Abstract
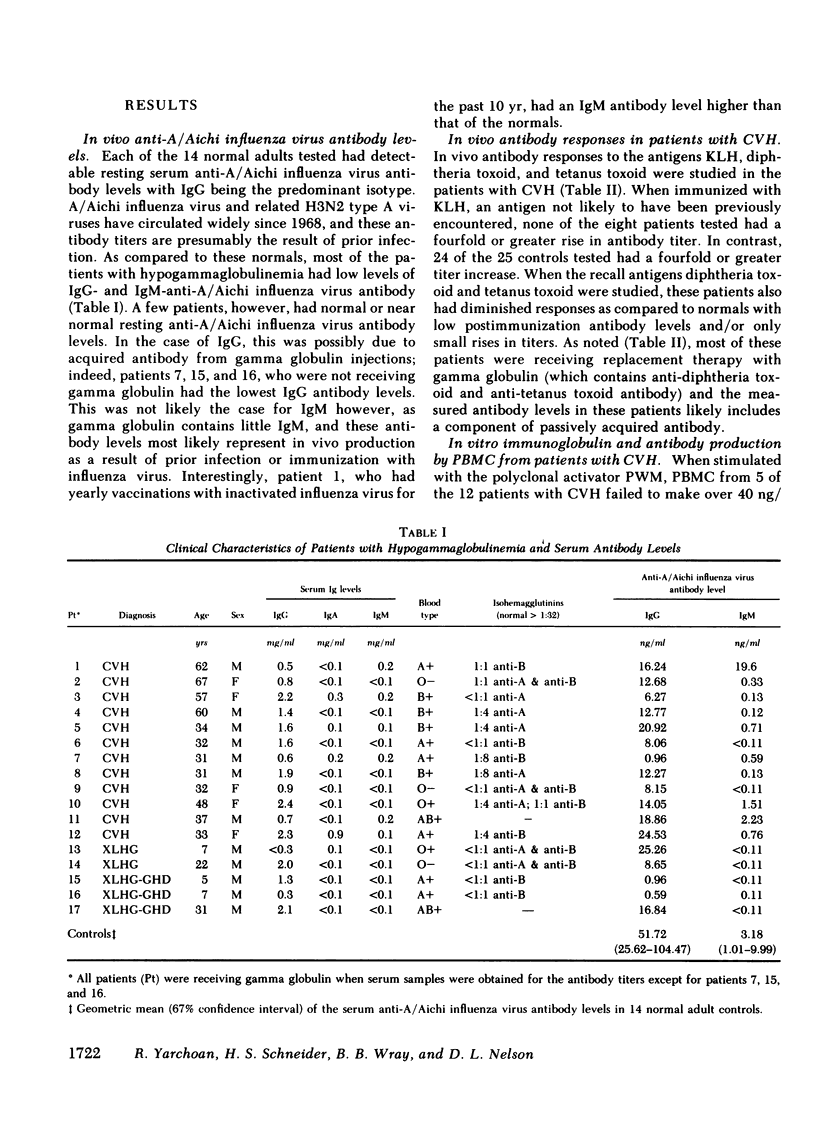
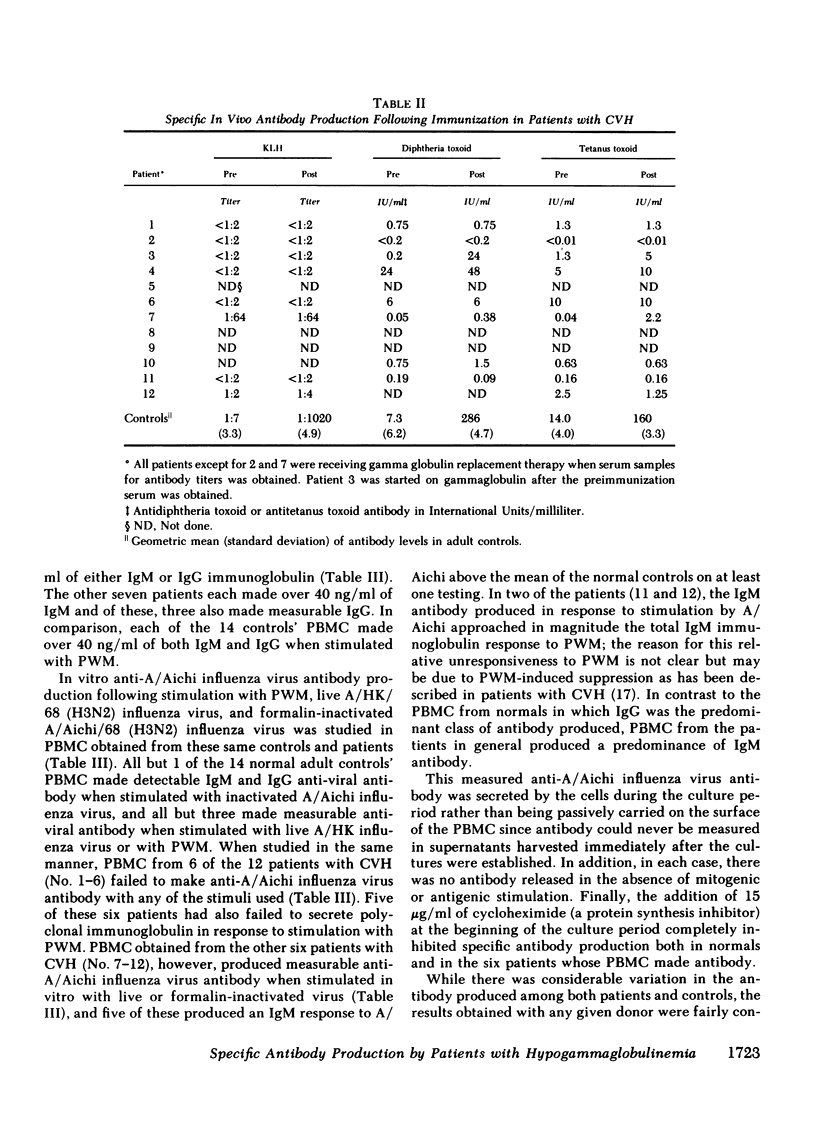
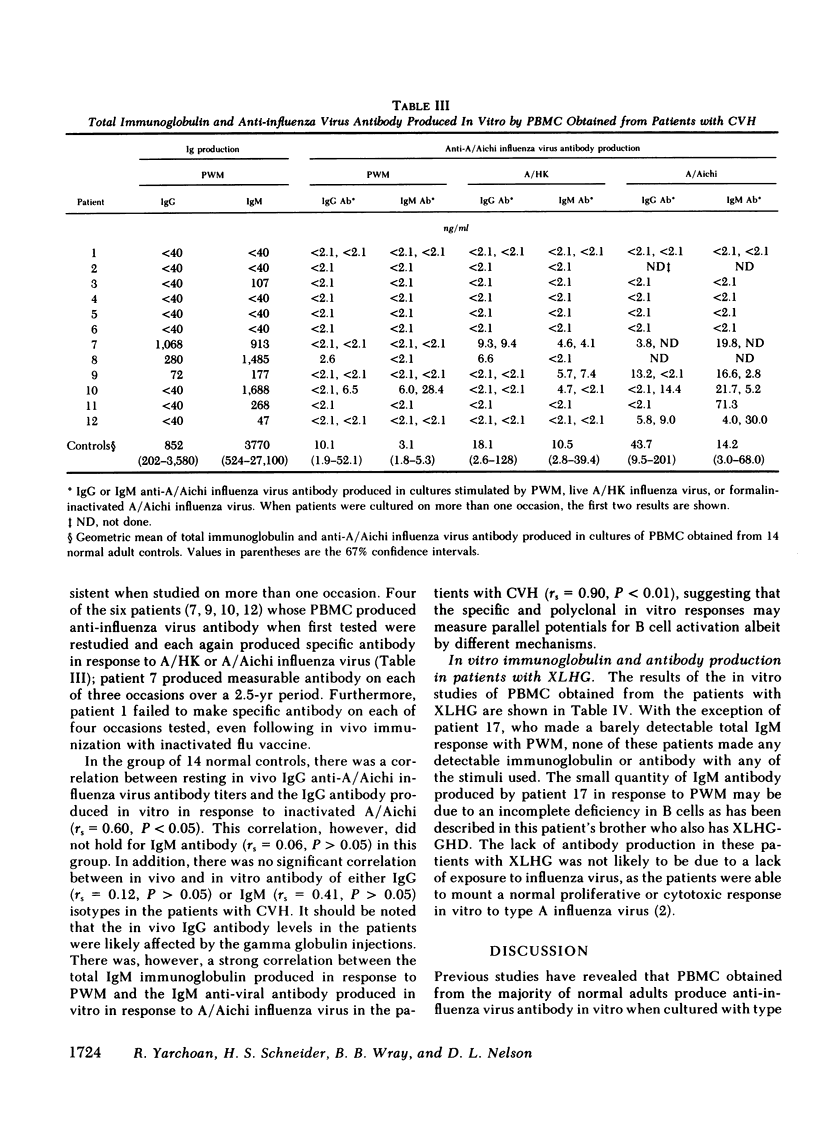
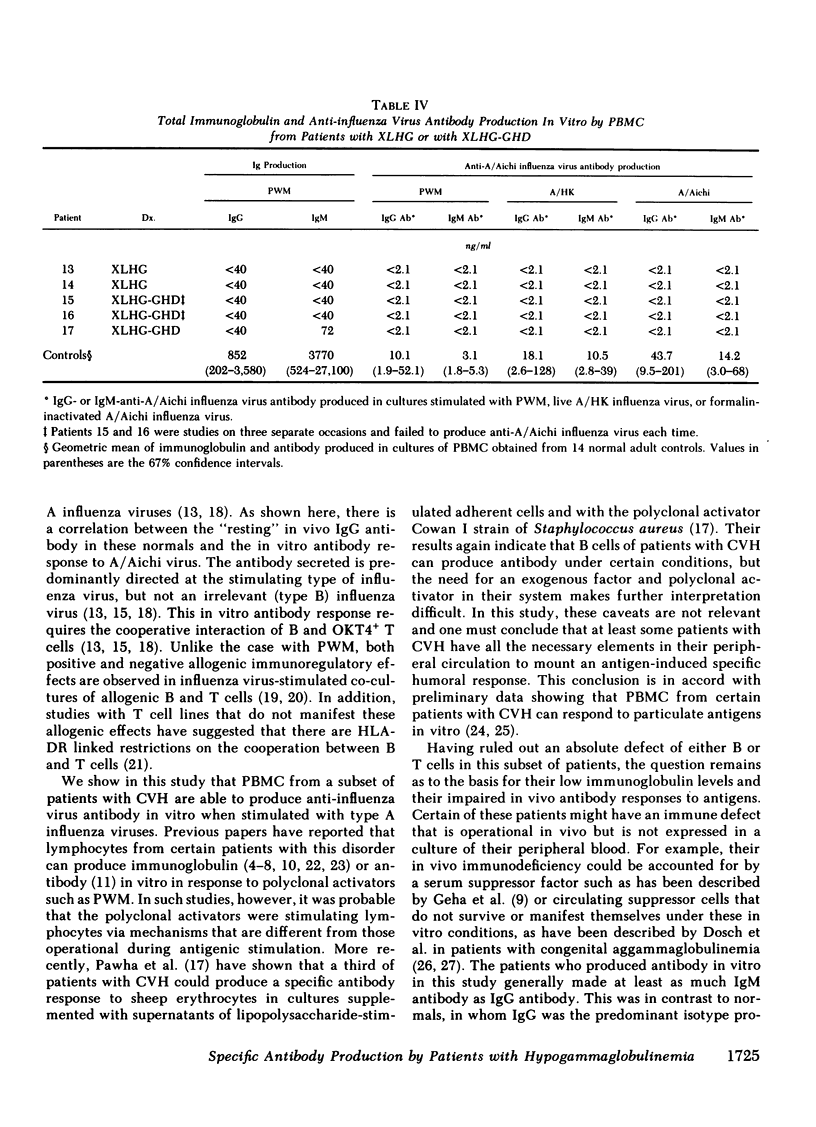
Specific anti-influenza virus antibody production in vitro was studied in peripheral blood mononuclear cells from 17 patients with hypogammaglobulinemia. Cells obtained from 6 of 12 patients with common variable hypogammaglobulinemia produced anti-influenza virus antibody, predominantly of the IgM isotype, when cultured in vitro with type A influenza virus. No antibody was produced in vitro, however, by cells from either of two patients with Bruton's type X-linked hypogammaglobulinemia or by cells from any of three patients with X-linked hypogammaglobulinemia and isolated growth hormone deficiency. These studies demonstrate that peripheral blood mononuclear cells from a subset of patients with common variable hypogammaglobulinemia retain the potential to produce specific antibody in response to antigenic stimulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callard R. E., Smith C. M. Histocompatibility requirements for T cell help in specific in vitro antibody responses to influenza virus by human blood lymphocytes. Eur J Immunol. 1981 Mar;11(3):206–212. doi: 10.1002/eji.1830110309. [DOI] [PubMed] [Google Scholar]
