Abstract
Food preferences (FP) predict food intake in childhood; however, the predictive power of FP may decline among girls as weight concerns (WC) and dietary restraint (DR) increase during preadolescence. To examine longitudinal change in the preference–intake (P–I) relation and assess whether this relation weakens among non-Hispanic white girls (n = 197) with a history of WC and DR from age 5 to 11. Girls’ preferences for and intake (kcal) of 10 palatable snack foods were assessed biennially. Height, weight, percent body fat (%BF), WC, and DR were measured. Individual correlation coefficients were calculated per girl to capture within-person P–I correlations at each time of measurement. Overall, FP predicted girls’ snack food calorie intakes between 5 and 11 years, but latent profile analysis (LPA) revealed three distinct patterns of change in P–I correlations over time: “strong/stable” P–I correlations were relatively high and became stronger with age; “increasing/later null” P–I correlations were initially weak and became stronger between 5 and 9 years, but dropped to near 0 at 11 years; “initially weak/later strong” P–I correlations were initially null and increased with age. Mixed models revealed that the “increasing/later null” group had greater increases in %BF, and higher WC, DR, and BMI percentiles from 5 to 11 years, compared to the other groups. In summary, FP predicted snack food calorie intake among most girls during childhood, but waned as a predictor of calorie intake at age 11 for a subset of girls with increasing %BF, and higher WC, DR, and BMIs.
Introduction
Food likes and dislikes are powerful predictors of food intake in childhood (1), however, this is problematic in the current obesogenic environment, where energy-dense, palatable snack foods are widely available. Though young children’s food choices are unlikely to be influenced by concerns about nutrition or weight status, limited evidence suggests that these factors can increasingly influence intake during childhood, particularly among girls (2). For example, dietary restraint (DR) (i.e., cognitive restriction of food intake) may influence food choices and intake among adolescent girls and adult women (3–5), such that preferred, palatable foods are not selected and/ or the amount consumed is intentionally restricted. Thus, the power of preferences to predict the intake of preferred, palatable snack foods may decline with age as DR and weight concerns (WC) emerge as potential influences on food selection and intake; however, this has yet to be examined empirically.
For girls, the power of food preferences (FP) to predict intake may begin to decline during early adolescence, triggered by the physical changes that accompany puberty and when cognitive abilities to delay gratification are more developed. During puberty, increases in body fat may prompt an increased focus on appearance, and subsequently increase girls’ WC and use of DR to limit the selection and consumption of preferred, palatable foods (6). In addition, improvements in cognitive self- regulation that occur in late childhood and early adolescence (7) may increase the likelihood that girls’ attempts to cognitively limit intake of palatable preferred foods will be successful. Although girls as young as 5 years old report attempts at dieting (8) and DR (2, 9), self-regulatory abilities in early childhood are limited (10, 11) and subsequently very young girls may be unlikely to have success in limiting their intake of preferred palatable foods (2).
The aims of the present study were to describe the longitudinal relation between snack FP and snack food calorie intake in girls from age 5 to 11, and investigate whether the power of preferences to predict snack food intake declines as WC and DR increase during preadolescence. In exploring our aims, we first examined the longitudinal relation between preferences and calorie intake (P–I) of snack foods for the total sample, and then used latent profile analysis (LPA) to identify subgroups of girls displaying distinct patterns of P–I change over time. Next, we assessed whether these patterns were related to girls’ childhood trajectories of WC and DR. It was hypothesized that during preadolescence, preference would wane as a predictor of food intake among girls with histories of WC and DR. We also examined whether the latent patterns were associated with longitudinal changes in BMI and percent body fat (%BF) from age 5 to 11.
Methods and Procedures
Subjects
Participants included 197 non-Hispanic white girls living in central Pennsylvania and recruited as a part of a longitudinal study on the health and development of young girls. Eligibility criteria for girls’ participation at the time of recruitment included living with two biological parents, the absence of severe food allergies or chronic medical problems affecting food intake, and the absence of dietary restrictions involving animal products. Families were recruited using flyers and newspaper advertisements. In addition, families with age-eligible female children within a 5-county radius received mailings and follow-up phone calls (Metromail, Liverpool, NY). At entry into the study, participants included 197 5-year-old girls (mean age 5.4 ± 0.4) and their parents, and were reassessed every 2 years (ages 7, 9, and 11). The final assessment included 177 families, representing a 90% retention rate. Attrition was primarily due to family relocation outside the study area.
Unless mentioned otherwise, all variables of interest were collected at each time point (5, 7, 9, and 11 years). We excluded children who had missing preference ranking or calorie intake data (n = 7, n = 4, n = 0, and n = 0 at ages 5, 7, 9, and 11, respectively), did not consume any of the foods offered (n = 3, n = 3, n = 11, and n = 12 at ages 5, 7, 9, and 11, respectively), or had difficulties completing the eating in the absence of hunger (EAH) protocol (n = 0, n = 6, n = 3, and n = 9 at ages 5, 7, 9, and 11, respectively). This slightly reduced the final sample for each time of measurement (age 5, n = 187; age 7, n = 177; age 9, n = 166; age 11, n = 155). The Pennsylvania State University institutional review board approved all study procedures, and parents provided consent for their family’s participation.
