Abstract
Aim
During adult cardiac arrest, rescuers frequently provide ventilations at rates exceeding those recommended by the American Heart Association (AHA). Excessive ventilation is associated with worse clinical outcome after adult cardiac arrest. This study is the first to characterize ventilation rate adherence to AHA guidelines during in-hospital pediatric cardiac arrest resuscitation.
Patients and methods
We prospectively enrolled children and adolescents (≥8 years of age) who suffered a cardiac arrest in a pediatric intensive care unit (PICU) or emergency department (ED) of a tertiary-care pediatric hospital. Ventilation rate (breaths per minute [bpm]) was monitored via changes in chest wall impedance (CWI) recorded by defibrillator electrode pads during cardiopulmonary resuscitation (CPR).
Results
Twenty-four CPR events were enrolled yielding 588 thirty-second CPR epochs. The proportion of CPR epochs with ventilation rates exceeding AHA guidelines (>10 bpm) was 63% (CI95 59–67%), significantly higher than our a priori hypothesis of 30% (p < 0.01). The proportion of CPR epochs with ventilation rates exceeding 20 bpm was 20% (CI95 17–23). After controlling for location of arrest and initial event rhythm, resuscitations that occurred on nights/weekends were 3.6 times (CI95: 1.6–7.9, p < 0.01) more likely to have a ventilation rate exceeding AHA guidelines.
Conclusions
During in-hospital pediatric cardiac arrest, rescuers frequently provide artificial ventilations at rates in excess of AHA guidelines, with twenty percent of CPR time having ventilation rates double that recommended. Excessive ventilation was particularly common during CPR events that occurred on nights/weekends.
Keywords: Pediatric, Cardiopulmonary resuscitation, Ventilation
1. Introduction
Thousands of children suffer a cardiac arrest event each year.1,2 Unfortunately, only between 3 and 17% of pediatric victims of cardiac arrest survive the event with favorable neurological outcome,3–5 with higher survival seen with in-hospital arrest. Given that survival after pediatric cardiac arrest can be improved, it is unfortunate that previous investigations have established that during both adult and pediatric resuscitation attempts, care frequently does not achieve recommended quality targets.6–9 In addition to non-compliance with national guidelines for chest compression (CC) rate, CC depth, and no flow time, rescuers are known to ventilate adult victims of cardiac arrest with rates in excess of those recommended by the American Heart Association (AHA).7,10,11 To our knowledge, ventilation rates delivered during pediatric cardiac arrest remains unknown.
While controlled ventilation (i.e., limited ventilation to maximize hemodynamics during the low flow state of cardiopulmonary resuscitation) is important during adult resuscitation, the question remains as to whether the same would apply during pediatric cardiac arrest. As pediatric arrests are often the consequence of tissue hypoxia due to respiratory failure,12,13 increasing ventilation in an attempt to reverse any physiologic derangements (e.g., hypoxemia) prior to arrest is appropriate. However, once cardiac arrest ensues (i.e., the heart stops beating and there is no blood flow), excessive ventilation during cardiopulmonary resuscitation (CPR) decreases venous return and coronary perfusion pressure leading to lower survival rates in animal models.10 As a result, the AHA recommends that pediatric cardiac arrest victims with an advanced airway receive rescue breaths at a rate of 8–10 breaths per minute (bpm) to improve the chance of successful resuscitation regardless of etiology.12 In response to resuscitation science highlighting the importance of this controlled ventilation during CPR, mechanisms to monitor ventilation rate have been developed.14 Changes in chest wall impedance (CWI) recorded by defibrillator pads can be used to monitor ventilation rates during active CPR.15,16
Our group previously reported the quality of CCs delivered during 20 pediatric in-hospital cardiac arrests and found that trained in-hospital providers frequently perform CCs that do not meet AHA guidelines (non-compliance rates ~30%).8 Given that defibrillator pads were applied in only a small number of events (i.e., had CWI measured), we were unable to report on ventilation quality at that time and it is thus the primary objective of this investigation. Based on our previous findings of non-compliance rates of ~30% for other pediatric CPR quality variables, we hypothesized that there would be an approximate 30% incidence of excessive ventilation (>10 bpm) during pediatric CPR events. Additionally, we acknowledged that in the pediatric pre-arrest state, many children require increased respiratory support as a means to prevent cardiac arrest. Therefore, we also hypothesized that the initial moments of pediatric cardiac arrest would suffer from higher ventilation rates as providers transition from a ventilation strategy to prevent arrest to one that increases the chance of successful resuscitation by maximizing CPR hemodynamics.
