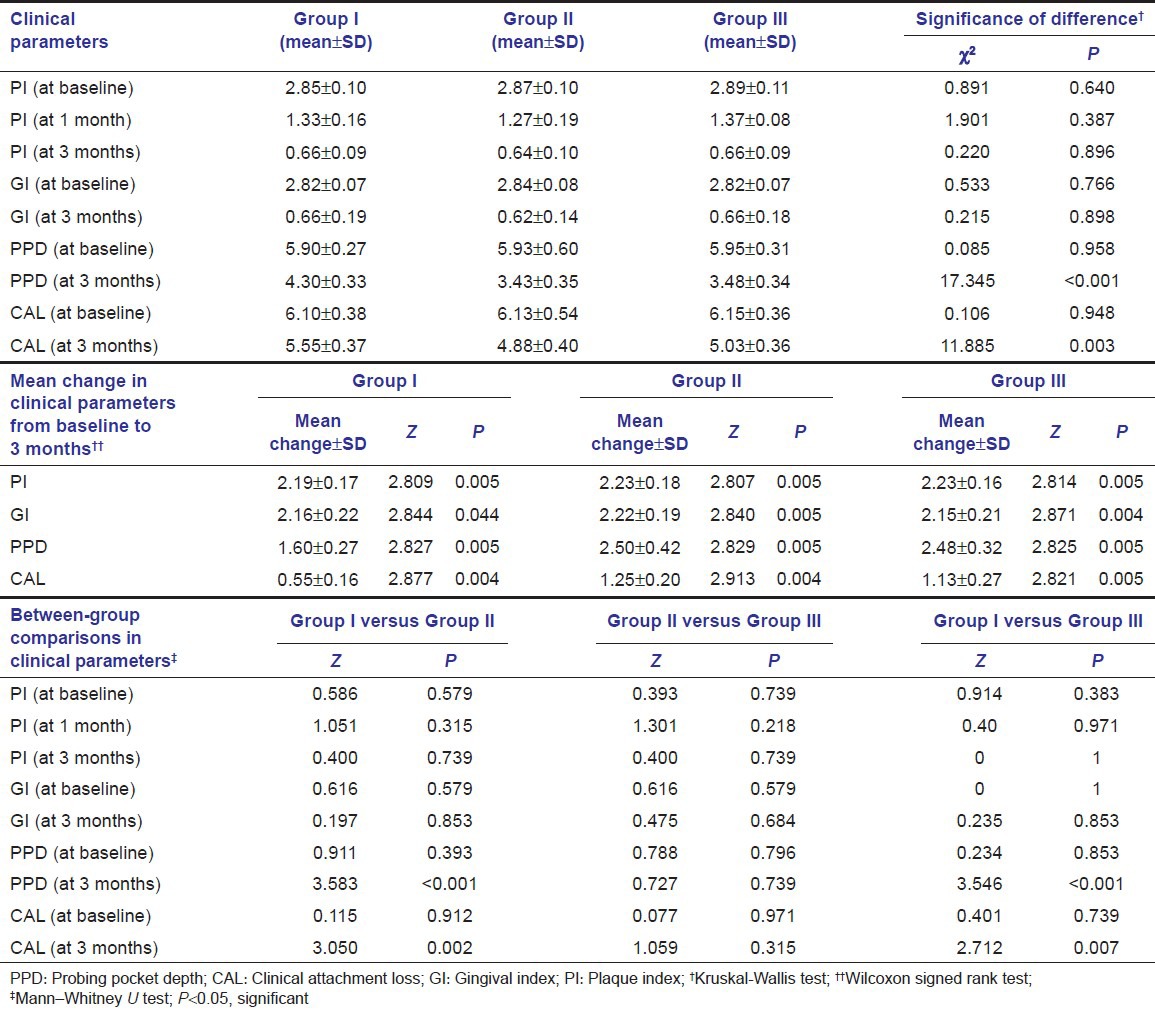
Clinical periodontal parameters in the three study groups are shown in Table 1. In all the three groups, minimum values for PI and GI were observed at 3 months and maximum at baseline. Statistically, there was no significant intergroup difference at different time intervals (P > 0.05). The mean change in the PI was statistically significant (P = 0.005) in all the three groups, at both the follow-up intervals as compared to baseline. Similarly, in all the three groups, the change in mean GI from baseline to 3 month follow-up was significant statistically (P < 0.05). In all the three groups, a significant reduction in PPD and gain in CAL were observed between baseline and 3 months follow-up (P < 0.05); however, at 3 months, change in PPD and CAL was more in Group II than Group III, but the difference was non-significant and Group I (control) showed less changes in PPD and CAL than both experimental groups.