- Callard R. E. Specific in vitro antibody response to influenza virus by human blood lymphocytes. Nature. 1979 Dec 13;282(5740):734–736. doi: 10.1038/282734a0. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Bockman D. E. Agammaglobulinaemia with B lymphocytes. Specific defect of plasma-cell differentiation. Lancet. 1971 Oct 9;2(7728):791–794. doi: 10.1016/s0140-6736(71)92742-5. [DOI] [PubMed] [Google Scholar]
- Dosch H. M., Gelfand E. W. Functional differentiation of B lymphocytes in agammaglobulinemia. III. Characterization of spontaneous suppressor cell activity. J Immunol. 1978 Nov;121(5):2097–2105. [PubMed] [Google Scholar]
- Dosch H. M., Percy M. E., Gelfand E. W. Functional differentiation of B lymphocytes in congenital agammaglobulinemia. I. Generation of hemolytic plaque-forming cells. J Immunol. 1977 Dec;119(6):1959–1964. [PubMed] [Google Scholar]
- Fischer A., Beverley P. C., Feldmann M. Long-term human T-helper lines producing specific helper factor reactive to influenza virus. Nature. 1981 Nov 12;294(5837):166–168. doi: 10.1038/294166a0. [DOI] [PubMed] [Google Scholar]
- Fleisher T. A., White R. M., Broder S., Nissley S. P., Blaese R. M., Mulvihill J. J., Olive G., Waldmann T. A. X-linked hypogammaglobulinemia and isolated growth hormone deficiency. N Engl J Med. 1980 Jun 26;302(26):1429–1434. doi: 10.1056/NEJM198006263022601. [DOI] [PubMed] [Google Scholar]
