Abstract
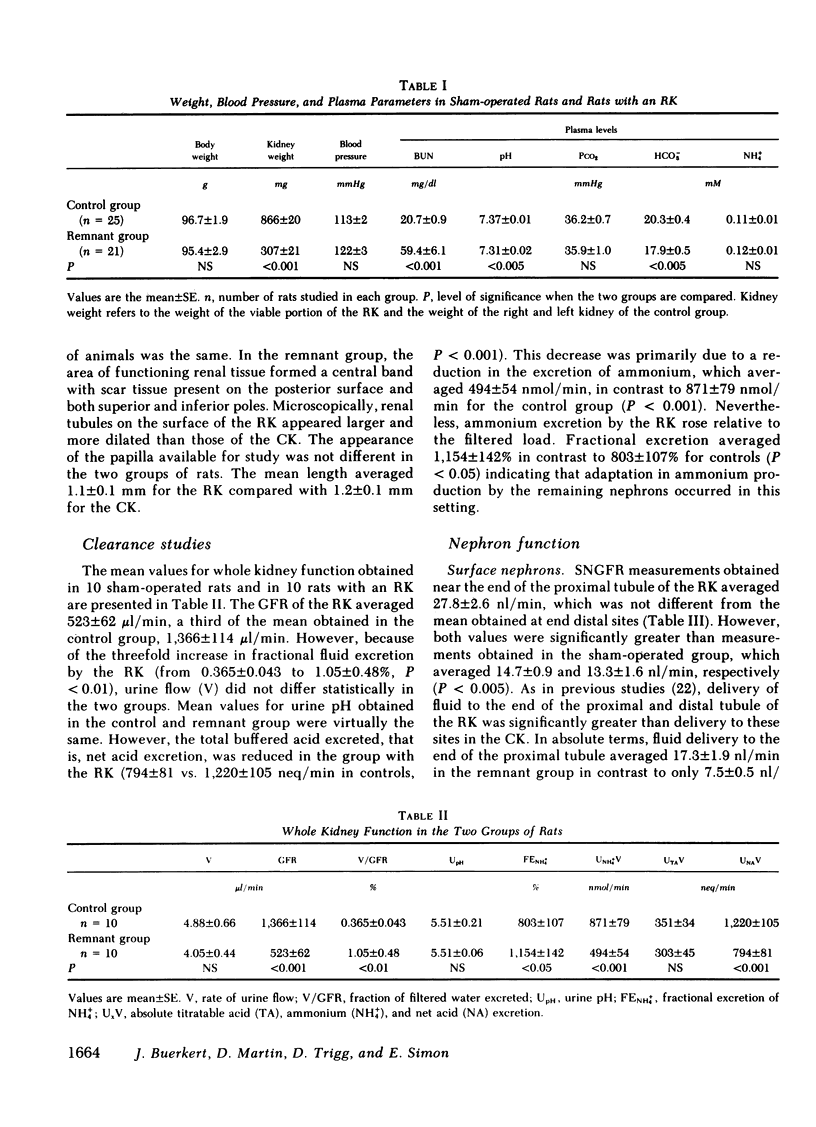
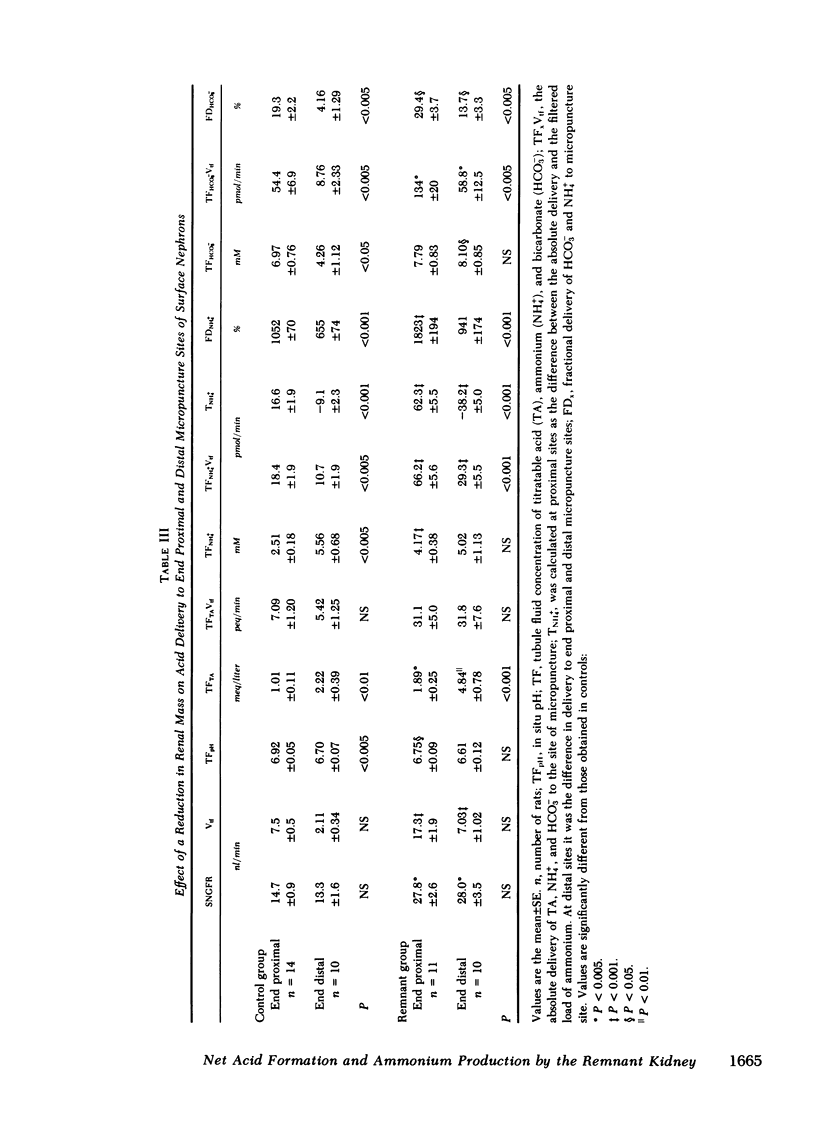
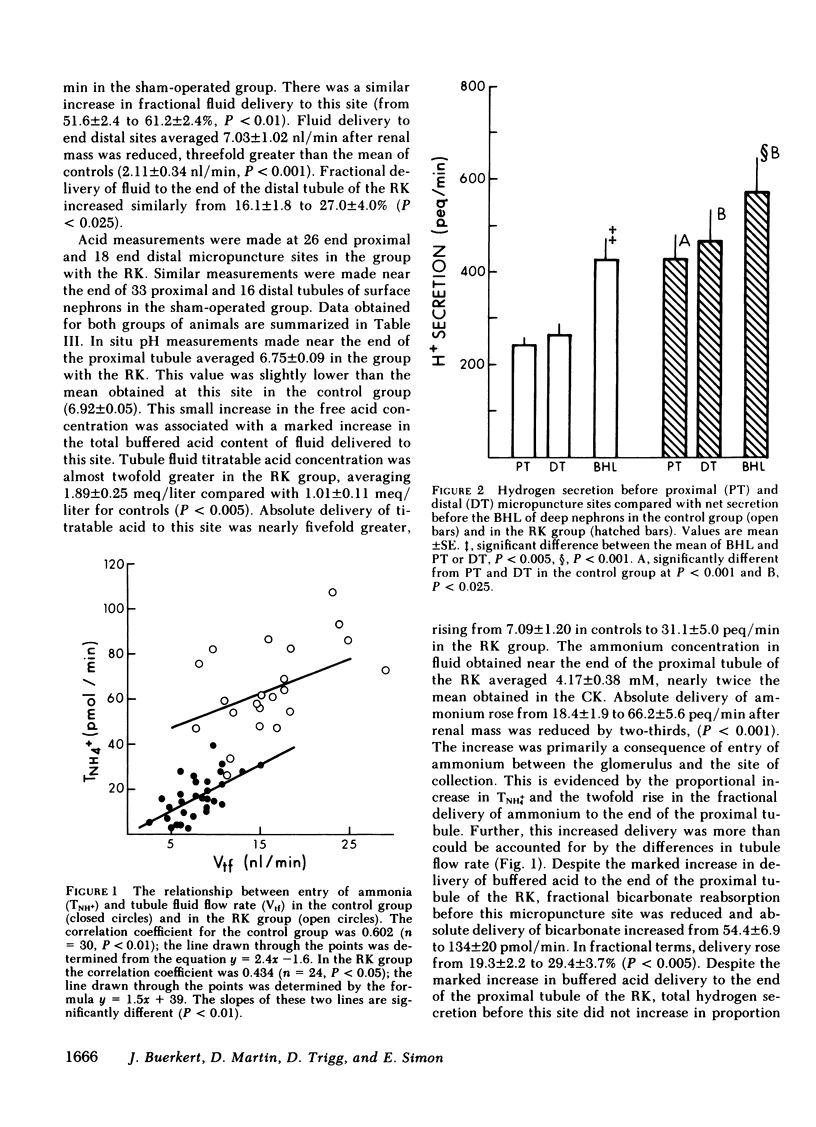
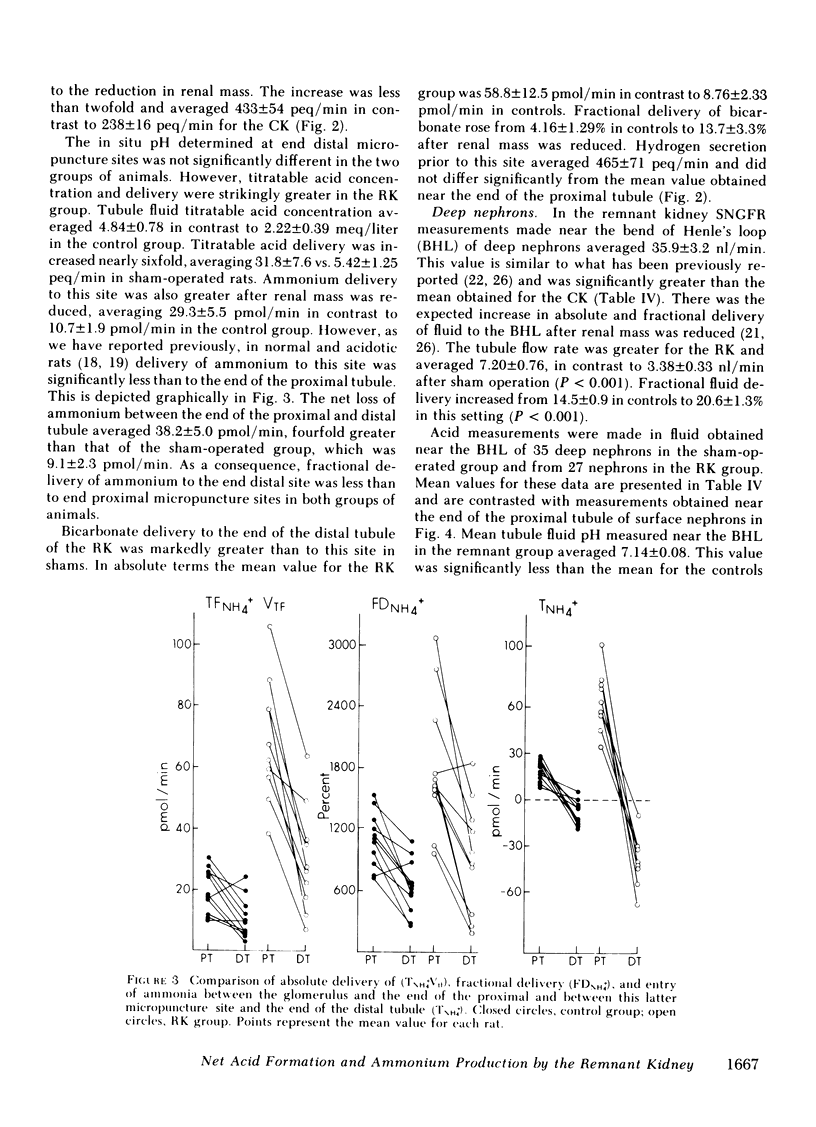
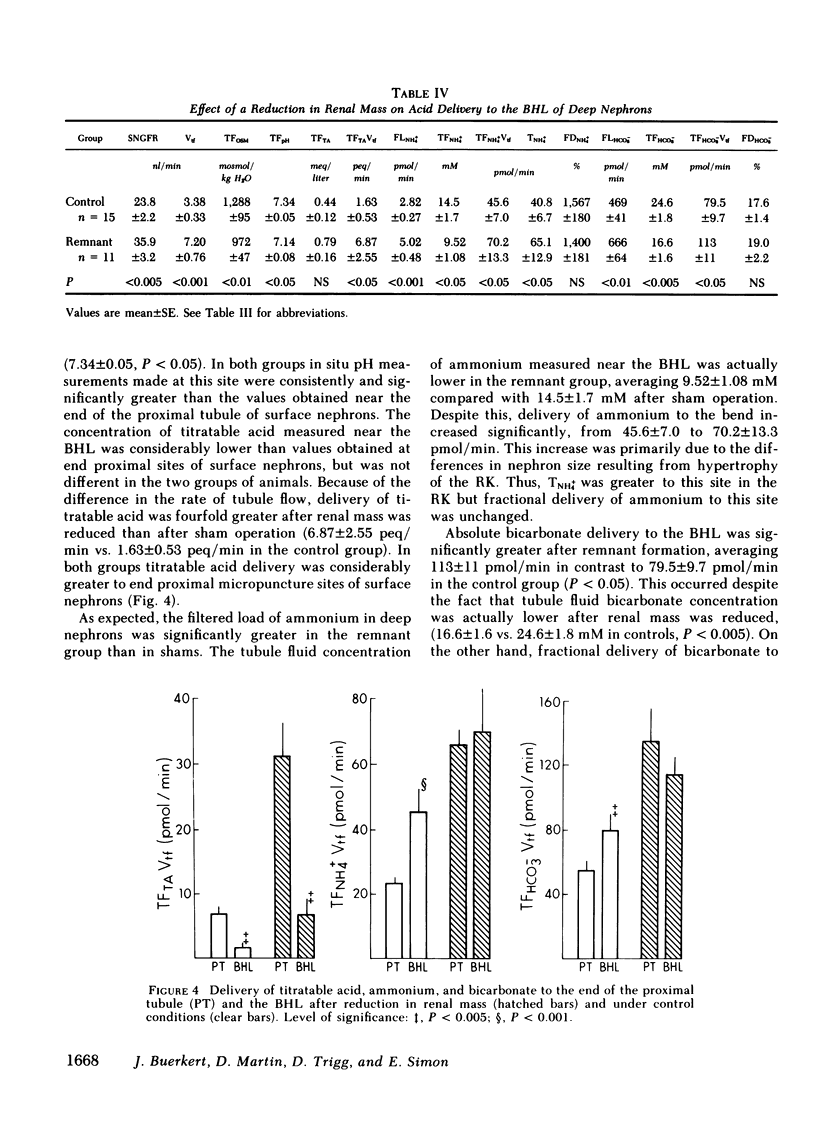
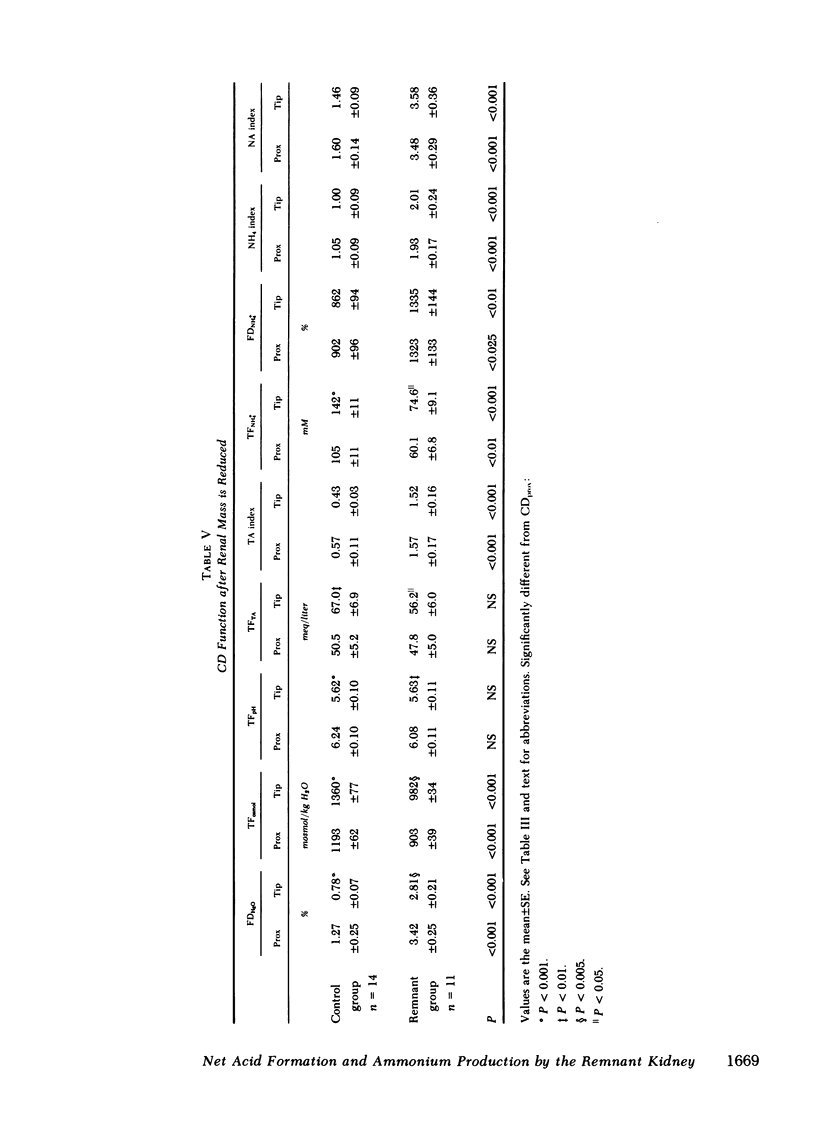
Papillary and surface micropuncture were used to study the handling of ammonium and the formation of net acid by surface nephrons, deep nephrons, and the terminal segment of collecting duct (CD) after renal mass was reduced by two-thirds. Net acid excretion by the remnant kidney (RK) was significantly reduced, averaging 794±81 neq/min (SE) compared with 1,220±105 neq/min after sham operation (P < 0.001), due to a decrease in ammonium excretion (494±54 vs. 871±79 nmol/min in controls, P < 0.001). Urinary pH and titratable acid excretion were not different in the two groups of animals. After RK formation, ammonium delivery to the end of the proximal tubule increased nearly threefold and averaged 66.2±5.6 compared with 18.4±2.9 pmol/min in controls, (P < 0.001). This greater delivery of ammonium was primarily due to renal tubule entry rather than to changes in the filtered load and was only partially related to the differences in flow rate. Ammonium processing by