2. Patients and methods
2.1. Protocol and consent
The primary objective of this prospective observational study is to quantitatively evaluate the frequency of ventilations delivered during in-hospital pediatric cardiac arrest. The study protocol including consent procedures was approved by the institutional review board at The Children's Hospital of Philadelphia. Data collection procedures were completed in compliance with the guidelines of the Health Insurance Portability and Accountability Act to ensure subject confidentiality. Written consent was obtained from all participating health care providers.
In our institution, responders to a cardiac arrest event include an intensive care (IC) or emergency medicine (EM) attending physician, IC or EM fellow physician, pediatric resident, critical care nurse, and a critical care respiratory therapist. The composition of the resuscitation team remains consistent at night and over the weekend. During a cardiac arrest event, it is our routine practice to disconnect an already intubated patient from the mechanical ventilator. Ventilations are typically provided manually by either the respiratory therapist or fellow physician.
2.2. Quantitative feedback system
The Heartstart MRx defibrillator with Q-CPR technology used in this investigation was jointly designed by Philips Healthcare (Andover, MA) and the Laerdal Medical Corporation (Stavanger, Norway) and is currently approved by the US Food and Drug Administration for patients ≥8 years of age. Data on CWI (Ohms) was obtained with self-adhesive defibrillator pads (HeartStart Pad, M3718A – Adult Radiotransparent Multifunction Electrode Pads, Philips Medical Systems, Seattle, WA) and continuously recorded and measured via the Philips HeartStart MRx/Q-CPR biphasic monitor/defibrillator (Philips Medical Systems, Andover, MA). As all patients weighed greater than 10 kg, adult pads were selected for all patients and were placed in standard anterior-apical position.
Changes in CWI measurements are used to obtain ventilation data, allowing the calculation of a ventilation rate in bpm. The accuracy of these methods has been validated in laboratory studies and used in previous adult investigations.7,9,17 As we have previously demonstrated,18 defibrillator software does have limitations in identifying lower tidal volume ventilations. Given this limitation, in our institution, it is the responsibility of the code leader to determine if the rate of artificial ventilations is appropriate. Providers were instructed to disregard any defibrillator-generated audio prompts to increase ventilation frequency. This recommendation was reinforced through our previously reported on-going daily CPR quality training program.19 In short, during each resuscitation event, the code leader, not the defibrillator software, was used to monitor and coach ventilation rate. No alteration was made to the standard of care. If the code leader perceived the ventilator frequency to be too fast or too slow, the provider delivering ventilations was coached to decrease or increase the ventilation rate.
2.3. Subject enrollment
Cardiac arrest events requiring CCs occurring in children ≥8 years of age in the pediatric intensive care unit (PICU) or the emergency department (ED) of a children's hospital were screened for inclusion in the study. Our 45-bed PICU consists of both medical and surgical patients. Cardiac surgical patients are cared for in a separate cardiac ICU and these patients were not included in our study. Only those CPR events where defibrillation pads were applied during the resuscitation (i.e., had CWI measured) were enrolled. Any ventilation data obtained prior to the initiation of CCs (pre-arrest) were excluded from the analysis.
2.4. Review of ventilation data
A Windows®-based software program, Q-CPR Review (Version 2.1.0.0, Laerdal Medical, Stavanger, Norway) was used for the initial detection of ventilation events. Although this software has the capability to interpret CWI data and identify ventilation events, previous investigations have established concern that software analysis may fail to detect all ventilation events.17 As such, each CPR event was manually reviewed by one author (AM) and these resulting ventilation rates were reported as our gold standard (Fig. 1). As a measure of manual review reliability, a random convenience sample of 5 (21%) CPR events was reviewed independently by a second author (AO). Concordance between reviewers was analyzed by kappa statistic.20
Fig. 1.