Measures
FP and intake
Girls’ FP and calorie intake were measured at all time points using the EAH protocol, a procedure developed in our laboratory to measure girls’ responsiveness to the presence of palatable foods in the absence of hunger (12, 13). For the present study, we employed data collected using this protocol to assess individual differences in the longitudinal P–I relationship. In the protocol, each girl was interviewed one-on-one in a quiet room following the consumption of a self-selected lunch, which consisted of generous portions of bread (2 slices), sandwich meat (4 slices), carrots (20 g), applesauce (4 oz), cheese (1 slice), cookies (2 medium), and milk (8 oz) at baseline. Girls first indicated the extent to which they were hungry using three cartoon figures depicting an empty stomach, half empty/half full stomach, and full stomach. Because the intention of the larger study was to measure EAH, girls who indicated that they were still hungry after lunch did not complete the protocol. Next, a rank-order FP assessment was performed to measure girls’ FP of 10 palatable energy-dense snack foods (popcorn, 3.1 kcal/g; pretzels, 3.7 kcal/g; chips, 5.7 kcal/g; chocolate chip cookies, 5.0 kcal/g; chocolate, 5.3 kcal/g; Skittles, 4.1 kcal/g; ice cream, 2.1 kcal/g; frozen yogurt, 1.4 kcal/g; Fig Newtons, 3.6 kcal/g; peanuts, 5.7 kcal/g). In the assessment, three nongendered faces were presented to depict “really yummy,” “really yucky,” or “just okay.” In self-selected order, girls’ categorized each food by first tasting it and then placing it in front of the face that best represented their preference for that food. After all the foods were categorized, girls’ sequentially rank-ordered the foods within each category by picking the yummiest in the “really yummy” category, removing that food, and then selecting the yummiest among the remaining foods. This procedure was repeated for the “just okay” foods and “really yucky” foods. Rank-order scores for the foods ranged from 1 (least preferred) to 10 (most preferred). Mean test–retest reliabilities of 0.58 and 0.81 have been reported in samples of preschool children (14, 15). In addition, Birch (1) found that FP predicted subsequent intake in 3–4-year-old children (r = 0.80), thus demonstrating predictive validity for the measure.
Following the preference assessment, each girl was shown various toys that were available for a play session. Generous portions of the same 10 sweet or savory snack foods, varying in sugar and fat content, were presented during the procedure. Each girl was told that she could play with the toys or eat any of the foods while the experimenter did some work in the adjacent room. The experimenter then left the room for 10 min. Calorie intakes of the lunch and snack foods were calculated by pre- and postweighing girls’ food intakes in combinations with manufacturers’ information on the energy content of each food. EAH intake was obtained by summing the total calorie content of all the snack foods consumed during the free access period. Total lunch calorie intake was similarly obtained by summing the amount of calories consumed during the lunch session. To compare the EAH calorie intake of the three least and most preferred foods, girls’ calorie intakes of their three lowest ranked foods were summed and calorie intakes of their three highest ranked foods were summed, respectively.
Anthropometrics
Height and weight were measured in triplicate using a Shorr Productions stadiometer (Irwin Shorr, Olney MD) and Seca Electronic Scale (Seca, Burmingham, UK) at ages 5–11, respectively, and were used to calculate BMI (weight (kg)/height (m2)). BMI percentiles were calculated using the 2000 CDC Growth Charts (16); BMI percentiles of ≥85th were used to classify girls as overweight and >95th as obese.
At ages 9 and 11, %BF was measured using dual-energy X-ray absorptiometry scans (Hologic QDR 4500W, Hologic, Bedford, MA). Because dual-energy X-ray absorptiometry was not used at earlier ages, triceps and subscapular skinfold measurements collected at ages 5 and 7 were used to compute %BF for ages 5 and 7, respectively. Based on the method described by Slaughter et al. (17), %BF was estimated using the equation: %BF = 1.46437 × (sum of skinfolds) – 0.01365 × (sum of skinfolds)2.
Weight concerns
The WC scale is a 5-item questionnaire that assesses fear of weight gain, worry about weight and body shape, the importance of weight, diet history, and perceived fatness (18). Only data collected at ages 9 and 11 are presented in the current paper. The WC scale was originally developed for adolescent girls, thus modifications were necessary in order to make the scale developmentally appropriate when girls were age 9; specifically, the number of response options was reduced from 5 to 3, all the questions and wording of the response options were simplified. At age 11, the original version of the scale was implemented. Because response options differed over time, the scale scores were standardized at each time point. In the present study, internal consistency coefficients for the total WC score were α = 0.64 and α = 0.77 at ages 9 and 11, respectively. The WC scale has been validated in other samples as young as 10 years (19) and preliminary analyses in the current sample revealed good convergent validity at age 9. Girls’ reported WC moderately correlated with their body dissatisfaction (r = 0.40, P < 0.01) and BMI percentile (r = 0.40, P < 0.01) at age 9.
Dietary restraint
Girl’s DR was measured using a modified version of the Dutch Eating Behavior Questionnaire (DEBQ) created by van Strien et al. (20). The measure consists of 33 items on a 5-point scale (never – often) and three subscales: restrained eating (10–items), emotional eating (13–items), and external eating (10–items). At age 9, the DEBQ was amended to be age appropriate for the study participants by changing the response options from a 5-point scale to a 3-point scale (yes, sometimes, no). Girls could also indicate not having experienced the situation or emotion in question. At age 11, the original version of the DEBQ was used. Because response options were different at ages 9 and 11, the subscale scores were standardized at each time point. Note that the current paper will only present data from ages 9 and 11 because the validity of the DR subscale in children younger than 9 years old is questionable (2). The DEBQ has been shown to be reliable and valid in several studies (21–23), including among samples as young as 8 years old (20). For the current sample, internal consistency scores at ages 9 and 11 were 0.87 and 0.93 for restrained eating.