deep nephrons was profoundly affected by a reduction in renal mass. Although absolute delivery of ammonium was greater to the bend of Henle's loop (BHL), the difference could be accounted for on the basis of an increase in nephron size. Thus, fractional delivery (FDNH+4) to this site was not different for the two groups of animals, averaging 1,567±180% in controls and 1,400±181% in the group with the RK. Hydrogen secretion in the proximal segments of deep and surface nephrons did not increase in proportion to the decrease in renal mass and as a consequence bicarbonate delivery to the end of the proximal tubule of surface nephrons and to the BHL of deep nephrons was increased.
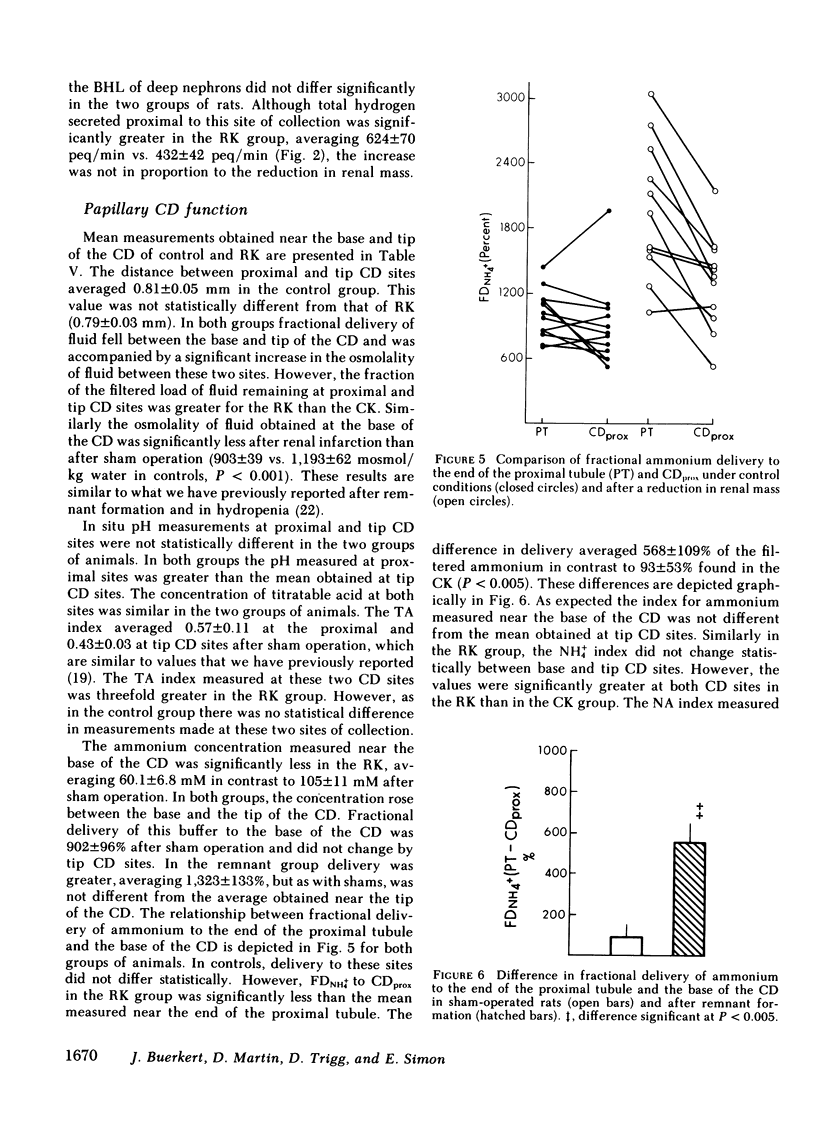
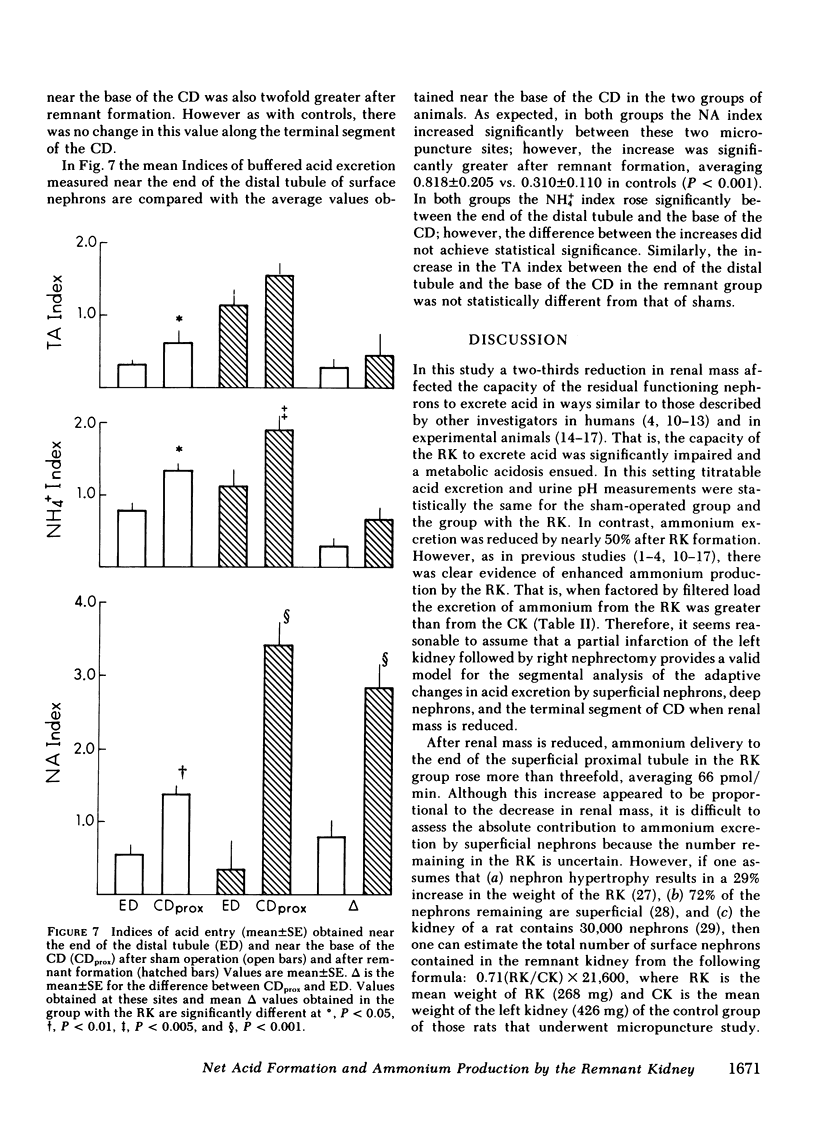
When renal mass was reduced FDNH+4 to the base of the terminal CD doubled but did not change by the tip. In both groups FDNH+4 to the base of the CD was greater than to the end of the distal tubule. However, the increase was the same. On the other hand, the increase in the net acid index between the end of the distal tubule and the base of the CD was profoundly greater in rats with an RK. This difference was primarily due to bicarbonate reabsorption rather