Table 1.
Clinical parameters, mean change, and between-group comparison of means of various parameters at different time intervals of the three study groups
Systemic/hematological parameters
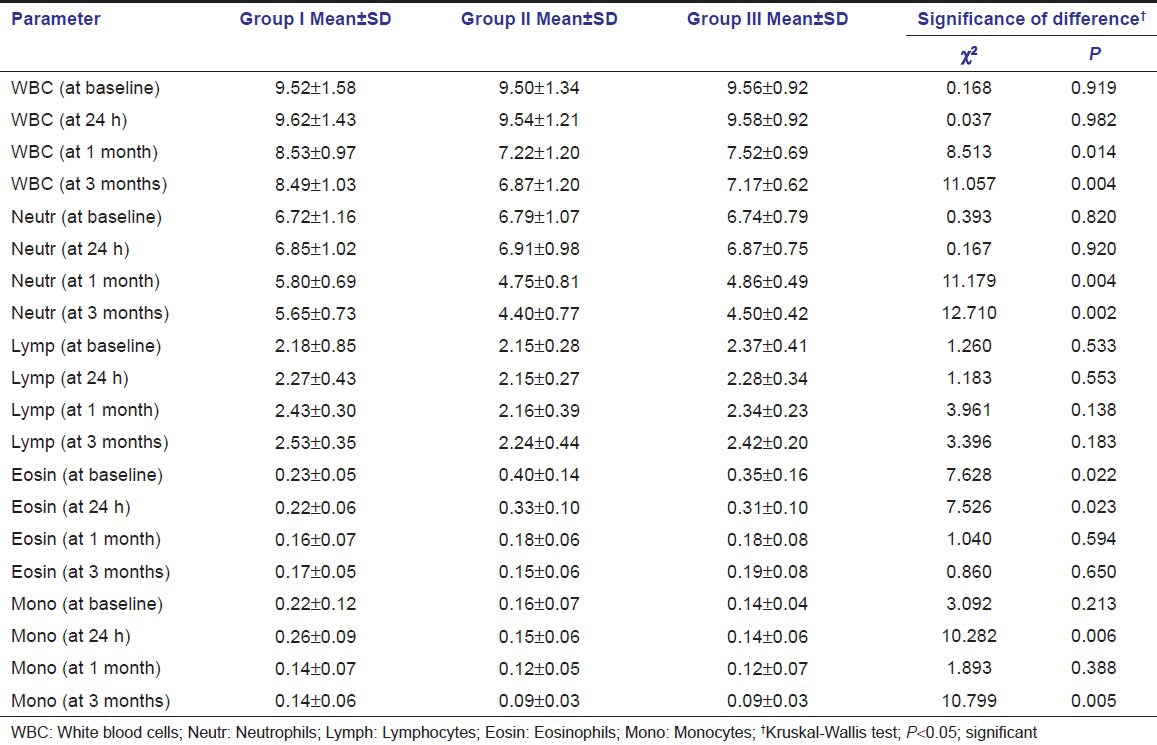
Among all the studied patients, only one patient revealed positive value for CRP at baseline, and hence could not be statistically analyzed. Mean values of systemic/hematological parameters at baseline, 24 h, 1 month, and 3 months, in three study groups, are shown in Table 2. In all the three groups, the peak values for WBC count were observed at 24 h and minimum at 3-month intervals; a significant difference in mean WBC count was observed in all the groups. Similarly, among all the three groups, the peak values were observed at 24 h, whereas minimum values were observed at 3 months for neutrophil, eosinophil, and monocyte counts, whereas slight decrease followed by increase in lymphocyte count was observed at follow-up intervals.
