Abstract
Background:
Transition of the normal oral epithelium to dysplasia and to malignancy is featured by increased cell proliferation. To evaluate the hypothesis of distributional disturbances in proliferating and stem cells in oral epithelial dysplasia and oral squamous cell carcinoma (OSCC).
Aim:
To evaluate layer wise expression of Ki-67 in oral epithelial dysplasia and in OSCC.
Materials and Methods:
Thirty histologically confirmed cases of oral epithelial dysplasia, fifteen cases of OSCC and five cases of normal buccal mucosa were immunohistochemically examined and nuclear expression of Ki-67 was counted according to basal, parabasal, and suprabasal layers in epithelial dysplasia and number of positive cells per 100 cells in OSCC as labeling index (LI).
Results:
Suprabasal expression of Ki-67 increased according to the severity of epithelial dysplasia and the difference was statistically significant (P < 0.001). The mean Ki-67LI was 12.78 for low risk lesions, 28.68 for high risk lesions, 39.45 for OSCC and 13.6 for normal buccal mucosa.
Conclusion:
The results of the present study demonstrate the use of proliferative marker Ki-67 in assessing the severity of epithelial dysplasia. Suprabasal expression of Ki-67 provides an objective criteria for determining the severity of epithelial dysplasia and histological grading of OSCC.
Keywords: Ki-67, oral epithelial dysplasia, oral squamous cell carcinoma
Introduction
Proliferation is considered to be a fundamental biological process because of the role it plays in the growth and maintenance of tissue homeostasis. It is well understood that transition of the normal oral epithelium to dysplasia to malignancy is featured by increased cell proliferation. Discovery of various proliferation markers has enabled the detection of the hyperactive state of the epithelium and has been suggested to be of prognostic significance.[1] The basal layer is the only proliferative compartment for normal oral epithelium, whereas in the rest of the epithelial layers, cellular maturation is produced without any proliferative activity. Hence, any sign of proliferative cellular activity beyond the basal layer should be considered as a warning sign.
The expression of the human Ki-67 protein is strictly associated with cell proliferation. During interphase, the antigen can be exclusively detected within the nucleus, whereas in mitosis most of the protein is relocated to the surface of the chromosomes. The fact that the Ki-67 protein is present during all active phases of cell cycle (G1, S, G2, and mitosis), but is absent from resting cells (G0), makes it an excellent marker for determining the so called growth fraction of a given population. The fraction of Ki-67 positive cells (the Ki-67 labeling index [LI]) is often correlated with the clinical course of the disease.[2]
The Ki-67 protein as a molecular marker of proliferating cells has been extensively examined in oral epithelial dysplasia and oral squamous cell carcinoma (OSCC), and the number of proliferating cells increased according to the grade of dysplasia. Various authors have showed Ki-67 to be over expressed in the suprabasal epithelium and its expression increased with the severity of dysplasia.[3] Several studies have also revealed that cell proliferation in invasive tumors measured by Ki-67 highly correlated with histologic grading in human oral squamous cell carcinoma (OSCC).[4]
These histological examinations, however, for proliferating cells and alteration of stem cells have mainly focused on the total number of positive cells within the epithelium as an index of malignancy rather than for architectural distribution within the altered epithelium.
To evaluate the hypothesis that the distributional alteration of proliferating cells and stem cells within epithelial dysplasia may be a useful index to estimate the grading and development of epithelial precursor lesions, we immunohistologically examined the detailed distribution patterns of proliferating cells by an antibody for Ki-67 protein in oral epithelial dysplasia and OSCC.
Materials and Methods
The study material comprised of archival formalin fixed paraffin embedded sections from Department of Oral and Maxillofacial Pathology Saraswati Dental College and Hospital, Lucknow. The study group included 30 cases of epithelial dysplasis, 15 cases of OSCC (5 well differentiated, 5 moderately differentiated, and 5 poorly differentiated) and 5 cases of normal buccal mucosa as control. Normal tonsil tissue with Ki-67 positivity was taken as positive control [Figure 1].
Figure 1.