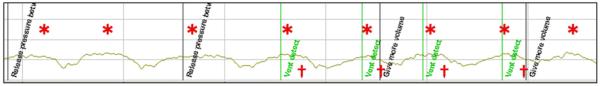
CWI tracing. *Ventilation events identified by manual review. †Ventilation events detected via software review and labeled as “Vent detect”.
2.5. Outcome variables/data analysis
In accordance with previous CPR quality investigations, all CPR events were subdivided into thirty-second (30 s) epochs.6,7 For ease of data analysis, all CPR epochs lasting less than 30 s were excluded from analysis. After this exclusion, the ventilation rate in breaths per minute was determined for each CPR epoch. Ventilation rates of CPR epochs were grouped for analysis as follows: (1) the first 5 min of each CPR event; (2) the remainder of the CPR event; and (3) the total duration of the CPR event (i.e., first 5 min plus the remainder of the event). Our primary outcome variable was the proportion of CPR time with excessive ventilation. Excessive ventilation was defined in the following manner: (1) a ventilation rate > 10 bpm (AHA-defined)12; and (2) a ventilation rate > 20 bpm. The latter was chosen as a meaningful clinical hemodynamic threshold (i.e., while a ventilation rate of 12 bpm exceeds AHA guidelines, the clinical effect is probably negligible; whereas rates exceeding 20 bpm likely have detrimental hemodynamic consequences for the cardiac arrest victim.10,21) Our primary hypothesis was that 30% of CPR epochs will have excessive ventilation using the AHA definition (>10 bpm).
In a secondary analysis, multivariable modeling using generalized estimating equations to adjust for within-event correlation between CPR epochs was used to evaluate the association of excessive ventilation with the following a priori clinical variables: (1) time of event: adult survival from in-hospital cardiac arrest is worse during nights and weekends as compared to weekdays22; (2) location of event: The Children's Hospital of Philadelphia has an on-going daily CPR quality training program19 primarily concentrated in the PICU; and (3) rhythm: in the pre-arrest state, bradycardia may be responsive to escalated respiratory support, consequently, after progression to a no-flow state (i.e., cardiac arrest) and the initiation of CPR, rescuers may continue with what would then be considered excessive ventilation. In an exploratory analysis, ventilation rates between events with return of spontaneous circulation (ROSC) and those without ROSC were compared using Wilcoxon ranksum. Categorical variables were compared using a χ2-test. P values less than 0.05 were considered significant. Statistical analysis was completed using the Stata-IC 10.0 statistical package (Stata Corp, College Station, TX).
3. Results
Between October 2006 and June 2009, quantitative CPR data was collected on 44 consecutive cardiac arrest events. CWI data were collected from 26 of the 44 events where defibrillator pads were applied. All subjects had an advanced airway in place at the time of cardiac arrest. Twenty-four of these events (92%) provided interpretable CWI waveforms and were included in ventilation quality assessment. Ventilation data recorded prior to the onset of CC (pre-arrest) were excluded. In total, 305 min of CPR (concurrent administration of CC and ventilations) were available for analysis. Events were further subdivided into 30 s CPR epochs (n = 621). 33 CPR epochs lasting less than 30 s were excluded, providing 588 analyzable CPR epochs. Please see Table 1 for the demographic data of enrolled patients.
Table 1.
Patient demographics.
| Age [mean (sd) years] | 15.1 ± 4.1 |
| Gender [n (%) male] | 12(50) |
| Time of daya [n (%) nights/weekends] | 12(50) |
| Initial rhythmb [n (%)] | |
| PEA/Asystole | 9(38) |
| Bradycardia | 5(21) |
| VT/VF | 7(29) |
| Unknown | 3(13) |
| Location [n (%) PICU] | 16(67) |
PEA – Pulseless Electrical Activity, VT – Ventricular Tachycardia, VF – Ventricular Fibrillation.