Table 2.
Systemic/hematological parameters in three study groups (in 103/μl)
Intra-group comparison
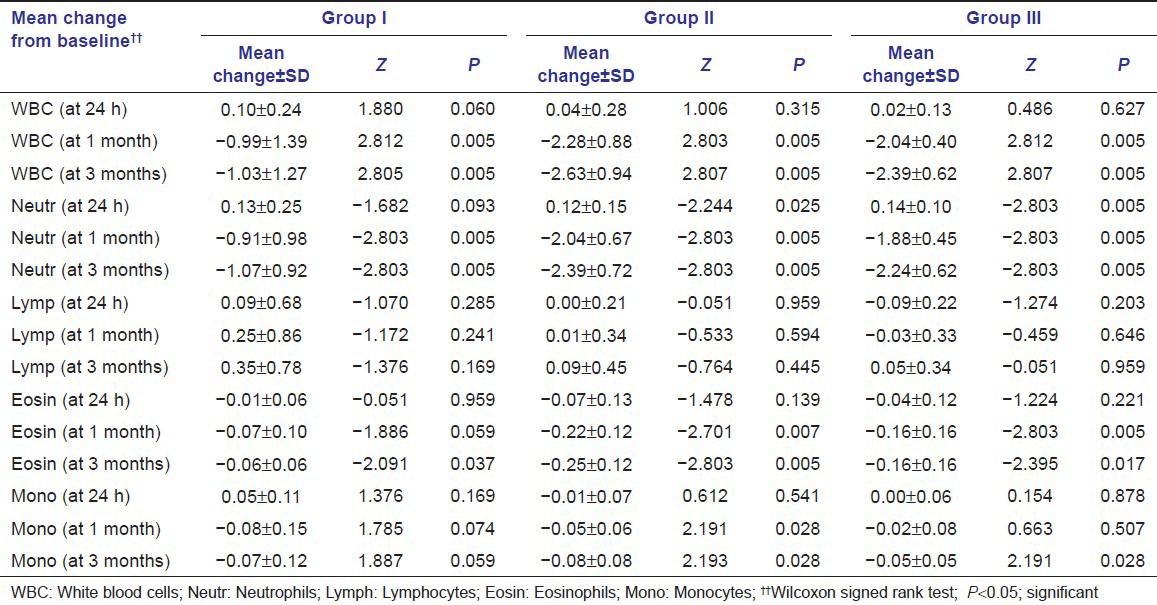
Mean change in systemic/hematological parameters in three study groups at different time intervals is shown in Table 3. In all the three groups, a significant change in mean values of WBC count was observed between baseline to 1 month and baseline to 3-month intervals (P < 0.05). Group II and Group III show more reduction in WBC count than Group I, and Group II showed more reduction than Group III, but it was not significant. At all the time intervals, the change in mean values of neutrophil count from baseline was significant in all the three groups (P < 0.05). It was observed that mean change in Group III is greater than Group II and Group I, and changes in Group II are greater than Group I.
Table 3.