than enhanced ammonium reentry. Indeed, >400% of the fractional ammonium delivered to the end of the proximal tubule was lost from the tubule fluid. The data suggest that the decrease in acid excretion by the RK is due to two factors. First, hydrogen secretion in the proximal segments of both nephron populations fails to increase in the proportion to the reduction in renal mass. Second, a reduced reentrapment of ammonia, rather than its impaired production, causes ammonium excretion to decrease.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrud J. A., Carrasquillo T., Cubria A., Rademacher D. R., Kurtzman N. A. Bicarbonate reabsorption in chronic renal failure. Kidney Int. 1976 Jun;9(6):481–488. doi: 10.1038/ki.1976.62. [DOI] [PubMed] [Google Scholar]
- BANK N., SCHWARTZ W. B. Influence of certain urinary solutes on acidic dissociation constant of ammonium at 37 degrees C. J Appl Physiol. 1960 Jan;15:125–127. doi: 10.1152/jappl.1960.15.1.125. [DOI] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Prasad J., Chambless S., Klahr S. Response of deep nephrons and the terminal collecting duct to a reduction in renal mass. Am J Physiol. 1979 May;236(5):F454–F464. doi: 10.1152/ajprenal.1979.236.5.F454. [DOI] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Ammonium handling by superficial and juxtamedullary nephrons in the rat. Evidence for an ammonia shunt between the loop of Henle and the collecting duct. J Clin Invest. 1982 Jul;70(1):1–12. doi: 10.1172/JCI110581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Segmental analysis of the renal tubule in buffer production and net acid formation. Am J Physiol. 1983 Apr;244(4):F442–F454. doi: 10.1152/ajprenal.1983.244.4.F442. [DOI] [PubMed] [Google Scholar]
- DENIS G., PREUSS H., PITTS R. THE PNH3 OF RENAL TUBULAR CELLS. J Clin Invest. 1964 Apr;43:571–582. doi: 10.1172/JCI104942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorhout-Mees E. J., Machado M., Slatopolsky E., Klahr S., Bricker N. S. The functional adaptation of the diseased kidney. 3. Ammonium excretion. J Clin Invest. 1966 Mar;45(3):289–296. doi: 10.1172/JCI105342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein F. O., Hayslett J. P. Role of medullary structures in the functional adaptation of renal insufficiency. Kidney Int. 1974 Dec;6(6):419–425. doi: 10.1038/ki.1974.127. [DOI] [PubMed] [Google Scholar]