- Galanaud P. In vitro antibody response to trinitrophenyl-polyacrylamide beads. Immunol Rev. 1979;45:141–161. doi: 10.1111/j.1600-065x.1979.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Schneeberger E., Merler E., Rosen F. S. Heterogeneity of "acquired" or common variable agammaglobulinemia. N Engl J Med. 1974 Jul 4;291(1):1–6. doi: 10.1056/NEJM197407042910101. [DOI] [PubMed] [Google Scholar]
- Herrod H. G., Buckley R. H. Use of a human plaque-forming cell assay to study peripheral blood bursa-equivalent cell activation and excessive suppressor cell activity in humoral immunodeficiency. J Clin Invest. 1979 May;63(5):868–876. doi: 10.1172/JCI109386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Woody J. N., Hartzman R. J., Eckels D. D. In vitro influenza virus-specific antibody production in man: antigen-specific and HLA-restricted induction of helper activity mediated by cloned human T lymphocytes. J Immunol. 1982 Oct;129(4):1465–1470. [PubMed] [Google Scholar]
- Mitsuya H., Osaki K., Tomino S., Katsuki T., Kishimoto S. Pathophysiologic analysis of peripheral blood lymphocytes from patients with primary immunodeficiency. I. Ig synthesis by peripheral blood lymphocytes stimulated with either pokeweed mitogen or Epstein-Barr virus in vitro. J Immunol. 1981 Jul;127(1):311–315. [PubMed] [Google Scholar]