Data analyses
Except where noted, all descriptives and data analyses were completed using SAS version 9.1 (SAS Institute, Cary, NC). Descriptives were computed for BMI percentiles, %BF, WC, DR, and EAH. In addition, girls’ mean calorie intake by preference rank-order (1–10) was computed for each time of measurement.
To assess the predictive relation between girls’ snack FP and calorie intake of snack foods, within-person polyserial correlation coefficients were computed between each girl’s preference rank-order scores and measured calorie intakes using LISREL 8.80 (Scientific Software International, Lincolnwood, IL). P–I correlations were computed for each time of measurement; a correlation coefficient equal to 1 indicates that girls’ preferences perfectly predicted calorie intake, whereas a coefficient equal to 0 indicates that preferences did not predict calorie intake. Next, a within-subjects repeated measures ANOVA (PROC mixed) was conducted to assess the longitudinal change in the P–I correlations from age 5 to 11; in other words, does the power of snack FP to predict calorie intake of snack foods change from childhood into preadolescence. The repeated measures ANOVA method is appropriate for longitudinal analysis, and accounts for the intraclass correlation between individuals.
To identify distinct patterns of change in P–I correlations, LPA was performed in Mplus (Muthen and Muthen, Los Angeles, CA) using P–I correlation coefficients obtained from 5, 7, 9, and 11 years data as indicators (24). Similar to latent class analysis, LPA is a mixture modeling technique that employs maximum likelihood algorithms to identify underlying subgroups in the data that are qualitatively distinct (25, 26). However, LPA can accommodate continuous data whereas categorical data are only appropriate in latent class analysis. Solutions were identified for models containing 1, 2, 3, 4, and 5 classes. Each model was run with 100 random starting values to find the best-fitting model. Model fit was assessed using the Akaike information criterion, Bayesian information criterion, and Lo–Mendell–Rubin Likelihood Ratio Test (27, 28). The lowest Akaike information criterion and Bayesian information criterion values indicate the best model fit. The LMR tests the parsimony of the current model against a model with one less class (e.g., 3 classes vs. 2 classes). A significant P value indicates that the current model is a significant improvement on the model containing one less class. Based on these three test statistics, a three-class model was the best-fitting. Next, the girls were assigned to one of the three classes using posterior probabilities; average posterior probabilities for the three classes were 0.89, 0.92, and 0.86, respectively. To aid the interpretation of these classes, mean calorie intakes of girls’ least preferred and most preferred foods were compared within each class per wave of measurement using paired sample t tests.
ANOVA was used to investigate our hypothesis that the P–I relation would weaken among girls with histories (i.e., at ages 9 and 11) of higher WC and DR. Specifically, mean differences in girls’ WC and DR at 9 and 11 years were examined with P–I class membership as the between-subjects factor. Next, random coefficient models were conducted to examine whether histories of BMI percentiles, %BF, and EAH differed by class membership. This method was selected to account for intraclass correlation within individuals. In all models, the factors were age (5, 7, 9, 11), class (1–3), and an age-by-class interaction. When the interaction term was significant, Tukey-adjusted paired comparisons were used to test for mean differences. In the model predicting change in EAH, girls’ lunch calorie intakes were entered as a covariate to control for the possibility that intake during lunch influenced EAH intake. For each mixed model, extreme outliers were identified using the Cook’s D statistic and then evaluated to determine whether they altered the significance of the model parameters. As a result, one girl was removed from the model predicting change in EAH.
Results
Subject characteristics
Girls were exclusively non-Hispanic, white, and resided in well-educated households. Descriptive statistics for BMI percentiles, %BF, WC, DR, and EAH are provided in Table 1. At age 5, the mean ± s.d. BMI percentile score was 60.5 ± 26.3 and ~20% of the sample had BMI percentiles >85th percentile. Obese and overweight proportions in the sample were similar to national estimates (29).
Table 1.
Characteristics of study sample by age
| Age 5 |
Age 7 |
Age 9 |
Age 11 |
|
|---|---|---|---|---|
| (n = 186) | (n = 176) | (n = 168) | (n = 155) | |
| BMI percentile | 60.5 ± 26.3 | 60.2 ± 27.7 | 66.5 ± 25.9 | 64.5 ± 27.8 |
| Overweight or obese (%) | 19.9 | 21.6 | 32.5 | 31.6 |
| Percent body fat | 20.8 ± 4.6 | 22.1 ± 5.8 | 26.8 ± 7.1 | 27.5 ± 7.2 |
| Weight concerns | 0.4 ± 0.3 | 0.7 ± 0.6 | ||
| Dietary restraint | 1.4 ± 0.4 | 1.8 ± 0.8 | ||
| EAH intake (kcal) | 127.6 ± 93.0 | 176.1 ± 123.3 | 227.8 ± 146.7 | 258.2 ± 199.2 |
Values are mean ± s.d. unless otherwise noted. BMI percentiles were calculated using the CDC growth charts (16); girls with BMI percentiles >85 were considered overweight or obese. Data for weight concerns and dietary restraint are only presented for ages 9 and 11 due to low construct validity at earlier ages. Ranges for weight concerns and dietary restraint subscales were 1–3 at age 9 and 1–5 at age 11 (shown unstandardized).