- Gonick H. C., Kleeman C. R., Rubini M. E., Maxwell M. H. Functional impairment in chronic renal disease. II. Studies of acid excretion. Nephron. 1969;6(1):28–49. doi: 10.1159/000179710. [DOI] [PubMed] [Google Scholar]
- Hayslett J. P. Functional adaptation to reduction in renal mass. Physiol Rev. 1979 Jan;59(1):137–164. doi: 10.1152/physrev.1979.59.1.137. [DOI] [PubMed] [Google Scholar]
- Hills A. G., Reid E. L. Renal ammonia balance. A kinetic treatment. Nephron. 1966;3(4):221–256. doi: 10.1159/000179537. [DOI] [PubMed] [Google Scholar]
- JACQUEZ J. A., POPPELL J. W., JELTSCH R. Solubility of ammonia in human plasma. J Appl Physiol. 1959 Mar;14(2):255–258. doi: 10.1152/jappl.1959.14.2.255. [DOI] [PubMed] [Google Scholar]
- Karlmark B. The determination of titratable acid and ammonium ions in picomole amounts. Anal Biochem. 1973 Mar;52(1):69–82. doi: 10.1016/0003-2697(73)90332-1. [DOI] [PubMed] [Google Scholar]
- Lubowitz H., Purkerson M. L., Rolf D. B., Weisser F., Bricker N. S. Effect of nephron loss on proximal tubular bicarbonate reabsorption in the rat. Am J Physiol. 1971 Feb;220(2):457–461. doi: 10.1152/ajplegacy.1971.220.2.457. [DOI] [PubMed] [Google Scholar]