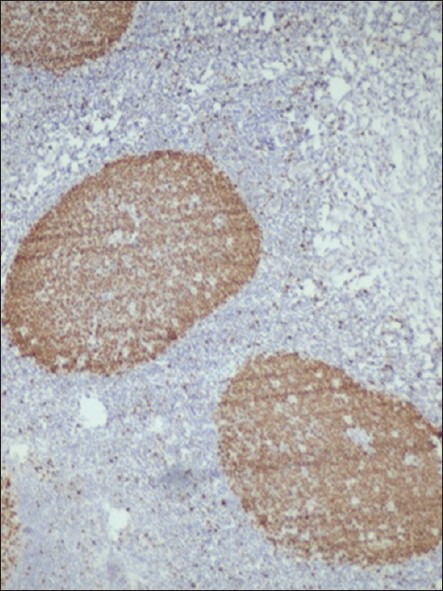
Photomicrograph showing Ki-67 expression in follicular area of tonsil (×200)
The 30 cases of oral epithelial dysplasia were further grouped according to binary system of grading proposed by Kujan et al.[5]
Low-riskgroup (15 cases) comprised of hyperkeratosis, hyperplasia, and mild epithelial dysplasia cases.
High-riskgroup (15 cases) comprised of moderate epithelial dysplasia, severe epithelial dysplasia, and carcinoma in situ cases.
Immunohistochemical procedure
Immunohistochemical (IHC) detection of Ki-67 was performed using DAKO-LSAB-2HRP detection system. For IHC staining the sections were cut at approximately 3 μm thickness using rotatory manual microtome. Two consecutive sections were cut from each block and one was stained with H and E and the other was used for IHC staining. For IHC, sections were placed on pre-coated slides and incubated for 1 h at 60°C in incubator. Later the sections were dewaxed in xylene (two changes) and then were rehydrated in graded alcohol.
Positive control consisted of paraffin embedded sections of tonsils with known antigenic reactivity to Ki-67 in the lymphoid follicles and a negative control was performed in all cases by omitting the step of primary antibody during the staining, which resulted in lack of staining in all cases.
For antigen retrieval, the sections were placed in a 1mM citrate buffer (pH 6) and microwave was used with cycles of high, medium high, low and very low each lasting for 5 min and then cooled to room temperature. The endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min followed by washing in 0.05mMTris-buffered saline (TBS) at pH 7.4. The sections were incubated with precisely diluted mouse monoclonal antibodies against Ki-67 (MIB-1predilutedDako, Japan) as primary antibodies for 1 h at 37°C. Subsequently, after washing in TBS, the sections were incubated with a secondary antibody conjugated with peroxidase-labeled dextran polymers (Envision + Dual Link/HRPSystem, Dako) for 30 min at room temperature. After rinsing with TBS, they were treated with 0.5 mg/ml3,3’-diaminobenzidinesolution containing 0.001% hydrogen peroxide to visualize reaction products, and counterstained with Mayer's hematoxylin for 3 min.
Counting criteria
The nuclear expression of Ki-67 was counted according to epithelial layers as the basal layer, nuclei positive just above the basement membrane; parabasal layer, nuclei positive within two layers above the basement membrane and next to the basal layer; and suprabasal layer, nuclei positive in a more upper layer above the parabasal layer, using a microscope at × 400. LI of Ki-67 was calculated by quantitatively assessing five non-overlapping fields at × 400 and photographs were taken using Olympus E-330 DSLR (manufacturer: Olympus Corporations of America) camera. The photographs were then analyzed using Image-Pro Express 6.0 for Windows manufacturer details: Media cybernetics, Inc. U.S.A (manufacturer: Media cybernetics, Inc. U.S.A). Total number of positive cells in each layer was counted in epithelial dysplasia and normal buccal mucosa as well as the total number of cells in each layer, i.e., basal, parabasal, and suprabasal [Figure 2].
Figure 2.
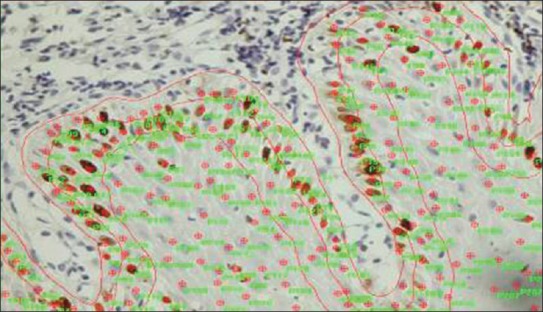
Photomicrograph showing Image Pro Express used for counting of Ki-67 positivecells in basal, parabasal, and suprabasal layers of epithelium
For OSCC total number of positive cells and also total number of cells were counted and the LI was calculated.