EAH, eating in the absence of hunger.
Snack FP and calorie intake
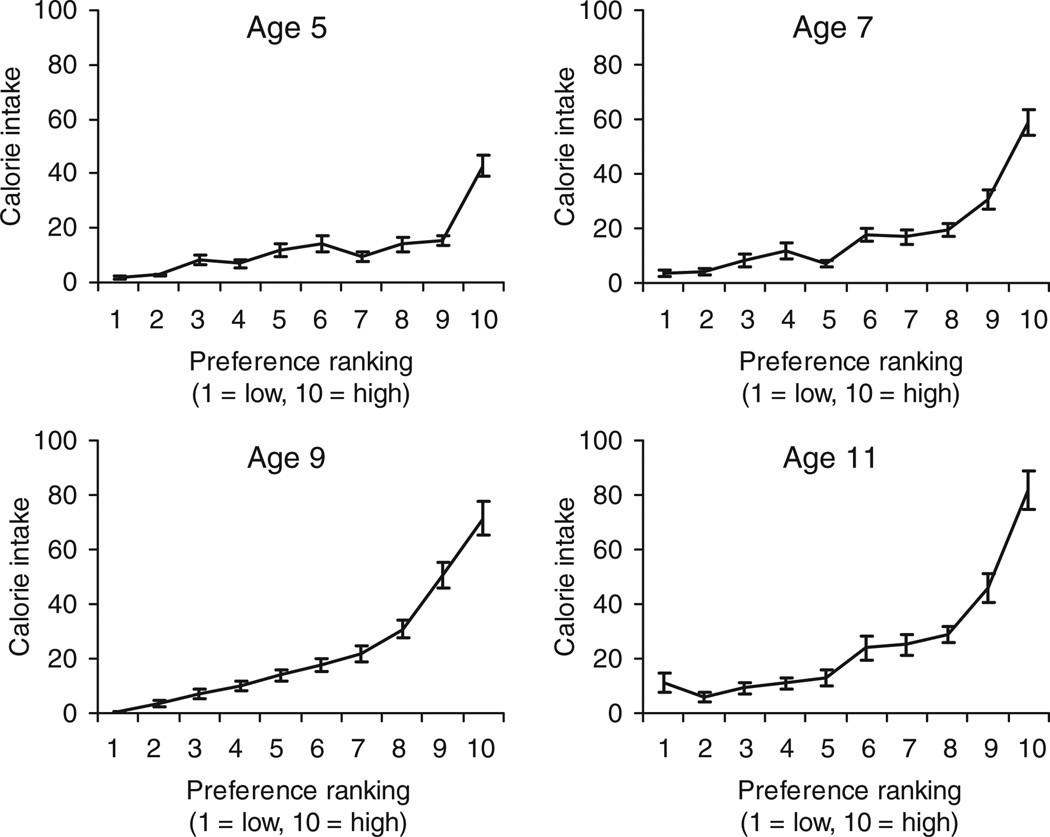
The descriptive relation between snack FP rank-orders (1–10) and calorie intakes of snack foods from age 5 to 11 is shown in Figure 1. At all ages, calorie intakes were greater for the highest ranked foods and lower for lowest ranked foods. Polyserial correlation was used to determine the predictive relation between girls’ preferences and calorie intakes of snack foods. For the total sample, girls’ preferences predicted their calorie intakes of snack foods at all ages: 5 (mean r = 0.33, s.d. = 0.30), 7 (mean r = 0.43, s.d. = 0.27), 9 (mean r = 0.52, s.d. = 0.23), and 11 (mean r = 0.45, s.d. = 0.29), though the interindividual variability of P–I correlations was high at each time point. Mean P–I correlation coefficients significantly increased between ages 5 and 11 from 0.33 to 0.45 (P < 0.001), suggesting that on average, snack FP became stronger predictors of snack food calorie intake with age.
Figure 1.
Descriptive relationship between girls’ preference rankings and calorie intake of 10 palatable snack foods. At each age, girls’ calorie intake was greater in higher ranked foods. Snack foods were peanuts, Skittles, pretzels, popcorn, chocolate, potato chips, ice cream, frozen yogurt, chocolate chip cookies, and Fig Newtons. Plotted values are mean kcal ± s.e.m.