- Lucci M. S., Pucacco L. R., Carter N. W., DuBose T. D., Jr Evaluation of bicarbonate transport in rat distal tubule: effects of acid-base status. Am J Physiol. 1982 Oct;243(4):F335–F341. doi: 10.1152/ajprenal.1982.243.4.F335. [DOI] [PubMed] [Google Scholar]
- MORRIN P. A., BRICKER N. S., KIME S. W., Jr, KLEIN C. Observations on the acidifying capacity of the experimentally diseased kidney in the dog. J Clin Invest. 1962 Jun;41:1297–1302. doi: 10.1172/JCI104592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacClean A. J., Hayslett J. P. Adaptive change in ammonia excretion in renal insufficiency. Kidney Int. 1980 May;17(5):595–606. doi: 10.1038/ki.1980.70. [DOI] [PubMed] [Google Scholar]
- Oelert H., Uhlich E., Hills A. G. Messungen des Ammoniakdruckes in den corticalen Tubuli der Rattenniere. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;300(1):35–48. [PubMed] [Google Scholar]
- Pennell J. P., Bourgoignie J. J. Adaptive changes of juxtamedullary glomerular filtration in the remnant kidney. Pflugers Arch. 1981 Jan;389(2):131–135. doi: 10.1007/BF00582103. [DOI] [PubMed] [Google Scholar]
- Pucacco L. R., Carter N. W. A glass-membrane pH microelectrode. Anal Biochem. 1976 Jun;73(2):501–512. doi: 10.1016/0003-2697(76)90200-1. [DOI] [PubMed] [Google Scholar]