Mean change in systemic parameters in three study groups (in 103/μl)
Inter-group comparison
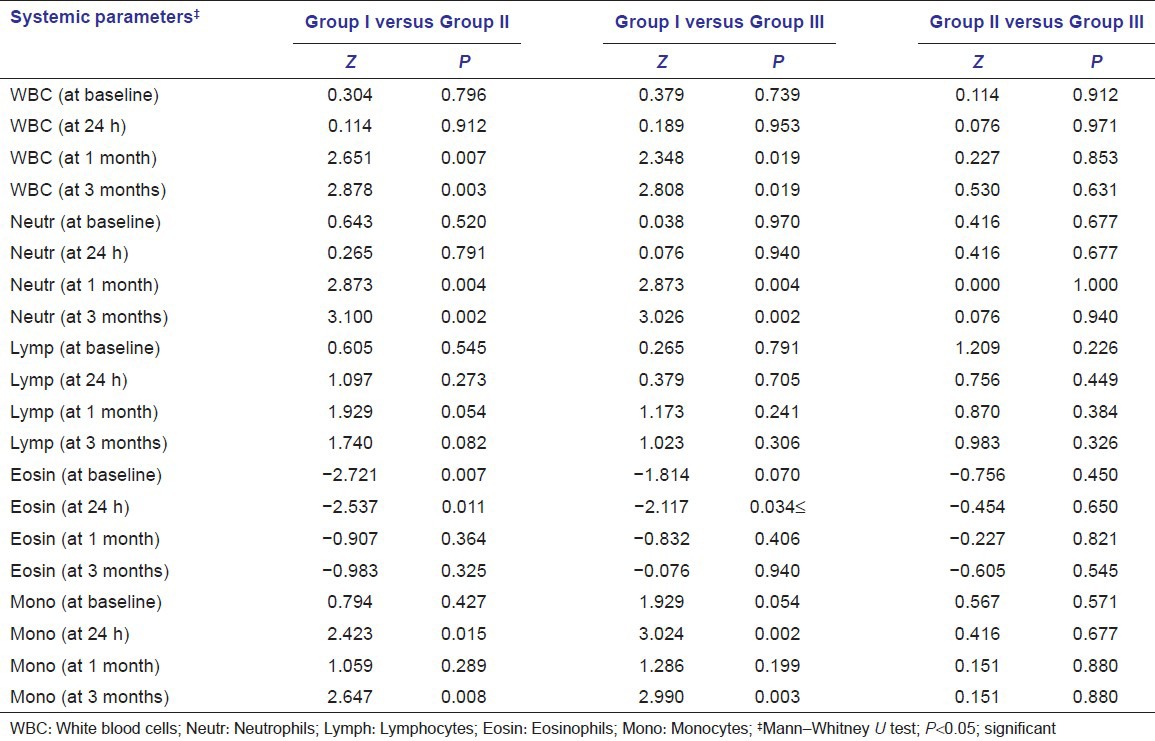
Between groups, comparison of means of various systemic parameters at different time intervals is shown in Table 4. For WBC and neutrophil counts, no significant difference was observed between Groups I and II, and Groups I and III at baseline, and at 24-h interval; however, the difference was significant statistically at 1-month and 3-month intervals. The mean levels of WBC count showed significant reduction in all three groups after treatment.
Table 4.
Between-group comparison of means of various systemic parameters at different time intervals
Discussion
The aim of this preliminary investigation was to evaluate the effects of NSP therapy, with or without the use of local drug delivery (LDD) as an adjunct to SRP, on the clinical periodontal and systemic or hematological parameters. It cannot be ascertained that no interaction between LDD and systemic/hematological parameters occurs, and thus it may be hypothesized that there is possibility of drug absorption from the local sites into the periodontal connective tissues, and hence, into the systemic circulation. Primary outcome of this study revealed that LDD (HA gel and CHX gel) used as an adjunct to SRP can significantly improve the benefits of SRP. Systemic/hematological markers associated with chronic periodontitis significantly improve after SRP with or without LDD. Secondarily, increase in CRP levels associated with moderate to severe chronic periodontitis is less than 6 mg/L, as inferred by our rapid chair side diagnostic test kit.
To overcome validity issues such as carry-over issues and asymmetric disease distribution[12] and to evaluate systemic/hematological inflammatory load due to periodontal disease and NSP treatment, whole mouth design was used for this study.
