Abstract
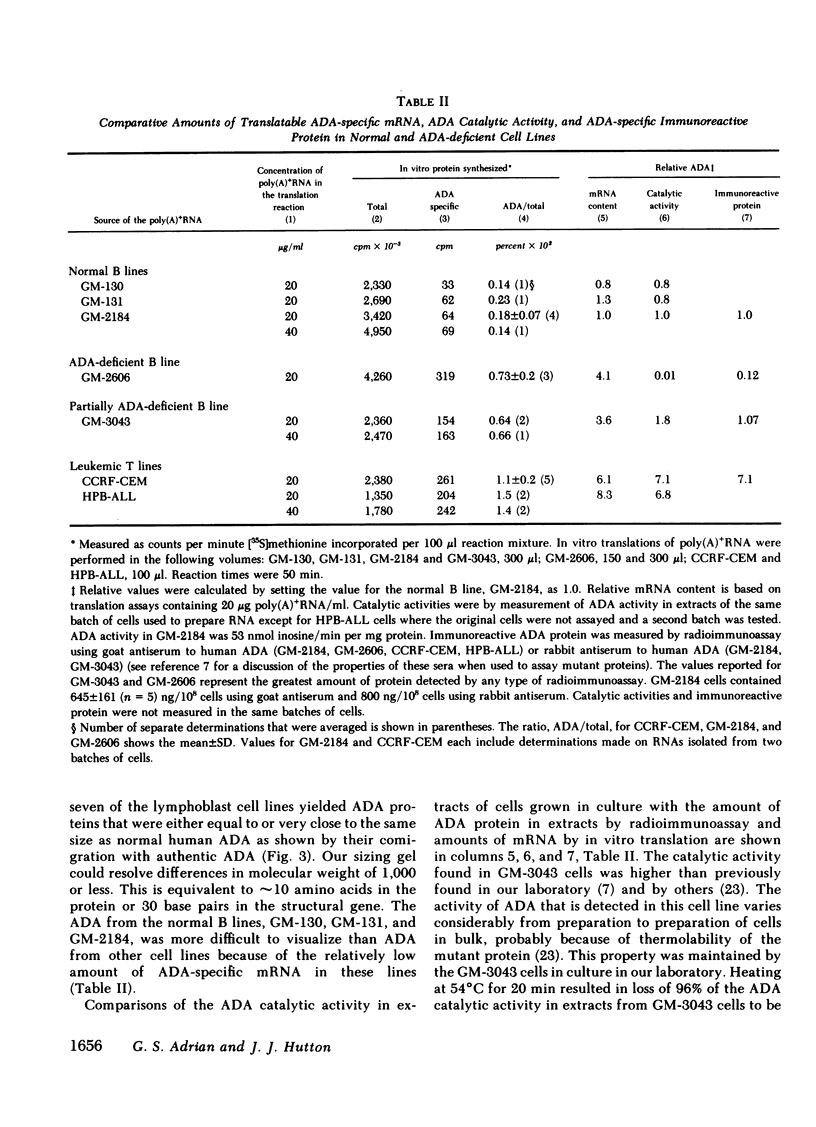
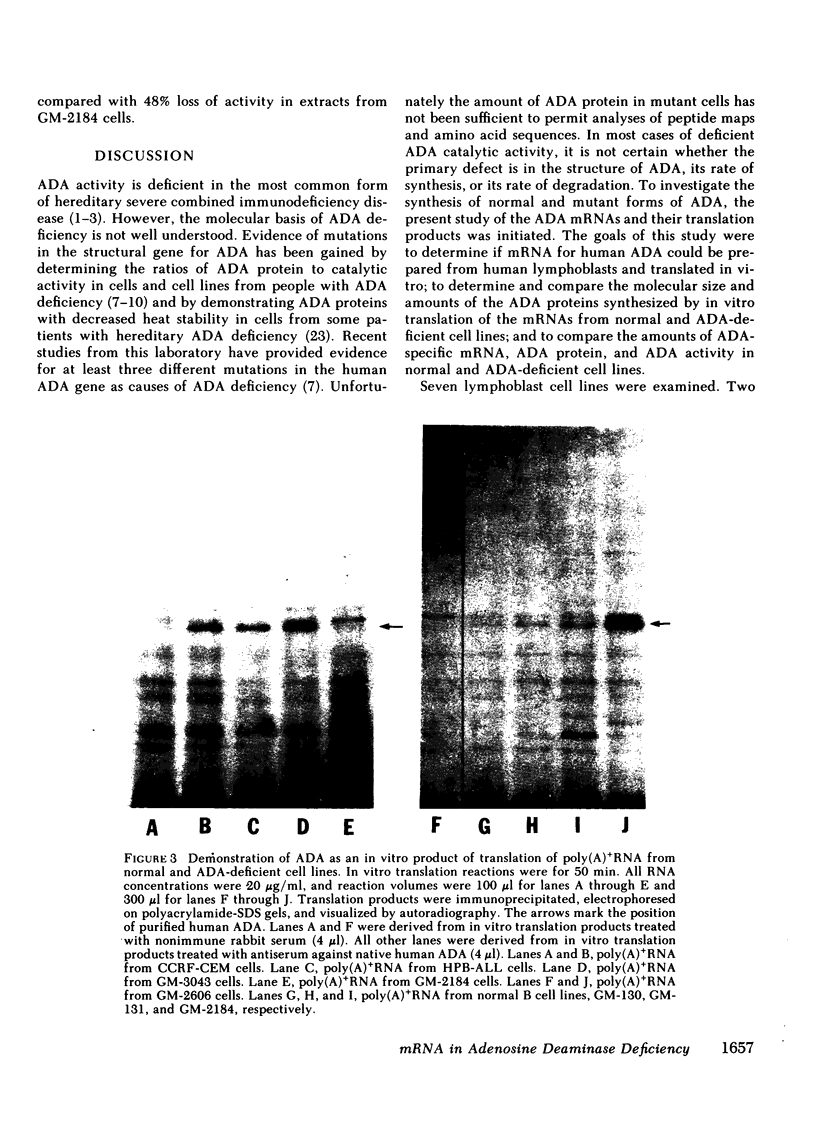
Hereditary deficiency of adenosine deaminase (ADA) usually causes profound lymphopenia with severe combined immunodeficiency disease. Cells from patients with ADA deficiency contain less than normal, and sometimes undetectable, amounts of ADA catalytic activity and ADA protein. The molecular defects responsible for hereditary ADA deficiency are poorly understood. ADA messenger RNAs and their translation products have been characterized in seven human lymphoblast cell lines derived as follows: GM-130, GM-131, and GM-2184 from normal adults; GM-3043 from a partially ADA deficient, immunocompetent !Kung tribesman; GM-2606 from an ADA deficient, immunodeficient child; CCRF-CEM and HPB-ALL from leukemic children. ADA messenger (m)RNA was present in all lines and was polyadenylated. The ADA synthesized by in vitro translation of mRNA from each line reacted with antisera to normal human ADA and was of normal molecular size. There was no evidence that posttranslational processing of ADA occurred in normal, leukemic, or mutant lymphoblast lines. Relative levels of specific translatable mRNA paralleled levels of ADA protein in extracts of the three normal and two leukemic lines. However, unexpectedly high levels of ADA specific, translatable mRNA were found in the mutant GM-2606 and GM-3043 lines, amounting to three to four times those of the three normal lines. Differences in the amounts of ADA mRNA and rates of ADA synthesis appear to be of primary importance in maintaining the differences in ADA levels among lymphoblast lines with structurally normal ADA. ADA deficiency in at least two mutant cell lines is not caused by deficient levels of translatable mRNA, and unless there is some translational control of this mRNA, the characteristic cellular ADA deficiency is most likely secondary to synthesis and rapid degradation of a defective ADA protein.