- Radda G. K., Ackerman J. J., Bore P., Sehr P., Wong G. G., Ross B. D., Green Y., Bartlett S., Lowry M. 31P NMR studies on kidney intracellular pH in acute renal acidosis. Int J Biochem. 1980;12(1-2):277–281. doi: 10.1016/0020-711x(80)90084-1. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ W. B., HALL P. W., 3rd, HAYS R. M., RELMAN A. S. On the mechanism of acidosis in chronic renal disease. J Clin Invest. 1959 Jan 1;38(1 Pt 1):39–52. doi: 10.1172/JCI103794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajo I. M., Goldstein M. B., Sonnenberg H., Stinebaugh B. J., Wilson D. R., Halperin M. L. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int. 1981 Sep;20(3):353–358. doi: 10.1038/ki.1981.146. [DOI] [PubMed] [Google Scholar]
- Schmidt R. W., Gavellas G. Bicarbonate reabsorption in experimental renal disease: effects of proportional reduction of sodium or phosphate intake. Kidney Int. 1977 Dec;12(6):393–402. doi: 10.1038/ki.1977.130. [DOI] [PubMed] [Google Scholar]
- Schoolwerth A. C., Sandler R. S., Hoffman P. M., Klahr S. Effects of nephron reduction and dietary protein content on renal ammoniagenesis in the rat. Kidney Int. 1975 Jun;7(6):397–404. doi: 10.1038/ki.1975.57. [DOI] [PubMed] [Google Scholar]