Both SRP alone (control group) and adjunctive use of HA and CHX gels (experimental groups) resulted in statistically significant reduction in the clinical periodontal parameters. Improved PI and GI were observed due to NSP treatment (SRP with or without LDD) modalities, along with improved oral hygiene practices commonly observed in the study subjects.[3] Similar to already published data,[2,13,14,15,16,17,18] this study also revealed significant improvement in the PPD and CAL after subgingival application of CHX gel as an adjunct to SRP. In contrast to Xu et al.,[19] but similar to our study, Jentsch et al.,[20] Johannsen et al.,[3] and Pistorius et al,[21] also reported significant improvement in gingival and periodontal health after subgingival application of HA as an adjunct. Improvement in clinical periodontal parameters in HA gel/SRP group may be attributed to its ability to promote regeneration and its anti-edema and anti-inflammatory properties that positively influence the local inflammation.[19] However, the difference between the two experimental materials was non-significant in our study.
Owing to its low-grade systemic inflammatory state, increased concentrations of hematological and systemic inflammatory markers is a well-established fact in the patients suffering from periodontitis, when compared with healthy subjects.[22,23] This study also demonstrated elevated systemic/hematological parameters within the limits of normalcy at baseline in all three groups. Also, local and systemic host responses have been proposed to be elicited after SRP that would have aided in eliminating local infection and promoting healing.[13] Results of this study revealed significant improvement in hematological parameters after the NSP therapy using both LDD as an adjunct to SRP and SRP alone. Comparisons of mean WBC count between experimental and control groups at different time intervals reveal no significant difference at baseline and at 24-h interval. However, the difference was significant statistically at 1-month and 3-month intervals. Comparison between different groups revealed more reduction in WBC counts in Group II (HA gel/SRP) followed by Group III (Xan-CHX gel/SRP), and least in Group I (SRP alone), both at 1 and 3 months. Although comparative difference between both experimental groups (Group II and III) was not significant, both experimental groups revealed significant difference from control group (SRP alone).
Similar to D’Aiuto et al.,[11] transient alterations of DLC count (103 /μL) were also observed in this study at 24 h after the NSP therapy in all the groups. Comparison of mean neutrophil between various groups at different time intervals shows no significant difference at baseline and at 24-h interval. At 1 month and 3 months, more reduction in neutrophil count was more in Group II (HA gel/SRP), followed by Group III (Xan-CHX gel/SRP) and least in Group I (SRP alone). No significant difference was observed between Groups II and III at any time interval. Christan et al.[9] also proposed that the significant reduction of leukocyte counts can be observed 3 months after the SRP.
No statistically significant difference was observed among experimental and control groups at any time interval for lymphocyte count; however, eosinophil count shows significant difference between experimental and control groups at 24 h only. For monocyte count, a statistically significant difference was observed between control and experimental groups at 24-h and 3-month intervals. None of the other differences were significant statistically. D’Aiuto et al.[11] also reported statistically significant increase in the number of circulating monocyte at 24 h, and decrease in lymphocyte count after 24 h followed by significant increase in lymphocyte number 1 month after SRP. Loos et al.,[23] Graziani et al.,[22] Sambashivaiah et al.,[24] and Rai and Anand,[7] reported improvement in hematological and inflammatory markers after SRP. D’Aiuto et al.[11] reported early increase in the hematological parameters in the first few days to a week after the periodontal therapy.
Significant difference in the systemic/hematological parameter between the control and experimental groups may be possibly due to the reduction in the systemic inflammatory load that occurs due to the cumulative effect of SRP and absorption of LDD from the local site. Furthermore, it might be noted that increase systemic inflammation after SRP, as reported by Graziani et al.,[22] may have been ameliorated by the anti-inflammatory effects of LDD.