Full text
PDF



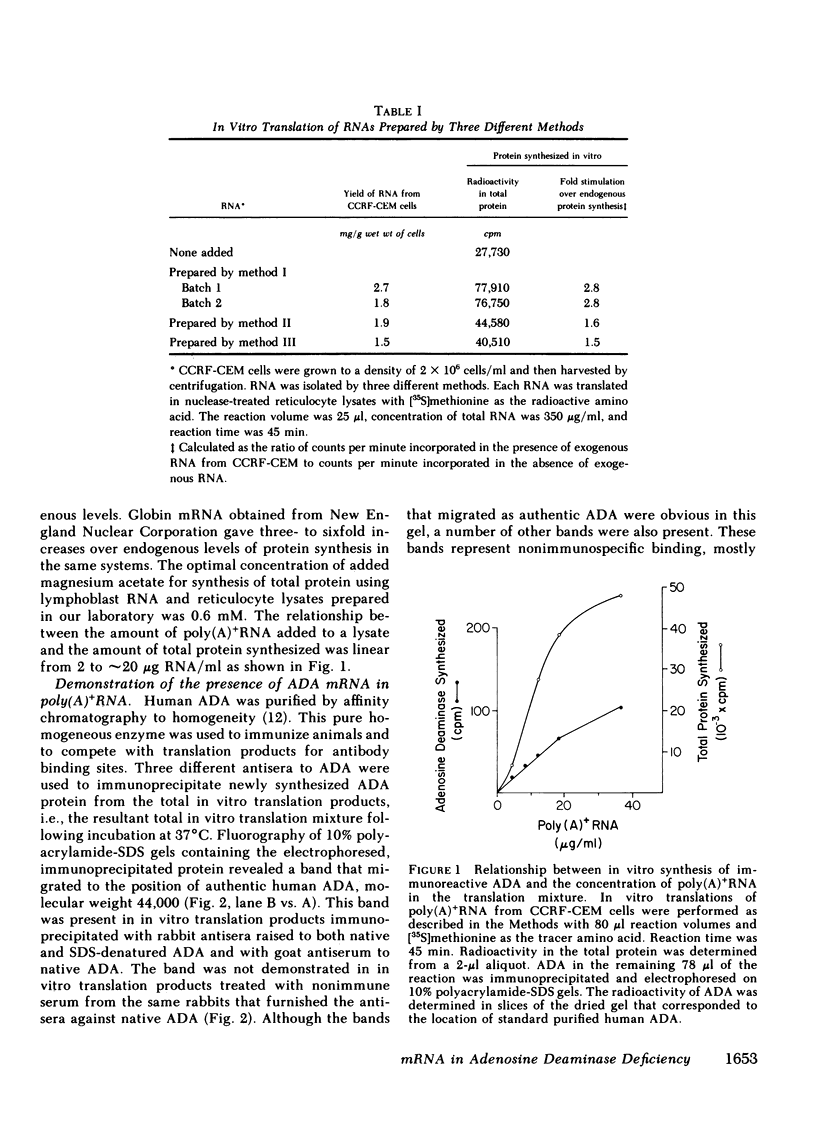
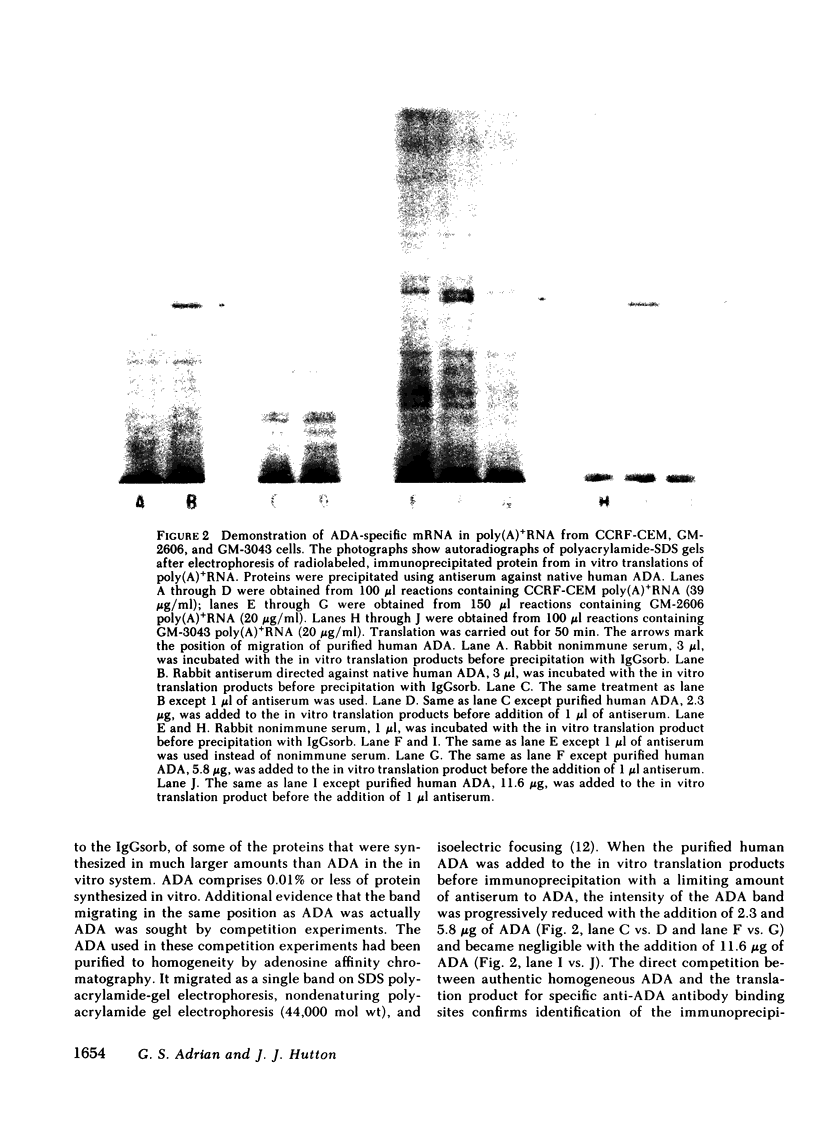




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Goldblum R., Seegmiller J. E. Quantitative immunoassay of adenosine deaminase in combined immunodeficiency disease. J Immunol. 1977 Jan;118(1):270–273. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Daddona P. E., Frohman M. A., Kelley W. N. Human adenosine deaminase and its binding protein in normal and adenosine deaminase-deficient fibroblast cell strains. J Biol Chem. 1980 Jun 25;255(12):5681–5687. [PubMed] [Google Scholar]
- Daddona P. E. Human adenosine deaminase. Properties and turnover in cultured T and B lymphoblasts. J Biol Chem. 1981 Dec 10;256(23):12496–12501. [PubMed] [Google Scholar]
- Daddona P. E., Kelley W. N. Analysis of normal and mutant forms of human adenosine deaminase - a review. Mol Cell Biochem. 1980 Feb 8;29(2):91–101. doi: 10.1007/BF00220303. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Goodridge A. G., Morris S. M., Jr, Goldflam T. Isolation, translation in vitro, and partial purification of messenger RNA for fatty acid synthase from uropygial gland. Methods Enzymol. 1981;71(Pt 100):139–150. doi: 10.1016/0076-6879(81)71021-8. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Beratis N., Rosen F. S. Characterization of residual enzyme activity in fibroblasts from patients with adenosine deaminase deficiency and combined immunodeficiency: evidence for a mutant enzyme. Proc Natl Acad Sci U S A. 1976 Jan;73(1):213–217. doi: 10.1073/pnas.73.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Roegner V., Jenkins T., Seaman C., Piomelli S., Borkowsky W. Erythrocyte adenosine deaminase deficiency without immunodeficiency. Evidence for an unstable mutant enzyme. J Clin Invest. 