- Murphy B. R., Chalhub E. G., Nusinoff S. R., Kasel J., Chanock R. M. Temperature-sensitive mutants of influenza virus. 3. Further characterization of the ts-1(E) influenza A recombinant (H3N2) virus in man. J Infect Dis. 1973 Oct;128(4):479–487. doi: 10.1093/infdis/128.4.479. [DOI] [PubMed] [Google Scholar]
- Pahwa S. G., Hoffman M. K., Pahwa R. N., Good R. A. Polyclonal and antigen-specific B-cell responses in patients with common variable immunodeficiency. J Clin Immunol. 1982 Jul;2(3):205–213. doi: 10.1007/BF00915223. [DOI] [PubMed] [Google Scholar]
- Pereira S., Webster D., Platts-Mills T. Immature B cells in fetal development and immunodeficiency: studies of IgM, IgG, IgA and IgD production in vitro using Epstein-Barr virus activation. Eur J Immunol. 1982 Jul;12(7):540–546. doi: 10.1002/eji.1830120703. [DOI] [PubMed] [Google Scholar]
- Preud'Homme J. L., Griscelli C., Seligmann M. Immunoglobulins on the surface of lymphocytes in fifty patients with primary immunodeficiency diseases. Clin Immunol Immunopathol. 1973 Jan;1(2):241–256. doi: 10.1016/0090-1229(73)90025-1. [DOI] [PubMed] [Google Scholar]
- Saiki O., Ralph P., Cunningham-Rundles C., Good R. A. Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6008–6012. doi: 10.1073/pnas.79.19.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. H., Tamaroff M., Saxon A. Inability of patients with common variable hypogammaglobulinemia to generate lymphjoblastoid B cells following booster immunization. Clin Immunol Immunopathol. 