Statistics
T-Test was used to compare the mean labeling indices among various study groups.
Results
The Ki-67 expression was detected in all cases studied [Table 1].
Table 1.
Mean labeling index indifferent groups
The Ki-67 expression was mainly present in the parabasal layer rather than in the basal layer [Figure 3].
Figure 3.
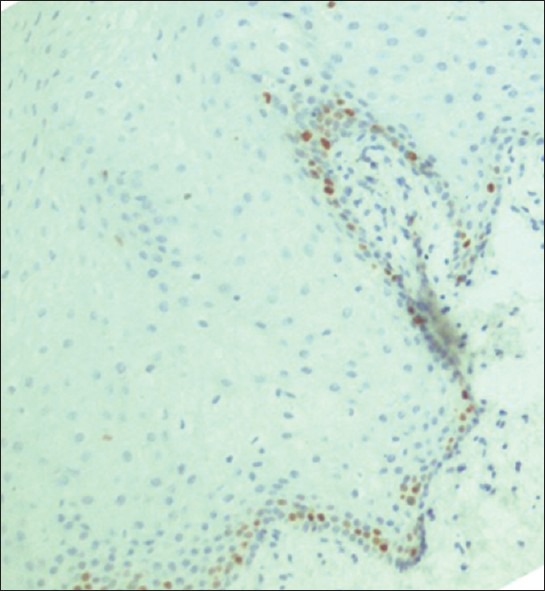
Photomicrograph showing Ki-67 expression in normal control: Parabasal expressionwas seen (×200)
Ki-67LI in the basal and suprabasal layers of epithelial dysplasia increased according to the severity of dysplasia in the suprabasal layer, while it was constant through every grade in the parabasal layer [Figure 4].
Figure 4.
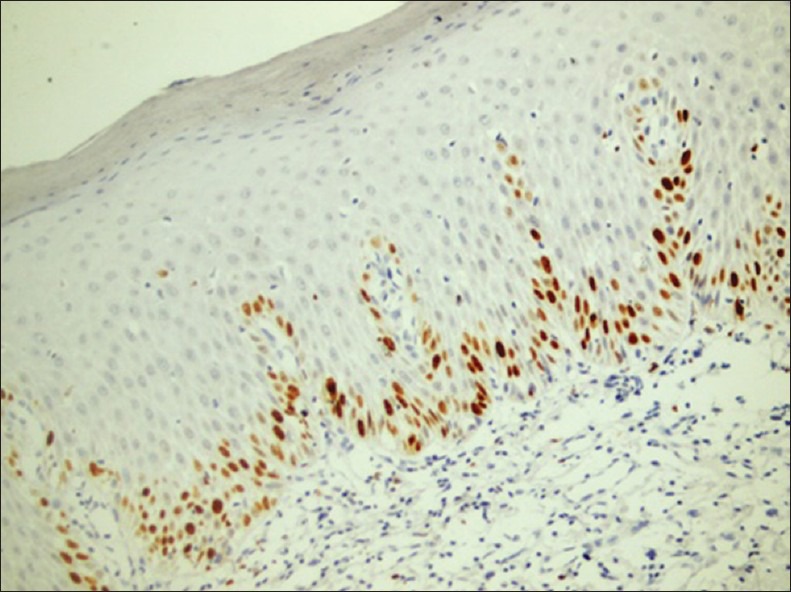
Photomicrograph showing Ki-67 expression in dysplatic epithelium: Basal, parabasal, and suprabasal expression is seen (×200)
In the low-risk and high-risk groups, maximum difference was observed between basal and suprabasal layers whereas in control group maximum difference was observed between parabasal and suprabasal layers [Graph 1].
Graph 1.
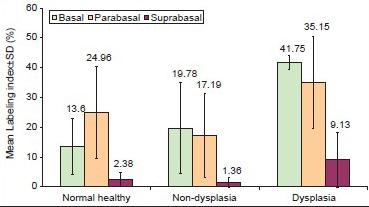
Layer wise mean Ki-67 labeling indices in various study groups with maximum parabasal expression seen in normal buccal mucosa and statistically increased suprabasal expression seen in dysplastic group from non-dysplastic group (P < 0.05)
In dysplastic and non-dysplastic groups, the difference between basal and suprabasal and between parabasal and suprabasal layers were significant statistically (P < 0.05), whereas in control group the difference between basal and suprabasal layers were significant statistically (P < 0.05) [Graph 1].