11:00 pm to 6:59 am Monday through Thursday nights and Friday 11:00 pm through 6:59 am Monday.
Percentages not equal to 100% due to rounding.
3.1. Primary outcome variable: proportion of CPR with excessive ventilation (Fig. 2)
Fig. 2.
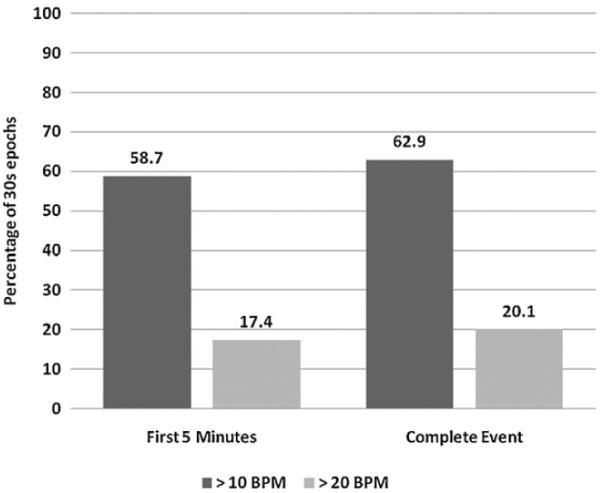
The proportion of CPR time with excessive ventilation (30 s epochs) with a ventilation rate >10 bpm (dark gray) and > 20 bpm (light gray). CPR time is reported as the first 5 min of the CPR event and the complete CPR event.
The proportion of CPR epochs with ventilation rates exceeding AHA guidelines (>10 bpm) during CPR was 63% (CI95 59–67%), significantly higher than our a priori hypothesis of 30% (p < 0.01). The proportion of CPR epochs with a ventilation rate exceeding 20 bpm was 20% (CI95 17–23%).
There was no difference between ventilation rates or proportion of CPR time with ventilation rates >10 bpm or >20 bpm between the first 5 min of the resuscitation compared to the remainder of the event (Table 2).
Table 2.
Comparison of ventilation rates and proportion of CPR time with a ventilation rate >10 bpm and >20 bpm between the first 5 min of the resuscitation compared to the remainder of the event.
| First five | Remainder | p | |
|---|---|---|---|
| Rate [(bpm; mean (sd))] | 14.8 ± 10.7 | 16.0 ± 11.5 | 0.28 |
| >10 bpm[%(Cl95)] | 59 (51–66) | 65 (60–69) | 0.18 |
| >20bpm[%(Cl95)] | 17 (12–23) | 21 (17–25) | 0.30 |
3.2. Excessive ventilation: clinical associations
After controlling for location of arrest and initial event rhythm, CPR epochs that occurred on nights/weekends were 3.6 times (CI95: 1.6–7.9, p < 0.01) more likely to have a ventilation rate in excess of 10 bpm and more than 5.5 times (1.3–23.8, p < 0.03) more likely to exceed 20 bpm. There was no significant difference between median event ventilation rates where ROSC was attained (median 11.1 bpm (IQR 6.6–28.0)) vs. those events where ROSC was not attained (median 12.8 bpm (IQR 8.1–19.1); p = 0.92).
3.3. Software review vs. manual event review
Given the insensitivity of the defibrillator software to detect ventilation events,18 we manually reviewed all CWI data. Prior to manual review, we utilized defibrillator software to review CWI data and determine the ventilation rate. There was a statistically significant difference between calculated ventilation rates by review method: software review median 10.1 bpm (IQR: 4.8–12.6) vs. manual review median 12.5 bpm (IQR 7.8–20.6); p = 0.03.
3.4. Reliability of manual event review
There was 80% agreement between reviewers in the random subset of events selected for reliability analysis (>10 bpm, kappa = 0.62; >20 bpm, kappa = 0.55). It should be noted that the results of the kappa statistic vary according to the prevalence of the finding. Because the incidence of excessive ventilation was high, this may result in a low kappa statistic, and thus, a lower reliability of measurement.