1979 Oct;64(4):1130–1139. doi: 10.1172/JCI109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins T., Lane A. B., Nurse G. T., Hopkinson D. A. Red cell adenosine deaminase (ADA) polymorphism in Southern Africa, with special reference to ADA deficiency among the !Kung. Ann Hum Genet. 1979 May;42(4):425–433. doi: 10.1111/j.1469-1809.1979.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Martin D. W., Jr, Gelfand E. W. Biochemistry of diseases of immunodevelopment. Annu Rev Biochem. 1981;50:845–877. doi: 10.1146/annurev.bi.50.070181.004213. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Ledbetter D. H., Hejtmancik J. F., Caskey C. T. In vitro translation of hypoxanthine/guanine phosphoribosyltransferase mRNA: characterization of a mouse neuroblastoma cell line that has elevated levels of hypoxanthine/guanine phosphoribosyltransferase protein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6977–6980. doi: 10.1073/pnas.78.11.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S., Tatsumi E., Baba M., Harada T., Yasuhira K. Two E-rosette-forming lymphoid cell lines. Int J Cancer. 1978 Feb 15;21(2):166–170. doi: 10.1002/ijc.2910210207. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Coleman P. F., Stark G. R. N-(Phosphonacetyl)-L-aspartate-resistant hamster cells overaccumulate a single mRNA coding for the multifunctional protein that catalyzes the first steps of UMP synthesis. J Biol Chem. 1979 Feb 10;254(3):974–980. [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ratner P. L., Fisher M., Burkart D., Cook J. R., Kozak L. P. The role of mRNA levels and cellular localization in controlling sn-glycerol-3-phosphate dehydrogenase expression in tissues of the mouse. J Biol Chem. 1981 Apr 10;256(7):3576–3579. [PubMed] [Google Scholar]