Longitudinal patterns in P–I correlations
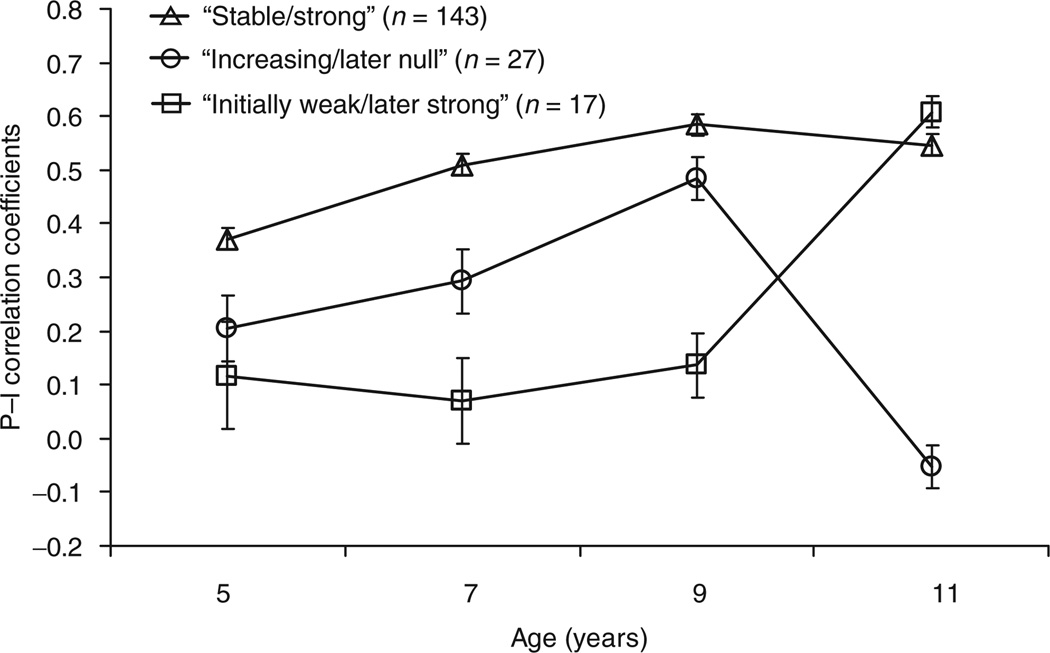
LPA was used to identify distinct patterns of change in P–I correlations from age 5 to 11. The fit statistics (26, 27) indicated a three class model, representing three patterns of change in the P–I relation as shown in Figure 2. For the first group of girls (n = 143, “strong/stable”), average P–I correlations were relatively strong and stable over time; P–I correlations were ≈0.40 at age 5 and exceeded 0.50 from ages 7 to 11. The second group included a smaller number of girls (n = 27, “increasing/later null”) and was characterized by a pattern of P–I correlations that increased from 0.21 at age 5 to 0.48 at age 9, respectively, but then dropped to near 0 (mean r = −0.05) at age 11.The third and smallest group of girls (n = 17, “initially weak/later strong”) was characterized by low mean P−I correlations at ages 5 (mean r = 0.12), 7 (mean r = 0.07), and 9 (mean r = 0.14), that increased substantially to 0.61 at age 11.
Figure 2.
Change in the predictive relation between snack food preferences and energy intake (kcal) of snack foods among girls from age 5 to 11 (n = 187). P−I correlations represent the association between food preferences and calorie intake; plotted values are means ± s.e.m. Three patterns in the P−I relation were identified by running a latent profile analysis on the P−I correlation coefficient data. In the “strong/stable” group (n=143), food preferences predicted intake from ages 5 to 11 and became stronger predictors with age; “increasing/later null” group (n = 27), food preferences were weak predictors of intake at age 5 and became better predictors with age, but stopped predicting intake at age 11; and, “initially weak/later strong” group (n = 17), food preferences were weak predictors of intake between ages 5 and 9, but became strong predictors at age 11.
Next, paired sample t tests were computed comparing girls’ average calorie intake of their least preferred and most preferred snack foods by class and age. As shown in Table 2, girls in the “strong/stable” group consistently consumed more calories from their most preferred snack foods than their least preferred snack foods between ages 5 and 11 (P’s < 0.001), suggesting that FP were a strong and stable predictor of calorie intake at all ages among these girls. Girls in the “increasing/later null” group consumed more calories from their most preferred snack foods than their least preferred snack foods at ages 5 (P < 0.05), 7 (P < 0.01), and 9 (P < 0.001), but not at age 11 (P = 0.74). In other words, preferences predicted calorie intake of snack foods at ages 5, 7, and 9, but no longer predicted calorie intake at age 11. Finally, girls in the “initially weak/later strong” group consumed more calories from their most preferred snack foods compared to their least preferred snack foods at age 11 only (P < 0.001); whereas, no significant differences were observed at ages 5 (P = 0.26), 7 (P = 0.56), and 9 (P = 0.11).
Table 2.
Mean (s.d.) kcal of least (LP) and most preferred (MP) foods by preference-intake (P-I) groups
| Age 5 |
Age 7 |
Age 9 |
Age 11 |
|||||
|---|---|---|---|---|---|---|---|---|
| P-I groups | LP | MP | LP | MP | LP | MP | LP | MP |
| Stable/strong (n = 143) | 12.0 (25.2)a | 76.8 (66.5) | 9.8 (26.4)a | 121.0 (88.7) | 6.0 (15.8)a | 160.5 (108.9) | 10.4 (32.4)a | 171.6 (130.1) |
| Increasing/later null (n = 27) | 18.7 (44.5) | 51.2 (55.7) | 29.0 (52.6)a | 95.5 (94.1) | 24.6 (48.0)a | 176.3 (144.7) | 105.4 (130.8) | 98.8 (152.6) |
| Initially weak/later strong (n = 17) | 17.9 (26.3) | 32.9 (38.8) | 54.9 (55.9) | 43.3 (41.0) | 32.4 (44.8) | 60.0 (52.9) | 3.0 (9.5)a | 142.9 (74.5) |
Individuals’ least preferred food kcal intake was obtained by summing caloric intakes of girls’ three lowest ranked foods; most preferred food kcal intake was obtained by summing caloric intakes of girls’ three highest ranked foods. P-I groups represent different trends in the relation between food preferences and intake from ages 5 to 11. “Stable/strong” group: food preferences predicted intake at all ages and became stronger predictors with age. “Increasing/later null” group: food preferences were weak predictors of intake at age 5 and became better predictors at ages 7 and 9, but did not predict intake at age 11. “Initially weak/later strong” group: food preferences did not predict intake between ages 5 and 9, but became strong predictors at age 11.