- Simpson D. P. Control of hydrogen ion homeostasis and renal acidosis. Medicine (Baltimore) 1971 Nov;50(6):503–541. doi: 10.1097/00005792-197111000-00002. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Hoffsten P., Purkerson M., Bricker N. S. On the influence of extracellular fluid volume expansion and of uremia on bicarbonate reabsorption in man. J Clin Invest. 1970 May;49(5):988–998. doi: 10.1172/JCI106318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg H., Cheema-Dhadli S., Goldstein M. B., Stinebaugh B. J., Wilson D. R., Halperin M. L. Ammonia addition into the medullary collecting duct of the rat. Kidney Int. 1981 Feb;19(2):281–287. doi: 10.1038/ki.1981.18. [DOI] [PubMed] [Google Scholar]
- Van Slyke D. D., Linder G. C., Hiller A., Leiter L., McIntosh J. F. THE EXCRETION OF AMMONIA AND TITRATABLE ACID IN NEPHRITIS. J Clin Invest. 1926 Feb;2(3):255–288. doi: 10.1172/JCI100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRONG O., DAVIES H. E. The excretion of acid in renal disease. Q J Med. 1959 Apr;28(110):259–313. [PubMed] [Google Scholar]
- Welbourne T., Weber M., Bank N. The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease. J Clin Invest. 1972 Jul;51(7):1852–1860. doi: 10.1172/JCI106987. [DOI] [PMC free article] [PubMed] [Google Scholar]