There was a statistically significant difference in the mean LI between low-risk and high-risk group while the difference was not statistically significant between normal epithelium and low-risk groups. The expression of Ki-67 increased progressively according to the grades of OSCC as reported in previous studies.
There was a significant difference between OSCC and normal epithelium and low-risk group while the difference was not significant between high-risk group and OSCC [Graph 2].
Graph 2.
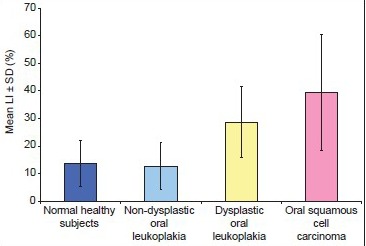
Mean labeling index in normal buccal mucosa, non-dysplastic, dysplastic lesions and OSCC
Discussion
This study demonstrates different expression patterns of Ki-67 as a marker for proliferating cells in different degrees of epithelial dysplasia and OSCC. The Ki-67 antigen is expressed in all the phases of the cellular cycle in proliferative cells, whereas it is not expressed in quiescent cells (G0 phase).[2] So, probably, the suprabasal expression of Ki-67 could well be a more reliable marker of epithelial dysplasia.
Oral epithelial dysplasia is often histologically recognized as the distribution disturbance of proliferating cells and stem cells within the stratified layers of the epithelium. In the present study, epithelial dysplasia group is subdivided as suggested by Kujan et al.[5] into low-risk group and high-risk groups.[5]
The LI of normal epithelium was found to be slightly more than the low-risk groups but statistically the differencewas not significant (P > 0.05). This finding is supported by Li et al. where the expression in epithelium of benign lymphoadenosis of oral mucosa (BLOM) without dysplasia was similar to that of the normal mucosa.[6] This was supported by the fact that the epithelial cells of BLOM without dysplasia had not undergone genetic transformation.
In normal oral epithelium, the proliferating cells were restricted mainly in the parabasal rather than basal layer as reported by Takeda et al.[7] Therefore, it could be considered that transient amplifying cells derived from stem cells might be present in the parabasal layer of normal epithelium. Asymmetric cell division in epithelial stem cells located in the basal layer may produce transient amplifying cells located in the parabasal layer in oral epithelium. It is well-recognized that the dispolarity of basal cells is a histological criterion and a hallmark of cellular atypia in epithelial dysplasia.
In the low-risk groups, the maximum expression of Ki-67 was in the basal layer followed by parabasal and then suprabasal, which showed the least expression. As there was no statistically significant difference between the low-risk group and the normal epithelium, it can be concluded that it is very difficult to predict the prognosis of a low-risk lesions at an early stage, as it is more or less, not very aggressive and has proliferative activity similar to normal epithelium.
In the low-risk and high-risk groups, maximum difference was observed between basal and suprabasal layers whereas in control group maximum difference was observed between parabasal and suprabasal layers. In the low-risk and high-risk groups the difference between basal and suprabasal and between parabasal and suprabasal layers were significant statistically (P < 0.05) whereas in control group the differences between parabasal and basal and basal and suprabasal layers were significant statistically (P < 0.05). According to Takeda et al. Ki-67-LI in the basal and suprabasal layers of epithelial dysplasias increased according to the grade of dysplasia, with constant expression through every in the parabasal layer up to carcinoma in situ.[7] This finding is in correlation with our study where expression increased in basal and suprabasal layers following the degree of dysplasia from non-dysplastic to dysplastic group.
The increased proliferating cell population in both basal and suprabasal layers of epithelial dysplasia in this study suggest that proliferating cells might increase not only in a superficial direction but also downward to the basal layer in epithelial dysplasia. The architectural disorganization of proliferating cells and stem cells in oral epithelium could be a useful index to estimate the grading of epithelial dysplasias if added to histomorphological examinations in H and E staining sections.
The expression of Ki-67 and the LI in OSCC increased according to histological grades of OSCC. In well differentiated 0SCC the expression was observed in peripheral areas of islands than the central areas of squamous maturation. This suggest that less differentiated cells are located in the peripheral layer and the central cells are highly differentiated with the ability of keratinization, thus, no expression of Ki-67 was observed in the central cells of the tumor island [Figure 5].