Significantly different from MP (paired sample t test), P < 0.05.
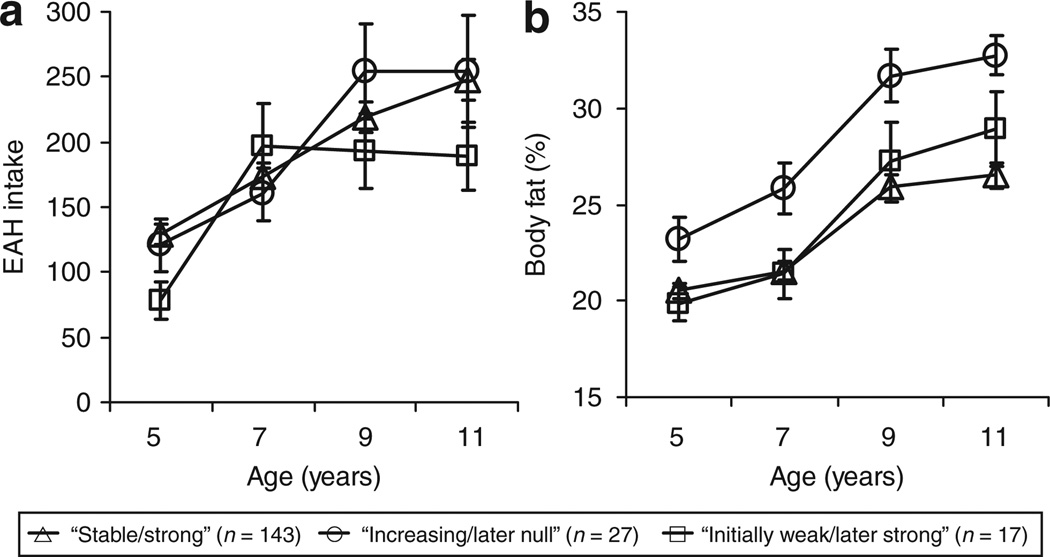
In light of the group differences in the calorie consumption of most and least preferred snack foods, a mixed model was run to determine whether total energy intake during the EAH procedure differed by group membership. The group effect (P = 0.56) and age-by-group interaction term (P = 0.26) did not reach statistically significance, though a significant age effect was observed (P < 0.001; Figure 3a). Overall, total calorie intake in the EAH protocol increased from 5 and 11 for the total sample. In addition, the significance level of the parameters did not change even after adjusting for the amount of calories consumed during the lunch before the EAH procedure. However, mean differences in lunch calorie intake were observed between the P-I groups. Girls in the “increasing/later null” group consumed more calories during lunch than girls in the “strong/stable” group at ages 5 (M = 304.5, s.d. = 143.9 vs. M = 225.9, s.d. = 115.8; P < 0.05), 7 (M = 408.9, s.d. = 156.2 vs. M = 289.1, s.d. = 131.2; P < 0.05), and 9 (M = 472.6, s.d. = 220.9 vs. M = 370.6, s.d. = 168.6; P < 0.05), and the “initially weak/later strong” did not differ from either group. In contrast, at age 11, all groups consumed an equal amount of calories (“increasing/later null”: M = 571.3, s.d. = 185.5; “strong/stable”: M = 535.1, s.d. = 175.7; “initially weak/later strong: M = 562.5, s.d. = 187.7; P > 0.05).
Figure 3.
Change in mean (±s.e.m.) eating in the absence of hunger (EAH) and % body fat among 187 girls following three distinct trajectories in the association between snack food preferences and calorie intake of snack foods from ages 5 to 11. In the “strong/stable” group (n = 143), food preferences predicted intake from ages 5 to 11 and became stronger predictors with age; “increasing/later null” group (n = 27), food preferences were weak predictors of intake at age 5 and became better predictors with age, but stopped predicting intake at age 11; and, “initially weak/later strong” group (n = 17), food preferences were weak predictors of intake between ages 5 and 9, but became strong predictors at age 11. The data were analyzed using mixed models: (a) main effect for age (P < 0.001) and (b) main effect for age (P < 0.001) and age-by-group interaction (P < 0.01).
Longitudinal P−I patterns and childhood histories of WC, DR, %BF, and BMI
ANOVA was used to identify mean differences in girls’ WC and DR at ages 9 and 11 by P-I groups. As shown in Table 3, the “increasing/later null” group reported greater DR than girls in the “stable/strong” and “initially weak/later strong” groups at ages 9 and 11 (P < 0.05). Similarly, girls in the “increasing/later null” group reported greater WC at ages 9 and 11 than those in the “stable/strong” group (P < 0.05), and the “initially weak/ later strong” was no different from either group.
Table 3.
