Abstract
PTMs (post-translational modifications) of lysine residues have proven to be major regulators of gene expression, protein–protein interactions, and protein processing and degradation. This is of particular importance in regulating the cytoskeleton, an enormously complex system of proteins responsible for cell motility, intracellular trafficking, and maintenance of cell form and structure. The cytoskeleton is present in all cells, including eukaryotes and prokaryotes, and comprises structures such as flagella, cilia and lamellipodia which play critical roles in intracellular transport and cellular division. Cytoskeletal regulation relies on numerous multi-component assemblies. In this chapter, we focus on the regulation of the cytoskeleton by means of PTMs of lysine residues on the cytoskeletal subunits and their accessory proteins. We specifically address the three main classes of cytoskeletal proteins in eukaryotes that polymerize into filaments, including microfilaments (actin filaments), intermediate filaments and microtubules. We discuss the identification and biological importance of lysine acetylation, a regulator of all three filament types. We also review additional lysine modifications, such as ubiquitination and SUMOylation, and their role in protein regulation and processing.
Introduction
The cytoskeleton is an enormously complex system of proteins responsible for cell motility and maintenance of cell form and structure. The ability of a cell to adopt various shapes and perform directed motility is a co-ordinated effort driven by many protein interactions. The cytoskeleton is present in all cells, including eukaryotes and prokaryotes, and comprises structures such as flagella, cilia and lamellipodia which play important roles in intracellular transport and cellular division. The eukaryotic cytoskeleton is comprised of three main types of proteins polymerized into filaments, classified as microfilaments (actin filaments), intermediate filaments and microtubules. Polymerized filaments can serve as molecular tracks on which protein motors take ‘steps’ and move cargo, including membrane-bound organelles and macromolecular complexes [1]. These tracks also serve to co-ordinate whole-cell locomotion.
Microfilaments (actin filaments)
Microfilaments are thin flexible linear polymers of actin subunits cross-linked into bundles. The ability of a cell to assume different shapes and perform directed motility is driven by the polymerization of actin filaments in the cytoskeleton. A series of actin-binding proteins give rise to an orthogonal network of these actin filaments at the leading edge which help to push the cell forward [1]. The ability of a cell to co-ordinate the assembly and disassembly of its actin cytoskeleton is essential for cell integrity, motility, membrane trafficking and shape changes [2]. Additionally, actin filaments co-operate with myosin molecules that attach to the filament producing two types of movements [3]. First, the myosin–actin interaction generates a force between actin filaments, producing contractions that pull up the rear of moving cells, pinch dividing cells in two, and change cellular shapes to form tissues, similar to muscle cell contractions [3]. Secondly, myosins serve as molecular motors carrying subcellular organelles and macro-molecular complexes of proteins and RNAs along actin filaments over short distances [3].
Intermediate filaments
The most complex of the cytoskeletal proteins, intermediate filaments, are comprised of at least 50 different proteins subcategorized into six broad types on the basis of tissue-specific expression, sequence similarity and protein structure [4]. Intermediate filaments help to organize the three-dimensional structure of cells, securing organelles and helping to prevent excessive stretching of cells by external forces. They also participate in anchoring cell–cell contacts and cell–matrix junctions, providing structural stability, flexibility and integrity of different cells and tissues [4].
Microtubules
Microtubules are essential cytoskeletal polymers that are made up of repeating α,β-tubulin heterodimers and are present in all eukaryotes. These rigid cylindrical polymers affect cell shape, cell transport, cell motility and cell division [5]. Microtubule motors power the beating of cilia and flagella, many organelle movements in animal cells and chromosomal movements during mitosis [6]. Similar to the actin–myosin relationship, motor proteins in the kinesin and dynein families move cargoes along microtubules or microtubules with respect to each other [5].
In general, cytoskeletal regulation relies on numerous multi-component assemblies. In the present chapter, we focus on the regulation of the cytoskeleton by means of PTM (post-translational modification) of lysine residues on the cytoskeletal subunits and their accessory proteins.
Evidence of lysine acetylation of cytoskeletal complexes
As discussed in previous chapters in this volume, acetylation is a reversible PTM of proteins and is a major regulator of gene expression. Technological limitations, cell and/or protein isoform specificity, and the reversible nature of lysine acetylation have complicated the analysis of the role of lysine acetylation in a variety of cellular activities. A previous study successfully identified 3600 lysine acetylation sites on 1750 proteins, concluding that lysine acetylation preferentially targets large macromolecular complexes involved in diverse cellular processes [7]. These include proteins associated with chromatin remodelling, cell cycle, splicing, nuclear transport and actin nucleation.