Figure 5.
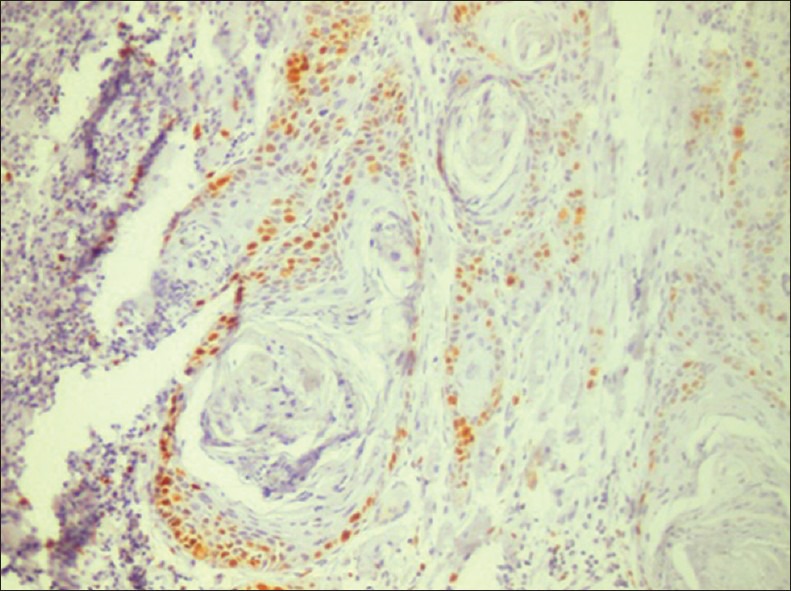
Ki-67 expression in oral squamous cell carcinoma: Peripheral expressionis seen around tumor islands (×200)
In moderately differentiated OSCC, Ki-67 expression when observed in both tumor islands and diffuse staining in other areas. The overall staining of Ki-67 in moderately differentiated tumors was more quantitatively than well differentiated whereas in poorly differentiated OSCC the staining of Ki-67 was diffuse and more intense as the cells were less differentiated and in more proliferating phase [Figure 6].
Figure 6.
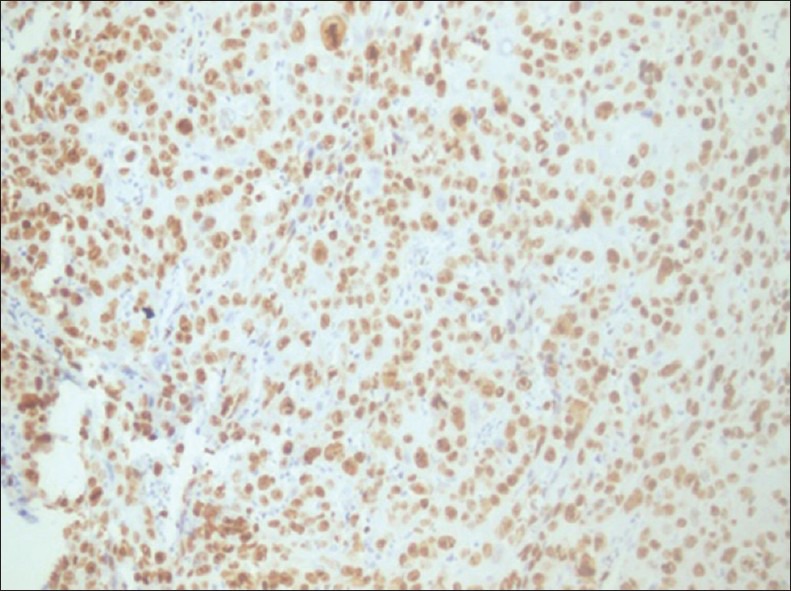
Ki-67 expression in oral squamous cell carcinoma: Diffuse expression is seen (×200)
The expression of Ki-67 is correlated with the grading of OSCC as it depicts the growth fraction of the tumor and its proliferative status. The fact that Ki-67 expression indicates the total fraction of proliferative cells in a tumor, the expression of Ki-67 increased according to the aggressiveness of the tumor.
A statistically significant difference between OSCC, low-risk group and normal healthy mucosa was observed in our study while the difference between high-risk group and OSCC was not significant thereby signifying the fact that dysplastic epithelium holds a high potential for malignant transformation and there is both architectural, cellular, and genetic changes which help in progression of dysplasia to OSCC.