Jentsch et al.[20] reported that absorption of HA through gingival epithelium or skin could not be found. However, their study used supragingival application of HA in gingivitis patients. It is well established that diseased pocket wall revealed progressive degenerative and necrotic changes in the epithelium lead to ulceration of the lateral wall, and thus exposure of the underlying inflamed connective tissue.[25] Also, inadvertent or unintentional curettage that followed SRP, resulting in removal of inflamed and pocket epithelium.[13] El-Sayed et al.[26] reported that removal of pocket lining facilitates placement of HA gel in direct contact with connective tissue to perform its alleged beneficial effect. Hence, subgingival application of LDD systems, immediately after thorough SRP, may lead to absorption of the drug from the local site, as reported in this study. Furthermore, coe-pak at the experimental sites may have reduced the chances of dilution and outflow of the drug by saliva and the gingival crevicular fluid.[27]
Similar to our study, Nagaral et al.[28] also revealed that estimation of serum CRP using a rapid chair side diagnostic test kit with detection limit of 6 mg/L is not of any significance in subjects with chronic periodontitis which signifies that increase in CRP levels associated with moderate to severe chronic periodontitis is less than 6 mg/L.
Multifold beneficial role of hyaluronic acid have been proposed. In the initial stage of inflammation, it bonds with the fibrin clot; stimulates production of cytokines by fibroblasts, keratinocytes, ameloblasts, and osteoblasts, which promote inflammation; and activates inflammatory cells, stimulating migration toward the lesion, phagocytosis, and clearing of pathogens. Increased osteoblast activity by stimulating differentiation and migration of mesenchymal cells, and the physiochemical properties to keep the growth factors responsible for tissue repair in situ by hyaluronic acid have been reported. It is bacteriostatic toward Actinobacillus actinomycetemcomitans, Prevotella intermedia, and Staphylococcus aureus.[29] These properties might reduce bacterial load in the initial stages of healing, and hence, may resulted in better improvement in HA gel/SRP group patients than SRP alone. Similarly, dual-staged released antimicrobial property and enhanced gel structure and substantivity due to cationic charges of the CHX that can interact with the anionic charges of the bioadhesive xanthan gum polymer in Xan-CHX gel[2] may be responsible for better results as compared to SRP alone.
Limitations of the study
To evaluate systemic inflammation, readily available hematological markers, in lieu of sensitive systemic inflammatory markers such as IL-1, TNF-alpha, and IL-6, were used. For the estimation of serum CRP, cost-effective rapid chair side diagnostic test kit with detection limit of 6 mg/L was used, instead of laboratory-assisted CRP detection kit with detection limit of 0.1 mg/L. Also, our results are based on short term, small sample size, and hence, it is suggested that further long-term, large-sample size clinical trial using more sensitive inflammatory markers may be required to quantify the anti-inflammatory effect of LDD.
Future research directions
Beside improvement in clinical periodontal parameters by SRP, LDD may influence systemic/hematological component of the patients. To the best of our knowledge, no published data have reported influence of LDD as an adjunct to SRP on systemic/hematological components, although several reports have confirmed that moderate to severe forms of periodontitis are associated with a mild systemic inflammatory response, as defined by raised serum concentrations of inflammatory markers (CRP, IL-6), and standard periodontal treatment has been shown to reduce the level of systemic inflammation, thus giving strength to the concept that periodontitis contributes to the systemic inflammatory burden of the individual.