4. Discussion
This study establishes the incidence of excessive ventilation in older children and adolescents during pediatric in-hospital cardiac arrest. In the pre-arrest state, rescuers may appropriately increase the respiratory support provided to pediatric patients. However, after a no-flow state occurs, ventilations should be limited to maximize coronary perfusion.10 Despite rescuer training and oversight by code leaders, providers at our institution frequently ventilated cardiac arrest patients with rates in excess of those recommended by AHA guidelines. Excessive ventilation was present at both the beginning and for the duration of resuscitation attempts. Moreover, 20% of CPR time had a ventilation rate >20 bpm, a rate that is double that recommended by the AHA. In short, excessive ventilation was common in our resuscitation attempts. While the clinical significance of excessive ventilations during CPR remains unclear, animal models of cardiac arrest suggest that an increased ventilation rate is associated with decreased survival.10 While determining the clinical significance of excessive ventilation during CPR remains a question to be answered, the primary aim of this study was to quantify deviation from AHA guidelines for ventilation rate during CPR.
During CPR, cardiac output is reduced to less than 25% of normal, leading to a decreased ventilation requirement during CPR. Porcine models of cardiac arrest using ventilation rates similar to those identified in human studies demonstrate that an increased ventilation rate leads to increased mean intra-thoracic pressure, and consequently, a decreased coronary perfusion pressure.21,23 In animal models, these physiological derangements are associated with a statistically significant decreased rate of survival.21,23
Adult studies of in-hospital cardiac arrest have documented decreased survival when events occur at night or on the weekend.22 Likewise, adult studies have shown that patients with intracerebral hemorrhage and pulmonary embolism admitted on the weekend have an increased rate of mortality as compared to those admitted during the week.24,25 In our group of patients, there was a statistically significant association with excessive ventilation and the time of event, with excessive ventilation being more likely to occur at night and on the weekends. While our study is underpowered to determine if there is a decreased rate of ROSC at night or on the weekends, the association remains interesting. With further investigation, it may become clear that quality improvement efforts should be targeted to include all time periods of hospital staffing.26
Now that excessive ventilation has been documented during pediatric resuscitation, efforts should be directed towards reduction of the ventilation rate in future events. In adult models, the use of automated feedback during CPR has resulted in a reduction in excessive ventilation rates.27 However, it is troubling that we observed such a high rate of excessive ventilation even when rescuers were assisted by defibrillator automated feedback. As an alternative, the use of a formal post-event CPR debriefing program can lead to a reduction in the ventilation rate seen during adult resuscitation attempts.28 It appears that similar targeted training programs would be desirable in pediatric institutions.
Defibrillator pads attached to the chest can monitor changes in CWI and thereby monitor the rate of ventilation.15 With the use of defibrillator pads during CPR, this method of ventilation monitoring seems practical, though not without technical challenge. Similar to adult studies, we noted noise within the CWI tracing (possibly from CCs or patient movement) and small impedance changes (possibly from low tidal volume ventilation).17 As a result, as seen in Fig. 1, software algorithms may fail to detect ventilation events. Supporting previous studies,17 we found a statistically significant difference between manual and software calculated ventilation rates.
There are several limitations to this study. First, as discussed above, there was variable quality to the CWI tracings. Additionally, we are lacking data on the quality of defibrillation pad attachment and the exact position of defibrillation pad attachment. However, all pads were placed in the anterior-apical position, limiting some of the variability in pad placement. Standard practice at our institution is to disconnect the patient from the mechanical ventilator during CPR and proceed with manual ventilation. However, we do not have data to confirm that this is the case for all data in our study. It is possible, though unlikely, that the observed excessive ventilation rates may have been delivered by a mechanical ventilator. Regardless of mode of delivery, the patient was excessively ventilated during the CPR. While our goal was to characterize the presence of excessive ventilation during pediatric CPR, we do not have a gold standard for this measurement. Although we attempted to evaluate concordance among event reviewers, we lack a precise mechanism to determine the true ventilation rate. In the future, alternative mechanisms to monitor the ventilation rate, such as capnography (End-tidal CO2) or airway pressure14,29 will be utilized. Alternatively, additional information about our existing data set could be gleaned from review of either audio or video transcripts.