Microfilaments (actin)
The ability of a cell to change and maintain its shape and perform directed motility, is largely driven by the polymerization of actin filaments. Thus identifying the PTMs on proteins responsible for the co-ordination of the actin cytoskeleton and the regulation of their interactions is important. The use of an antibody specifically targeted against acetyl-lysine to enrich acetylated peptides following a trypsin digestion of whole-cell lysates from a human myeloid leukaemia cell line, as well as stable-isotope labelling with amino acids in cell culture, resulted in the discovery of many proteins containing acetylation sites using high-resolution MS (mass spectrometry) [7]. Among the thousands of proteins discovered, a significant number of proteins controlling actin-based cell motility were identified. Six of the seven subunits of the Arp2/3 (actin-related protein 2/3) complex, a well-studied actin nucleation complex that forms new actin filaments off of the sides of existing actin filaments, are acetylated on lysine residues [7]. Cortactin, a regulator of the Arp2/3 complex, which is of particular importance in invadopodia function [8], was also shown to be acetylated, as were the actin/Arp2/3-interacting proteins cofilin and coronin [7,9].
Intermediate filaments
Although intermediate filaments contain more than 50 genes encoding six classes of proteins in vertebrates, all intermediate filament proteins share a similar structure consisting of a rod-like domain with variable head and tail domains at the two ends. Intermediate filaments have up to 16 coiled-coil domains, with the subunits organized into thin rope-like polymers. The central rod domain is a major driving force during the assembly of all intermediate filament proteins. The end domains are important sites of regulation and interaction with other cellular elements and additionally contribute to assembly [10]. All major classes of filaments have been implicated as mechanical scaffolds in differentiated cell types. Defects in structure or organization cause a loss of cellular integrity following exposure to shearing forces [11]. Some of the intermediate filament family members have been found to be acetylated. Vimentin, a class III filament found in mesenchymal cells, is acetylated on several lysine residues (Lys104, Lys120, Lys139, Lys235, Lys292, Lys373 and Lys445) [7] and cytokeratin 8, a class II basic keratin found in epithelial cells, is acetylated on three lysine residues (Lys10, Lys471 and Lys482) [12].
Microtubules
Of the three filaments described here, tubulin acetylation is the most extensively studied and best characterized. In fact, tubulin was the first acetylated protein described in the cytoplasm [12]. Acetylation/deacetylation of tubulin regulates microtubule function such that overexpression of HDAC (histone deacetylase) 6, which mediates tubulin deacetylation, increases chemotactic movement of NIH 3T3 cells [13], whereas inhibition of HDAC6 inhibits cell migration [14]. The acetyltransferase ELP3 (elongation protein 3), a subunit in the transcriptional elongator complex, was shown to acetylate tubulin, which is essential for the maturation of cortical neurons [15]. Evidence for the imporantance of ELP3 in neuronal development is further supported by RNAi (RNA interference) supression screens for tubulin-acetylation regulators in Caenorhabditis elegans [16]. Additional proteins, including GCN5 (general control of amino acid synthesis 5) [17] and MEC17 (mechanosensory abnormal 17) [18], have also been identified as acetyltransferases capable of acetylating tubulin.
Biological significance of lysine acetylation of cytoskeletal complexes
Microfilaments (actin)
To understand actin regulation, it is important to understand the process of actin polymerization. Actin is present in two forms: monomeric or G-actin (globular actin) polymerizes into the second F-actin (filamentous actin) creating a double helix which can be bundled into fibres. There are three major isoforms of actin. β- and γ-actin form stress fibres and are important for cell shape and cell movement, whereas α-actin comprises the microfilaments found in muscle cells which, together with myosin, directs muscle contraction and also cytoplasmic streaming in non-muscle cells [12]. Proteomic approaches have shown that all three actin isoforms can be acetylated [7,19] and acetylation of Lys61 in γ-actin may result in stabilization of actin stress fibres [19].