Girls’ reports of weight concerns and dietary restraint (standardized) by preference–intake (P–I) groups at 9 and 11 years
| Stable/strong (n = 143) | Increasing/later null (n = 27) | Initially weak/later strong (n = 17) | F value | |
|---|---|---|---|---|
| Weight concerns, age 9 | −0.10 (0.95)a | 0.57 (1.12)b | −0.48 (0.66)a,b | 5.09** |
| Weight concerns, age 11 | −0.10 (0.96)a | 0.42 (1.09)b | −0.50 (0.69)a,b | 3.09* |
| Dietary restraint, age 9 | −0.11 (0.90)a | 0.88 (1.18)b | −0.48 (0.66)a | 14.21*** |
| Dietary restraint, age 11 | −0.07 (0.99)a | 0.50 (1.01)b | −0.50 (0.69)a | 5.45*** |
Values are means (s.d.). Different subscripts indicate significant group differences (P < 0.05) based on paired comparisons. Scores on the weight concerns and dietary restraint subscales were standardized due to differences in response scales at ages 9 and 11. Ranges for the weight concerns and dietary restraint subscales were 1–3 at age 9, and 1–5 at age 11. P-I groups represent different trends in the relation between food preferences and intake from ages 5 to 11. “Stable/strong” group: food preferences predicted intake at all ages and became stronger predictors with age. “Increasing/later null” group: food preferences were weak predictors of intake at age 5 and became better predictors at ages 7 and 9, but did not predict intake at age 11. “Initially weak/later strong” group: food preferences did not predict intake between ages 5 and 9, but became strong predictors at age 11.
Mixed models were used to examine whether longitudinal change in %BF and BMI percentiles from age 5 to 11 differed by group membership. From age 5 to 11 years, %BF increased for the total sample (P > 0.001), though the age-by-class interaction term was significant (P > 0.01). As shown in Figure 3b, girls in the “increasing/later null” had greater increases in %BF (B = 0.78, P < 0.01) compared to girls in the “stable/strong” group; moreover, adjusted paired comparisons revealed that the “increasing/later null” group had greater %BF at ages 7, 9, and 11 than girls in both groups. Similar results were observed for BMI percentiles. A significant group effect was present (P < 0.05) such that the “increasing/later null” group had greater BMI percentiles than the “stable/strong” group at all ages (P < 0.05); however, no age-by-group interaction was observed (P = 0.97).
Discussion
In general, girls’ preferences for snack foods predicted self-selected calorie intake of snack foods between ages 5 and 11, and became better predictors of snack food intake with age. However, interindividual variability in P−I correlations was high and results of a LPA revealed three distinct patterns of P−I change over time. For most girls, preferences for palatable snack foods were strong and stable predictors of snack food calorie intakes between 5 to 11 years, suggesting that, at least for palatable snack foods, this relationship remains stable into preadolescence for most girls. Snack FP no longer predicted snack food calorie intake at age 11 for a small subset of girls, who had a history of higher weight status, WC, and DR; thereby providing support for our hypothesis that the P−I relation would weaken among girls with a history of WC and DR. At age 11, girls in the “increasing/later null” group, reduced their consumption of highly preferred snacks and increased their consumption of less preferred snacks, a finding that may reflect attempts to limit their intake of preferred, palatable foods. However, despite their greater DR at age 11, their total calorie intake of snack foods matched the calorie intakes of girls in the other two groups at age 11, suggesting that attempts to limit their intake of preferred foods did not result in a reduced amount of total energy consumed from snacks.
To our knowledge, the present study is the first to describe patterns of change in P−I correlations from early childhood into early adolescence. The results of the LPA revealed three distinct patterns of change among girls during this period; the first pattern, demonstrating that preferences were strong predictors of snack food calorie intake for most girls from early childhood into preadolescence. This finding is consistent with previous research showing that preferences predict intake among children (1, 30, 31), and suggests that snack FP are powerful predictors of snack food intake among girls who, on average, reported lower DR and WC, and had lower BMIs and %BF.
The LPA revealed a second pattern, the “increasing/later null” group, whereby P−I correlations increased with age and then sharply declined to near zero as girls entered preadolescence, indicating that by preadolescence, snack FP no longer predicted snack food calorie intake. These girls had greater increases in DR and WC relative to the other groups, thereby supporting our hypothesis that the P−I relation would weaken among girls with a history of high DR and WC. These girls also had higher BMI percentiles and increasing %BF from age 5 to 11, which may have put them at risk for developing greater DR (32, 33) and WC (34). Moreover, the finding that these girls consumed an equal amount of calories from their most and least preferred snack foods at age 11, suggests that they were perhaps trying to limit their intake of their most preferred foods by consuming a significant quantity of their least preferred foods. Yet, despite their attempts at DR, these girls still consumed a similar amount of total calories compared to girls in the other groups who reported lower DR. It’s possible that the “increasing/later null” girls might have been able to consume fewer calories if snack foods low in energy density (e.g., fruits) were made available in the EAH procedure; however, this procedure may better reflect the current obesogenic environment—characterized by the wide availability of palatable snack foods—in which these girls may normally eat. Taken together, these girls may have had some success in limiting their selection and calorie intake of preferred, palatable foods, but no success in limiting their total calorie intake of snack foods. However, this group is a small minority of the total sample (14%), suggesting that preferences for palatable snack foods remain a powerful predictor of snack food calorie intake into preadolescence for most girls.