We report on ventilation frequency performed with Q-CPR technology assistance and after the initiation of a bedside CPR retraining program.19,30 Ventilation quality without the assistance of automated feedback, or in an environment not primed to train and focus attention on the quality of CPR, was not assessed. Under the circumstances of intense training and attention to CPR quality, excessive ventilation still occurred. Our results are in accordance with adult units focusing on CPR quality, whose documented ventilation rate was greater than 20 bpm for approximately 60% of CPR duration.7 In addition, we should emphasize that we analyzed only ventilation rate. In future studies we hope to further characterize ventilation rate by describing inflation rate and tidal volume of rescue breath ventilations. While we have demonstrated an excessive rate of ventilation in this study, it is probable that a measure combining ventilation rate, tidal volume, and mean airway pressure is more important in determining resuscitation quality and/or outcome.
5. Conclusion
During pediatric cardiac arrest, rescuers provide artificial ventilations at rates in excess of those recommended by the AHA (>10 bpm) with 20 percent of CPR time exceeding 20 bpm. Further, rates of ventilation were higher during resuscitations that occurred during nights and weekends. Future studies should be directed at accurately monitoring the frequency of ventilations delivered during pediatric CPR, training frontline providers to concentrate on this component of high quality CPR.
Acknowledgments
Unrestricted research grant support: Vinay Nadkarni and Dana Niles from the Laerdal Foundation for Acute Care Medicine; Benjamin Abella from Philips Healthcare and Cardiac Science Corporation.
Financial disclosure This study was supported by the Laerdal Foundation for Acute Care Medicine and the Endowed Chair of Pediatric Critical Care Medicine at the Children's Hospital of Philadelphia. Robert M. Sutton receives funding from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (Award Number K23HD062629).
Abbreviations
- AHA
American Heart Association
- CPR
cardiopulmonary resuscitation
- CWI
chest wall impedance
- CC
chest compression
- bpm
breaths per minute
- ROSC
return of spontaneous circulation
- IC
intensive care
- EM
emergency medicine
Footnotes
A Spanish translated version of the summary of this article appears as Appendix in the final online version at doi:10.1016/j.resuscitation.2011.03.020.
Conflicts of interest statement No additional conflict of interest exists.
References
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the resuscitation outcomes consortium epistry-cardiac arrest. Circulation. 2009;119:1484–91. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid response teams: a systematic review and meta-analysis. Arch Intern Med. 2010;170:18–26. doi: 10.1001/archinternmed.2009.424. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue AJ, Nadkarni V, Berg RA, et al. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–22. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Meaney PA, Nadkarni VM, Cook EF, et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118:2424–33. doi: 10.1542/peds.2006-1724. [DOI] [PubMed] [Google Scholar]
- 5.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–7. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation. 2005;111:428–34. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 7.Abella BS, Alvarado JP, Myklebust H, et al. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA. 2005;293:305–10. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 8.Sutton RM, Maltese MR, Niles D, et al. Quantitative analysis of chest compression interruptions during in-hospital resuscitation of older children and adolescents. Resuscitation. 2009;80:1259–63. doi: 10.1016/j.resuscitation.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293:299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 10.Aufderheide TP, Lurie KG. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32(Suppl.):S345–51. doi: 10.1097/01.ccm.0000134335.46859.09. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill JF, Deakin CD. Do we hyperventilate cardiac arrest patients? Resuscitation. 2007;73:82–5. doi: 10.1016/j.resuscitation.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: pediatric advanced life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(Suppl. 3):S876–908. doi: 10.1161/CIRCULATIONAHA.110.971101. [DOI] [PubMed] [Google Scholar]