Not only actin itself, but several regulatory proteins of the actin cytoskeleton, are modified by acetylation. Cortactin, mentioned previously as a regulator of the actin nucleator Arp2/3, can be acetylated on nine different lysine residues and, when acetylated, its translocation to the cell periphery is inhibited and actin-binding capacity is reduced [12,20]. The decrease in nucleation and actin dynamics at the cell periphery alters cell motility. In contrast, deacetylation of cortactin may induce reduced cell migration [20,21]. Actin dynamics is also regulated by small GTPases of the Rho family of proteins: RhoA in stress fibre formation, Rac1 in lamellipodia formation and Cdc42 (cell-division cycle 42) in filopodia formation [12]. The activation of these proteins sequentially depends on the action of GDIs (guanine-nucleotide-dissociation inhibitors), GAPs (GTPase-activating proteins) and GEFs (guanine-nucleotide-exchange factors). Acetylation of RhoGDIα leads to enhanced stress fibre and filopodia formation [19].
Muscle contraction is also regulated by acetylation. By immunohistochemical and electron microscopic analyses, HDAC3 was localized to sarcomeres, the basic unit of a muscle, and was capable of deacetylating MHC (myosin heavy-chain) isoforms [9]. This study also demonstrated that the motor domains of both cardiac α- and β-MHC isoforms are reversibly acetylated, and that lysine acetylation increased the actin-sliding velocity of α- and β-myosin by more than 20% compared with their respective non-acetylated isoforms, and that myosin acetylation is sensitive to cardiac stress [9].
Intermediate filaments
As discussed, some of the intermediate filament family members have been found to have acetylated lysine residues, including vimentin and cytokeratin. Although the role of these acetylations remains unclear, acetylation is recognized as an important PTM of proteins that regulates protein function. In contrast with tubulin and actin, however, acetylation has been shown to destabilize the polymer [22,23]. For example, the increase of acetylation was coupled with a breakdown in intermediate filaments in the cytoskeleton [22].
Microtubules
Two tubulin isoforms, α- and β-, form heterodimers serving as the foundation of microtubules. Although acetylation on Lys40 of α-tubulin has long been known, recent evidence has identified several additional acetylated lysine residues in different tubulin isoforms [7]. While controversy exists regarding the functional role of acetylation at Lys40, it is accepted that acetylated microtubules represent a subpopulation of more stable microtubles in the cell [12]. Three cell types contain a population of heavily acetylated microtubules in their cytoplasm: neurons, platelets and megakaryocytes; in addition, substructures formed by microtubules, including primary cilia, flagella, mitotic spindles and midbodies, are also heavily acetylated, offering an hypothesis on the significance of acetylation. The commonality of all of these structures is the need for microtubule bundles, thus acetylation may increase bundling efficiency and stability [12]. Biological evidence supports the hypothesis that acetylation accompanies bundle formation [24–28].
As mentioned earlier, microtubules can serve as molecular tracks on which the motor proteins kinesin, which travels in a positive direction (usually from the centre of the cell towards the periphery), and dynein, with an opposite direction of travel, transport cargo throughout the cell. Studies have shown that binding and mobility of kinesin and dynein is enhanced by the acetylation of tubulin [29,30].
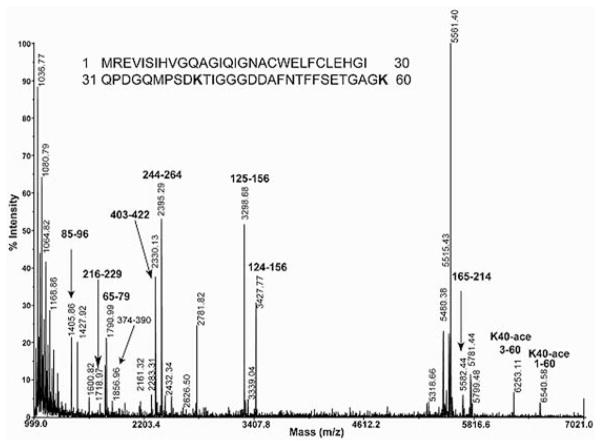
MS has been used to confirm α-tubulin acetylation. As shown in Figure 1 [31], acetylation of Lys40 of Toxoplasma gondii α-tubulin interferes with the standard trypsin cleavage site (after lysine and arginine residues). The result is the appearance of a longer peptide with an additional +42 Da mass, indicating the addition of an acetyl group. The predicted mass of the peptide containing amino acid residues 1–60 is 6498.29 Da and the corresponding peptide measured by MS, and labelled K40-ace 1–60 in Figure 1, indicates a measured mass of 6540.30 Da, demonstrating the addition of 42 Da, consistent with acetylation [31].