[11] This study signifies that SRP, with or without LDD, reduces the systemic inflammatory load associated with immuno-inflammatory response to bacterial plaque in chronic periodontitis patients. However, these results can be used as preliminary data to allow designing large-scale interventional study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Cosyn J, Wyn I. A systematic review on the effects of the chlorhexidine chip when used as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J Periodontol. 2006;77:257–64. doi: 10.1902/jop.2006.050216. [DOI] [PubMed] [Google Scholar]
- 2.Paolantonio M, D’Ercole S, Pilloni A, D’Archivio D, Lisanti L, Graziani F, et al. Clinical, microbiologic, and biochemical effects of subgingival administration of a Xanthan-based chlorhexidine gel in the treatment of periodontitis: A randomized multicenter trial. J Periodontol. 2009;80:1479–92. doi: 10.1902/jop.2009.090050. [DOI] [PubMed] [Google Scholar]
- 3.Johannsen A, Tellefsen M, Wikesjö U, Johannsen G. Local delivery of hyaluronan as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J Periodontol. 2009;80:1493–7. doi: 10.1902/jop.2009.090128. [DOI] [PubMed] [Google Scholar]
- 4.Ide M, Jagdev D, Coward PY, Crook M, Barclay GR, Wilson RF. The short-term effects of treatment of chronic periodontitis on circulating levels of endotoxin, C-reactive protein, tumor necrosis factor-alpha, and interleukin-6. J Periodontol. 2004;75:420–8. doi: 10.1902/jop.2004.75.3.420. [DOI] [PubMed] [Google Scholar]
- 5.Gani DK, Lakshmi D, Krishnan R, Emmadi P. Evaluation of C-reactive protein and interleukin-6 in the peripheral blood of patients with chronic periodontitis. J Indian Soc Periodontol. 2009;13:69–74. doi: 10.4103/0972-124X.55840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fredriksson MI, Figueredo CM, Gustafsson A, Bergström KG, Asman BE. Effect of periodontitis and smoking on blood leukocytes and acute-phase proteins. J Periodontol. 1999;70:1355–60. doi: 10.1902/jop.1999.70.11.1355. [DOI] [PubMed] [Google Scholar]
- 7.Rai B, Anand SC. After scaling and root planing lower systemic inflammatory and thrombotic marker of cardiovascular risk. Middle-East J Sci Res. 2007;2:54–6. [Google Scholar]
- 8.Hussain Bokhari SA, Khan AA, Tatakis DN, Azhar M, Hanif M, Izhar M. Non-surgical periodontal therapy lowers serum inflammatory markers: A pilot study. J Periodontol. 2009;80:1574–80. doi: 10.1902/jop.2009.090001. [DOI] [PubMed] [Google Scholar]
- 9.Christan C, Dietrich T, Hägewald S, Kage A, Bernimoulin JP. White blood cell count in generalized aggressive periodontitis after non-surgical therapy. J Clin Periodontol. 2002;29:201–6. doi: 10.1034/j.1600-051x.2002.290303.x. [DOI] [PubMed] [Google Scholar]
- 10.Spolsky VW. Epidemiology of gingival and periodontal diseases. In: Carranza FA, Newman MG, editors. Clinical Periodontology. 8th ed. Banglore: Prism Books Pvt Ltd; 1996. p. 65. [Google Scholar]
- 11.D’Aiuto F, Nibali L, Mohamed-Ali V, Vallance P, Tonetti MS. Periodontal therapy: A novel non-drug-induced experimental model to study human inflammation. J Periodontal Res. 2004;39:294–9. doi: 10.1111/j.1600-0765.2004.00741.x. [DOI] [PubMed] [Google Scholar]
- 12.Hujoel PP, Loesche WJ. Efficiency of split-mouth designs. J Clin Periodontol. 1990;17:722–8. doi: 10.1111/j.1600-051x.1990.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta R, Pandit N, Aggarwal S, Verma A. Comparative evaluation of subgingivally delivered 10% doxycycline hyclate and xanthan-based chlorhexidine gels in the treatment of chronic periodontitis. J Contemp Dent Pract. 2008;9:25–32. [PubMed] [Google Scholar]
- 14.Kranti K, Seshan H, Sameer Z. Clinical evaluation of topical subgingival application of biodegradable xanthan based 1.5% chlorhexidine gel for treatment on periodontal pockets. J Adv Dent Res. 2010;1:47–54. [Google Scholar]