- 13.Moler FW, Meert K, Donaldson AE, et al. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med. 2009;37:2259–67. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terndrup TE, Rhee J. Available ventilation monitoring methods during pre-hospital cardiopulmonary resuscitation. Resuscitation. 2006;71:10–8. doi: 10.1016/j.resuscitation.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Losert H, Risdal M, Sterz F, et al. Thoracic impedance changes measured via defibrillator pads can monitor ventilation in critically ill patients and during cardiopulmonary resuscitation. Crit Care Med. 2006;34:2399–405. doi: 10.1097/01.CCM.0000235666.40378.60. [DOI] [PubMed] [Google Scholar]
- 16.Risdal M, Aase SO, Stavland M, Eftestol T. Impedance-based ventilation detection during cardiopulmonary resuscitation. IEEE Trans Biomed Eng. 2007;54:2237–45. doi: 10.1109/tbme.2007.908328. [DOI] [PubMed] [Google Scholar]
- 17.Edelson DP, Eilevstjonn J, Weidman EK, Retzer E, Hoek TL, Abella BS. Capnography and chest-wall impedance algorithms for ventilation detection during cardiopulmonary resuscitation. Resuscitation. 2010;81:317–22. doi: 10.1016/j.resuscitation.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts K, Srinivasan V, Niles DE, et al. Does change in thoracic impedance measured via defibrillator electrode pads accurately detect ventilation breaths in children? Resuscitation. 2010;81:1544–9. doi: 10.1016/j.resuscitation.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Niles D, Sutton RM, Donoghue A, et al. “Rolling Refreshers”: a novel approach to maintain CPR psychomotor skill competence. Resuscitation. 2009;80:909–12. doi: 10.1016/j.resuscitation.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 21.Yannopoulos D, Tang W, Roussos C, Aufderheide TP, Idris AH, Lurie KG. Reducing ventilation frequency during cardiopulmonary resuscitation in a porcine model of cardiac arrest. Respir Care. 2005;50:628–35. [PubMed] [Google Scholar]
- 22.Peberdy MA, Ornato JP, Larkin GL, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785–92. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 23.Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960–5. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
- 24.Crowley RW, Yeoh HK, Stukenborg GJ, Medel R, Kassell NF, Dumont AS. Influence of weekend hospital admission on short-term mortality after intracerebral hemorrhage. Stroke. 2009;40:2387–92. doi: 10.1161/STROKEAHA.108.546572. [DOI] [PubMed] [Google Scholar]
- 25.Aujesky D, Jimenez D, Mor MK, Geng M, Fine MJ, Ibrahim SA. Weekend versus weekday admission and mortality after acute pulmonary embolism. Circulation. 2009;119:962–8. doi: 10.1161/CIRCULATIONAHA.108.824292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckett DJ, Gordon CF, Paterson R, et al. Improvement in out-of-hours outcomes following the implementation of Hospital at Night. QJM. 2009;102:539–46. doi: 10.1093/qjmed/hcp056. [DOI] [PubMed] [Google Scholar]
- 27.Abella BS, Edelson DP, Kim S, et al. CPR quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73:54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Edelson DP, Litzinger B, Arora V, et al. Improving in-hospital cardiac arrest process and outcomes with performance debriefing. Arch Intern Med. 2008;168:1063–9. doi: 10.1001/archinte.168.10.1063. [DOI] [PubMed] [Google Scholar]
- 29.Morley PT. Monitoring the quality of cardiopulmonary resuscitation. Curr Opin Crit Care. 2007;13:261–7. doi: 10.1097/MCC.0b013e32814b05bd. [DOI] [PubMed] [Google Scholar]
- 30.Sutton RM, Niles D, Meaney PA, et al. Booster” training: Evaluation of instructorled bedside cardiopulmonary resuscitation skill training and automated corrective feedback to improve cardiopulmonary resuscitation compliance of Pediatric Basic Life Support providers during simulated cardiac arrest. Pediatr Crit Care Med. 2011;12:1–6. doi: 10.1097/PCC.0b013e3181e91271. [DOI] [PMC free article] [PubMed] [Google Scholar]