Figure 1. MS analysis of acetylation of lysine on T. gondii tubulin.
Trypsin specifically cleaves peptide bonds after lysine and arginine residues. The resulting peptides are labelled by their sequence location (e.g. 85–96, 216–229 etc.). K40-ace 3–60 and K40-ace 1–60 are peptides containing acetylated Lys40. Acetylation on Lys40 modifies the lysine residue making it unrecognizable by trypsin, resulting in a blocked cleavage site; thus tryptic peptides with one extra missed cleavage (1–60) and (3–60) are observed in the mass spectrum of a trypsin digestion of T. gondii α-tubulin. Additionally, the mass of these two peptides is +42 Da larger than their predicted masses of 6498.29 and 6210.91 respectively, confirming the presence of acetylation. Reprinted, with permission, from Hui Xiao, Kamal El Bissati, Pascal Verdier-Pinard, Berta Burd, Hongshan Zhang, Kami Kim, Andras Fiser, Ruth Hogue Angeletti and Louis M. Weiss (2010), Post-translational modifications to Toxoplasma gondii α- and β-tubulins include novel C-terminal methylation, Journal of Proteome Research, 9(1), pp. 359–372, Copyright (2010) American Chemical Society.
Additional lysine modifications and other PTMs of cytoskeletal complexes
The reversible acetylation/deacetylation of lysine residues is a common protein regulator in eukaryotes. Although many other PTMs exist on lysine residues, including methylation, acylation, ubiquitination, SUMOylation and deamination, acetylation and ubiquitination are the most commonly characterized lysine modification in cytoskeletal proteins. It should be noted that there are many other PTMs occurring on other residues in cytoskeletal proteins and they will be mentioned briefly; however, we will primarily focus on lysine-specific modifications.
Microfilaments (actin)
Actin filament formation, polymerization and depolymerization is vital during cell migration and cell division. At the end of mitosis, formins contribute to the generation of actin filaments that form a contractile ring and divide the cell into two daughter cells. Questions still remain about how the formins are regulated or turned off. Recent evidence suggests that there is an Ub (ubiquitin)-mediated degradation of formin at the completion of cell division [32]. Actin itself is also post-translationally regulated. A study of the mammalian cytoskeletal proteins β- and γ-actin revealed that β-actin is N-terminally arginylated, regulating its function [33]. Arginylation is a post-translational process which transfers arginyl from tRNA on to the N-terminus of proteins, forming a peptide bond. In actin, arginylation follows acetylation and is the critical next step in N-terminal processing needed for actin functioning in vivo. Arginylated β-actin can regulate actin filament properties, β-actin localization and lamella formation in motile cells [34]. Arginylated γ-actin, unlike β-actin, proves to be highly unstable and is selectively ubiquitinated and degraded in vivo. Despite the high degree of sequence homology between β- and γ-actin, differences in the nucleotide coding sequence between the two isoforms yielded different protein levels in vivo. Because γ-actin accumulates more slowly than β-actin, it is likely that this slower processing exposes a normally hidden lysine residue for ubiquitination, leading to the preferential degradation of γ-actin upon arginylation [33].
Intermediate filaments
Among the many diverse functions of intermediate filaments is their participation in the integrity of the nucleus of eukaryotes. The nuclear lamina is a fibrous network inside the nucleus composed of intermediate filaments, or lamins, and membrane-associated proteins (nuclear lamin-associated membrane proteins). Alterations in nuclear morphology are observed in various physiological processes and pathologies, namely cell differentiation and cancers. While the molecular mechanisms that define nuclear shape are unclear, data have indicated that both depletion and overexpression of lamins result in cancers with aberrant nuclear shapes [35]. Non-lysine PTMs have proven highly significant in regulating intermediate filament function and are briefly mentioned, including farnesylation of the C-terminal part of lamins, shown to be important for modifying nuclear shape [36]. In another study, it was shown that phosphorylation of GFAP (glial fibrillary acidic protein), the principal intermediate filament protein of mature astrocytes in the central nervous system, contributes to stabilization of GFAP in both cytosolic and cytoskeletal fractions [37].