- Siciliano M. J., Bordelon M. R., Kohler P. O. Expression of human adenosine deaminase after fusion of adenosine deaminase-deficient cells with mouse fibroblasts. Proc Natl Acad Sci U S A. 1978 Feb;75(2):936–940. doi: 10.1073/pnas.75.2.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A., Sun L., Witte O. N., Baltimore D. Biosynthesis of murine terminal deoxynucleotidyltransferase. J Biol Chem. 1980 Jan 25;255(2):791–796. [PubMed] [Google Scholar]
- Thibault J., Vidal D., Gros F. In vitro translation of mRNA from rat pheochromocytoma tumors, characterization of tyrosine hydroxylase. Biochem Biophys Res Commun. 1981 Apr 15;99(3):960–968. doi: 10.1016/0006-291x(81)91256-0. [DOI] [PubMed] [Google Scholar]
- Thompson L. F., Seegmiller J. E. Adenosine deaminase deficiency and severe combined immunodeficiency disease. Adv Enzymol Relat Areas Mol Biol. 1980;51:167–210. doi: 10.1002/9780470122969.ch4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wiginton D. A., Coleman M. S., Hutton J. J. Purification, characterization and radioimmunoassay of adenosine deaminase from human leukaemic granulocytes. Biochem J. 1981 May 1;195(2):389–397. doi: 10.1042/bj1950389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Hutton J. J. Immunoreactive protein in adenosine deaminase deficient human lymphoblast cell lines. J Biol Chem. 1982 Mar 25;257(6):3211–3217. [PubMed] [Google Scholar]
- Yoshida A. Amino acid substitution (histidine to tyrosine) in a glucose-6-phosphate dehydrogenase variant (G6PD Hektoen) associated with over-production. J Mol Biol. 1970 Sep 28;52(3):483–490. doi: 10.1016/0022-2836(70)90414-6. [DOI] [PubMed] [Google Scholar]