In our study, in low-risk and high-risk groups a maximum difference was observed between basal and suprabasal layers with increased expression in the suprabasal layer as the lesion progress from low-risk to high-risk group thus, supporting the fact that increased suprabasal expression is a marker of dysplasia. This was supported by Gonzαlez-Moles et al. who reported that increased proliferation in parabasal layers of premalignant oral epithelium is likely related to loss of heterozygosity in 3p, 9p, and 17p, which behaves as a marker of precancerous fields and increases the risk of developing multiple tumors.[8] Hypothetically, a pool of highly proliferative parabasal cells may represent a target for additional late oncogenic events that could endow subclones of cells from the field with invasive capacity. Hence, an increase in the proliferation rate of cell clones on the path to malignancy appears to be important for a precancerous field to generate a tumor.
The findings of Gonzαlez-Moles et al.[8] are supported by Li et al.,[6] where basal and superficial layers showed the clearest difference between the normal and abnormal tissues. This was because of higher number of cells and the relatively high proliferative activity in the dysplastic leukoplakias and hence they concluded that the superficial layer and the basal layer seem to be the most adequate tissue components to investigate the possible modulation of cell proliferation in precursor lesions.
Macluskey et al. showed statistically significant correlation with mean Ki-67LI in healthy tissue, dysplasia, and in carcinomas[9] thereby suggesting that epithelial proliferation may continue to increase during the transition from dysplasia to carcinoma, but this is likely to occur at a slow rate. Hence, Ki-67 expression is not a good indicator of neoplastic transformation. This finding is consistent with the present study where no statistically significant difference was observed between high-risk group and OSCC.
In conclusion, suprabasal expression of Ki-67 provides objective criteria for determining the severity of dysplasia and histological grading of OSCC and can be used as an objective marker to evaluate the grading of epithelial dysplasia and OSCC. Studies with larger sample size are needed to get a cut-off value for distinguishing between non-dysplastic and dysplastic oral epithelium using Ki-67 as an objective marker and can aid in early detection of oral premalignant and malignant lesions.
Acknowlegement
Dr. Vini Tondon (MD, Pathology) for her guidance and support during the research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Pitiyage G, Tilakaratne WM, Tavassoli M, Warnakulasuriya S. Molecular markers in oral epithelial dysplasia: Review. J Oral Pathol Med. 2009;38:737–52. doi: 10.1111/j.1600-0714.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 2.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Moles MA, Ruiz-Avila I, Rodriguez-Archilla A, Martinez-Lara I. Suprabasal expression of Ki-67 antigen as a marker for the presence and severity of oral epithelial dysplasia. Head Neck. 2000;22:658–61. doi: 10.1002/1097-0347(200010)22:7<658::aid-hed3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Tumuluri V, Thomas GA, Fraser IS. Analysis of the Ki-67 antigen at the invasive tumour front of human oral squamous cell carcinoma. J Oral Pathol Med. 2002;31:598–604. doi: 10.1034/j.1600-0714.2002.00042.x. [DOI] [PubMed] [Google Scholar]
- 5.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42:987–93. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Li SX, Yu SF, Sun KH. Characteristics of benign lymphoadenosis of oral mucosa. World J Gastroenterol. 2005;11:4536–40. doi: 10.3748/wjg.v11.i29.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda T, Sugihara K, Hirayama Y, Hirano M, Tanuma JI, Semba I. Immunohistological evaluation of Ki-67, p63, CK19 and p53 expression in oral epithelial dysplasias. J Oral Pathol Med. 2006;35:369–75. doi: 10.1111/j.1600-0714.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 8.González-Moles MA, Bravo M, Ruiz-Avila I, Acebal F, Gil-Montoya JA, Brener S, et al. Ki-67 expression in non-tumour epithelium adjacent to oral cancer as risk marker for multiple oral tumours. Oral Dis. 2010;16:68–75. doi: 10.1111/j.1601-0825.2009.01611.x. [DOI] [PubMed] [Google Scholar]
- 9.Macluskey M, Ogden GR, Green M, Chisholm DM, Schor SL, Schor AM. The association between epithelial proliferation and disease progression in the oral mucosa. Oral Oncol. 1999;35:409–14. doi: 10.1016/s1368-8375(99)00014-7. [DOI] [PubMed] [Google Scholar]