SUMOylation is a reversible process involving the addition and removal of SUMO (small ubiquitin-related modifier) polypeptides on lysine residues, playing roles in many biological processes, including the cell cycle, transcriptional regulation, chromatin integrity and the DDR (DNA-damage response). This important regulatory modification has also been found in many intermediate filament proteins. SUMOylated IFB-1, a cytoplasmic intermediate filament protein in C. elegans, regulates filament assembly via maintaining a cytoplasmic pool of unpolymerized IFB-1 [38]. SUMOylation in many mammalian cytoplasmic intermediate filaments has been implicated in human diseases [11,39].
Microtubules
The structural unit of microtubules, the α- and β-tubulin heterodimer, undergoes multiple PTMs. Besides the acetylation of lysine, well-characterized tubulin PTMs include detyrosination, glycylation and glutamylation. Tubulin detyrosination is the removal of the C-terminal tyrosine of α-tubulin, leading to what is referred to as Glutubulin with a glutamate residue as the new C-terminus [40]. Detyrosinated tubulin can recycle a tyrosine residue and return it to its original state [41]. It can also undergo subsequent elimination of the penultimate glutamate residue on α-tubulin, resulting in formation of Δ2-tubulin [42,43]. Polyglutamylation and polyglycylation are two polymodifications occurring at the C-terminal end of tubulin, in which a chain of glutamate or glycine residues of various lengths adds to single or multiple glutamate residues on the primary sequence of tubulin [44–46].
Most of these PTMs are enzymatically reversible and can serve as fine regulators of diverse microtubule functions within the cell. For example, Glu-tubulin has been associated with microtubule stability, with higher abundance in differentiating cells than dividing cells [47,48]. Spastin and katanin are MAPs (microtubule-associated proteins) that function in microtubule severing and are regulated by the polyglutamylation of tubulin [49]. Tau is another member of the MAP family that is regulated by tubulin polyglutamylation [50,51]. MAP1a and the motor protein kinesin are also regulated by polyglutamylation [52,53], each with its preferred glutamylation length.
Summary.
PTMs on lysine residues are major regulators of gene expression, protein–protein interactions, and protein processing and degradation.
In the cytoskeleton, in particular, acetylation of lysine residues has a major role in the association of proteins with cytoskeletal filaments, as well as the formation of specialized cytoskeletal structures.
Other modifications, such as methylation, acylation, ubiquitination, SUMOylation and deamination are also seen on lysine residues and are associated with changes in stability and function of various cytoskeleton structures.
Acknowledgments
The work of the authors is supported by the National Institutes of Health [grant numbers AI31788 and AI39454 (to L.M.W.)].
References
- 1.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 2.Millard TH, Sharp SJ, Machesky LM. Signalling to actin assembly via the WASP (Wiskott–Aldrich syndrome protein)-family proteins and the Arp2/3 complex. Biochem J. 2004;380:1–17. doi: 10.1042/BJ20040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamcheu JC, Siddiqui IA, Syed DN, Adhami VM, Liovic M, Mukhtar H. Keratin gene mutations in disorders of human skin and its appendages. Arch Biochem Biophys. 2011;508:123–137. doi: 10.1016/j.abb.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nogales E. Structural insights into microtubule function. Annu Rev Biochem. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- 6.Dutcher SK. The tubulin fraternity: alpha to eta. Curr Opin Cell Biol. 2001;13:49–54. doi: 10.1016/s0955-0674(00)00173-3. [DOI] [PubMed] [Google Scholar]