1980 Jul;16(3):336–343. doi: 10.1016/0090-1229(80)90139-7. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S., Krakauer R., MacDermott R. P., Durm M., Goldman C., Meade B. The role of suppressor cells in the pathogenesis of common variable hypogammaglobulinemia and the immunodeficiency associated with myeloma. Fed Proc. 1976 Jul;35(9):2067–2072. [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Wu L. Y., Lawton A. R., Cooper M. D. Differentiation capacity of cultured B lymphocytes from immunodeficient patients. J Clin Invest. 1973 Dec;52(12):3180–3189. doi: 10.1172/JCI107518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan R., Biddison W. E., Schneider H. S., Nelson D. L. Human T-cell subset requirements for the production of specific anti-influenza virus antibody in vitro. J Clin Immunol. 1982 Apr;2(2):118–125. doi: 10.1007/BF00916895. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Murphy B. R., Strober W., Clements M. L., Nelson D. L. In vitro production of anti-influenza virus antibody after intranasal inoculation with cold-adapted influenza virus. J Immunol. 1981 Nov;127(5):1958–1963. [PubMed] [Google Scholar]
- Yarchoan R., Murphy B. R., Strober W., Schneider H. S., Nelson D. L. Specific anti-influenza virus antibody production in vitro by human peripheral blood mononuclear cells. J Immunol. 1981 Dec;127(6):2588–2594. [PubMed] [Google Scholar]