- 15.Cosyn J, Sabzevar MM. A systematic review on the effects of subgingival chlorhexidine gel administration in the treatment of chronic periodontitis. J Periodontol. 2005;76:1805–13. doi: 10.1902/jop.2005.76.11.1805. [DOI] [PubMed] [Google Scholar]
- 16.Paolantonio M, D’Angelo M, Grassi RF, Perinetti G, Piccolomini R, Pizzo G, et al. Clinical and microbiologic effects of subgingival controlled-release delivery of chlorhexidine chip in the treatment of periodontitis: A multicenter study. J Periodontol. 2008;79:271–82. doi: 10.1902/jop.2008.070308. [DOI] [PubMed] [Google Scholar]
- 17.Oosterwaal PJ, Mikx FH, van ‘t Hof MA, Renggli HH. Comparison of the antimicrobial effect of the application of chlorhexidine gel, amine fluoride gel and stannous fluoride gel in debrided periodontal pockets. J Clin Periodontol. 1991;18:245–51. doi: 10.1111/j.1600-051x.1991.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 18.Vinholis AH, Figueiredo LC, Marcantonio Júnior E, Marcantonio RA, Salvador SL, Goissis G. Subgingival utilization of a 1% chlorhexidine collagen gel for the treatment of periodontal pockets. A clinical and microbiological study. Braz Dent J. 2001;12:209–13. [PubMed] [Google Scholar]
- 19.Xu Y, Höfling K, Fimmers R, Frentzen M, Jervøe-Storm PM. Clinical and microbiological effects of topical subgingival application of hyaluronic acid gel adjunctive to scaling and root planing in the treatment of chronic periodontitis. J Periodontol. 2004;75:1114–8. doi: 10.1902/jop.2004.75.8.1114. [DOI] [PubMed] [Google Scholar]
- 20.Jentsch H, Pomowski R, Kundt G, Göcke R. Treatment of gingivitis with hyaluronan. J Clin Periodontol. 2003;30:159–64. doi: 10.1034/j.1600-051x.2003.300203.x. [DOI] [PubMed] [Google Scholar]
- 21.Pistorius A, Martin M, Willershausen B, Rockmann P. The clinical application of hyaluronic acid in gingivitis therapy. Quintessence Int. 2005;36:531–8. [PubMed] [Google Scholar]
- 22.Graziani F, Cei S, Tonetti M, Paolantonio M, Serio R, Sammartino G, et al. Systemic inflammation following non-surgical and surgical periodontal therapy. J Clin Periodontol. 2010;37:848–54. doi: 10.1111/j.1600-051X.2010.01585.x. [DOI] [PubMed] [Google Scholar]
- 23.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–34. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 24.Sambashivaiah S, Rebentish PD, Kulal R, Bilchodmath S. One stage vs two stage non-surgical periodontal therapy and their effect on WBC count. Int J Clin Dent Sci. 2010;1:38–44. [Google Scholar]
- 25.Carranza FA, Camargo PM. The periodontal pocket. In: Newman MG, Takei HH, Klokevold PR, Carranza FA, editors. Carranza's Clinical Periodontology. 11th ed. New Delhi: Elsevier Publication; 2011. pp. 127–39. [Google Scholar]
- 26.Fawzy El-Sayed KM, Dahaba MA, Aboul-Ela S, Darhous MS. Local application of hyaluronan gel in conjunction with periodontal surgery: A randomized controlled trial. Clin Oral Investig. 2012;16:1229–36. doi: 10.1007/s00784-011-0630-z. [DOI] [PubMed] [Google Scholar]
- 27.Abrishami M, Iramloo B, Ansari G, Eslami G, Bagheban AA, Anaraki M. The effect of locally delivered xanthan-based CHLO-SITE gel with scaling and root planing in the treatment of chronic periodontitis: Microbial findings. Dent Res J. 2008;5:47–52. [Google Scholar]
- 28.Nagarale G, Ravindra S, Thakur S, Setty S. Efficacy of a chairside diagnostic test kit for estimation of C-reactive protein levels in periodontal disease. J Indian Soc Periodontol. 2010;14:213–6. doi: 10.4103/0972-124X.76919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanden Bogaerde L. Treatment of infrabony periodontal defects with esterified hyaluronic acid: Clinical report of 19 consecutive lesions. Int J Periodontics Restorative Dent. 2009;29:315–23. [PubMed] [Google Scholar]