- 7.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 8.Clark ES, Weaver AM. A new role for cortactin in invadopodia: regulation of protease secretion. Eur J Cell Biol. 2008;87:581–590. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samant SA, Courson DS, Sundaresan NR, Pillai VB, Tan M, Zhao Y, Shroff SG, Rock RS, Gupta MP. HDAC3-dependent reversible lysine acetylation of cardiac myosin heavy chain isoforms modulates their enzymatic and motor activity. J Biol Chem. 2011;286:5567–5577. doi: 10.1074/jbc.M110.163865. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Coulombe PA, Ma L, Yamada S, Wawersik M. Intermediate filaments at a glance. J Cell Sci. 2001;114:4345–4347. doi: 10.1242/jcs.114.24.4345. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- 12.Sadoul K, Wang J, Diagouraga B, Khochbin S. The tale of protein lysine acetylation in the cytoplasm. J Biomed Biotechnol. 2011;2011:970382. doi: 10.1155/2011/970382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 14.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of α-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 16.Solinger JA, Paolinelli R, Kloss H, Scorza FB, Marchesi S, Sauder U, Mitsushima D, Capuani F, Sturzenbaum SR, Cassata G. The Caenorhabditis elegans elongator complex regulates neuronal α-tubulin acetylation. PLoS Genet. 2010;6:e1000820. doi: 10.1371/journal.pgen.1000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conacci-Sorrell M, Ngouenet C, Eisenman RN. Myc-nick: a cytoplasmic cleavage product of Myc that promotes α-tubulin acetylation and cell differentiation. Cell. 2010;142:480–493. doi: 10.1016/j.cell.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. MEC-17 is an α-tubulin acetyltransferase. Nature. 2010;467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Zhang M, Dong H, Yong S, Li X, Olashaw N, Kruk PA, Cheng JQ, Bai W, Chen J, et al. Deacetylation of cortactin by SIRT1 promotes cell migration. Oncogene. 2009;28:445–460. doi: 10.1038/onc.2008.388. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, et al. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drake PJ, Griffiths GJ, Shaw L, Benson RP, Corfe BM. Application of high-content analysis to the study of post-translational modifications of the cytoskeleton. J Proteome Res. 2009;8:28–34. doi: 10.1021/pr8006396. [DOI] [PubMed] [Google Scholar]
- 23.Leech SH, Evans CA, Shaw L, Wong CH, Connolly J, Griffiths JR, Whetton AD, Corfe BM. Proteomic analyses of intermediate filaments reveals cytokeratin 8 is highly acetylated: implications for colorectal epithelial homeostasis. Proteomics. 2008;8:279–288. doi: 10.1002/pmic.200700404. [DOI] [PubMed] [Google Scholar]
- 24.Naranatt PP, Krishnan HH, Smith MS, Chandran B. Kaposi’s sarcoma-associated herpesvirus modulates microtubule dynamics via RhoA-GTP-diaphanous 2 signaling and utilizes the dynein motors to deliver its DNA to the nucleus. J Virol. 2005;79:1191–1206. doi: 10.1128/JVI.79.2.1191-1206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iliev AI, Djannatian JR, Opazo F, Gerber J, Nau R, Mitchell TJ, Wouters FS. Rapid microtubule bundling and stabilization by the Streptococcus pneumoniae neurotoxin pneumolysin in a cholesterol-dependent, non-lytic and Src-kinase dependent manner inhibits intracellular trafficking. Mol Microbiol. 2009;71:461–477. doi: 10.1111/j.1365-2958.2008.06538.x. [DOI] [PubMed] [Google Scholar]
- 26.Tonami K, Kurihara Y, Aburatani H, Uchijima Y, Asano T, Kurihara H. Calpain 6 is involved in microtubule stabilization and cytoskeletal organization. Mol Cell Biol. 2007;27:2548–2561. doi: 10.1128/MCB.00992-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa-Goto K, Tanaka K, Ueno T, Kurata T, Sata T, Irie S. p180 is involved in the interaction between the endoplasmic reticulum and microtubules through a novel microtubule-binding and bundling domain. Mol Biol Cell. 2007;18:3741–3751. doi: 10.1091/mbc.E06-12-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falconer MM, Vielkind U, Brown DL. Establishment of a stable, acetylated microtubule bundle during neuronal commitment. Cell Motil Cytoskeleton. 1989;12:169–180. doi: 10.1002/cm.970120306. [DOI] [PubMed] [Google Scholar]
- 29.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeWard AD, Alberts AS. Ubiquitin-mediated degradation of the formin mDia2 upon completion of cell division. J Biol Chem. 2009;284:20061–20069. doi: 10.1074/jbc.M109.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang F, Saha S, Shabalina SA, Kashina A. Differential arginylation of actin isoforms is regulated by coding sequence-dependent degradation. Science. 2010;329:1534–1537. doi: 10.1126/science.1191701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karakozova M, Kozak M, Wong CC, Bailey AO, Yates JR, III, Mogilner A, Zebroski H, Kashina A. Arginylation of β-actin regulates actin cytoskeleton and cell motility. Science. 2006;313:192–196. doi: 10.1126/science.1129344. [DOI] [PubMed] [Google Scholar]
- 34.Polychronidou M, Grosshans J. Determining nuclear shape: the role of farnesylated nuclear membrane proteins. Nucleus. 2011;2:17–23. doi: 10.4161/nucl.2.1.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ralle T, Grund C, Franke WW, Stick R. Intranuclear membrane structure formations by CaaX-containing nuclear proteins. J Cell Sci. 2004;117:6095–6104. doi: 10.1242/jcs.01528. [DOI] [PubMed] [Google Scholar]
- 36.Takemura M, Gomi H, Colucci-Guyon E, Itohara S. Protective role of phosphorylation in turnover of glial fibrillary acidic protein in mice. J Neurosci. 2002;22:6972–6979. doi: 10.1523/JNEUROSCI.22-16-06972.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaminsky R, Denison C, Bening-Abu-Shach U, Chisholm AD, Gygi SP, Broday L. SUMO regulates the assembly and function of a cytoplasmic intermediate filament protein in C. elegans. Dev Cell. 2009;17:724–735. doi: 10.1016/j.devcel.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omary MB. “IF-pathies”: a broad spectrum of intermediate filament-associated diseases. J Clin Invest. 2009;119:1756–1762. doi: 10.1172/JCI39894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallak ME, Rodriguez JA, Barra HS, Caputto R. Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett. 1977;73:147–150. doi: 10.1016/0014-5793(77)80968-x. [DOI] [PubMed] [Google Scholar]
- 40.Ersfeld K, Wehland J, Plessmann U, Dodemont H, Gerke V, Weber K. Characterization of the tubulin-tyrosine ligase. J Cell Biol. 1993;120:725–732. doi: 10.1083/jcb.120.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafanechere L, Job D. The third tubulin pool. Neurochem Res. 2000;25:11–18. doi: 10.1023/a:1007575012904. [DOI] [PubMed] [Google Scholar]
- 42.Verdier-Pinard P, Pasquier E, Xiao H, Burd B, Villard C, Lafitte D, Miller LM, Angeletti RH, Horwitz SB, Braguer D. Tubulin proteomics: towards breaking the code. Anal Biochem. 2009;384:197–206. doi: 10.1016/j.ab.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, MacDonald TL, Sundberg RJ, Rebhun LI, Frankfurter A. Characterization of post-translational modifications in neuron-specific class III β-tubulin by mass spectrometry. Proc Natl Acad Sci USA. 1991;88:4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edde B, Rossier J, Le Caer JP, Desbruyeres E, Gros F, Denoulet P. Post-translational glutamylation of α-tubulin. Science. 1990;247:83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- 45.Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bre MH. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science. 1994;266:1688–1691. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- 46.Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated α tubulin are distributed differently in vivo. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- 47.Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci USA. 1987;84:9040–9044. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacroix B, van Dijk J, Gold ND, Guizetti J, Aldrian-Herrada G, Rogowski K, Gerlich DW, Janke C. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol. 2010;189:945–954. doi: 10.1083/jcb.201001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pusztai L, Jeong JH, Gong Y, Ross JS, Kim C, Paik S, Rouzier R, Andre F, Hortobagyi GN, Wolmark N, Symmans WF. Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial. J Clin Oncol. 2009;27:4287–4292. doi: 10.1200/JCO.2008.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spicakova T, O’Brien MM, Duran GE, Sweet-Cordero A, Sikic BI. Expression and silencing of the microtubule-associated protein Tau in breast cancer cells. Mol Cancer Ther. 2010;9:2970–2981. doi: 10.1158/1535-7163.MCT-10-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonnet C, Boucher D, Lazereg S, Pedrotti B, Islam K, Denoulet P, Larcher JC. Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J Biol Chem. 2001;276:12839–12848. doi: 10.1074/jbc.M011380200. [DOI] [PubMed] [Google Scholar]
- 52.Larcher JC, Boucher D, Lazereg S, Gros F, Denoulet P. Interaction of kinesin motor domains with α- and β-tubulin subunits at a tau-independent binding site. Regulation by polyglutamylation. J Biol Chem. 1996;271:22117–22124. doi: 10.1074/jbc.271.36.22117. [DOI] [PubMed] [Google Scholar]
- 53.Xiao H, El Bissati K, Verdier-Pinard P, Burd B, Zhang H, Kim K, Fiser A, Angeletti RH, Weiss LM. Post-translational modifications to Toxoplasma gondii α- and β-tubulins include novel C-terminal methylation. J Proteome Res. 2010;9:359–372. doi: 10.1021/pr900699a. [DOI] [PMC free article] [PubMed] [Google Scholar]