We expected that girls would not begin to attempt to limit their intake in challenging obesogenic food environments until preadolescence, perhaps triggered by the normative weight gain that accompanies puberty in girls (6) and/or when their self-regulatory abilities are more developed (2). However, the current findings show that higher BMI and %BF were present among the “increasing/later null” group as early as 7 years, a time of measurement when 84% of this group met the Tanner stage 1 of breast development. Moreover, further analysis using a mixed model revealed overlapping longitudinal change in breast development among the P-I groups (P > 0.05), thereby suggesting that the increasing %BF, WC, and DR observed in the “increasing/later null” was not due to this group maturing earlier than the other groups. It is possible that girls’ success at limiting their intake of preferred, palatable foods was in part due to cognitive advances in behavioral inhibition processes that occur during late childhood and preadolescence. The cognitive ability to self-regulate behaviors develops substantially between 5 and 11 years (7), so that girls may not be able to effectively resist the immediate gratification of eating palatable snack foods until preadolescence. For example, girls as young as 5 years old report DR (9); however, another study suggests that reports of DR by young children may reflect a positive response bias or intent or desire to control food intake rather than actual behavioral inhibition (2). Because the present study did not assess girls’ motivations for food choices during the EAH procedure, it is unclear whether girls’ attempts to limit their intakes of preferred, palatable foods at 11 years were due to enhanced self-regulation abilities. However, our findings nevertheless highlight when girls who report DR may begin to limit their caloric intake of palatable snack foods.
It is noteworthy that the P−I correlations observed here were generally lower than those reported by Birch (1), a study that used the same preference assessment procedure with younger children and different, less palatable foods. The weaker P−I correlations observed in the present study may have been a consequence of (i) the use of a set of relatively homogeneous palatable snack foods that were generally preferred, and (ii) girls having eaten lunch just before completing the preference assessment, which may have attenuated intake of snack foods. Though satiety most likely reduced the amount of calories girls consumed in the free access procedure, they still ate on average 100–300 calories at each time of measurement.
The present study has several strengths and limitations. First, this is the first study to show that snack FP are, on average, stable predictors of snack food intake from 5 to 11 years. Furthermore, by using LPA, we identified three distinct patterns of change in the P−I relationship, among which snack FP declined as a predictor of intake in a small group of girls who reported higher WC and DR. Second, the present study used the EAH procedure to measure preferences and intake, a procedure that presented a challenge to sated girls who wished to practice DR. As a result, the procedure allowed us to identify girls who were able to restrict or limit their intake of preferred, palatable snack foods. Third, the present study used the preference food assessment to measure preferences at all waves, a valid and reliable measure of FP (1, 14, 15). Fourth, girls’ intakes of snack foods were weighed in a laboratory setting, thereby minimizing measurement error. Lastly, dual-energy X-ray absorptiometry scans and skinfold measurements were used to estimate %BF, which allowed us to examine longitudinal changes in body fat. The study also had a few limitations. Preference and intake data were collected in a laboratory setting, were limited to snack foods, and thus may not fully reflect “real world” eating behaviors. As a result, it is possible that girls in the “increasing/later null” group altered their eating behaviors in the EAH procedure at age 11 by eating less of their preferred foods because they figured out the purpose of the measure. However, the higher WC and DR reported among this group at ages 9 and 11 adds validity to the group structure we identified using LPA. In addition, the sample was homogenous— female, white, and from middle-class families—and thus may not generalize to other populations.
The present findings have clinical implications. Our data suggest that snack FP are strong predictors of snack food intake throughout middle childhood into preadolescence for most girls. Given children’s predispositions to prefer sweet and salty tastes, and to learn to like energy-dense foods, children’s preferences for palatable, energy-dense snack foods may be difficult to modify. Therefore, one approach to reducing children’s intake of energy-dense snacks may be to focus on improving children’s preference for low-calorie snacks. For example, feeding strategies such as repeated exposure and pairing low energy-dense foods with positive social contexts (e.g., adult attention, verbal praise) have been shown to increase children’s preferences for a variety of low energy-dense foods (35–37). Thus, parental and caregiver feeding practices may be an effective target for improving the quality of children’s snack food intake.
In conclusion, our study provides evidence that, on average, snack FP predict the calorie intakes of snack foods across childhood. However, interindividual variability in the P−I relationship was high and LPA revealed three patterns in this relationship over time. For most girls, snack FP predicted intake from 5 to 11 years; however, the power of preferences to predict snack food intake decreased during preadolescence for girls with a history of higher DR, WC, %BF, and BMIs. Overall, these findings highlight the relatively stability of preference as a predictor of snack food intake among most girls from childhood into preadolescence, as well as providing some initial evidence for the emerging role of DR and WC on the intake of preferred palatable snack foods for girls with a history of higher weight status.
Acknowledgments
We are thankful for the support from the National Institutes of Health (NIH; grants: M01 RR10732 and HD32973), and Bunton Waller Fellowship Award, and NIH Ruth L. Kirschstein National Research Service Award (grant: 1 F31 HL092721-01). We thank Michele Marini for her excellent technical assistance.
Footnotes
Disclosure
The authors declared no conflict of interest.
REFERENCES
- 1.Birch L. Preschool children’s food preferences and consumption patterns. J Nutr Educ. 1979;11:189–192. [Google Scholar]
- 2.Shunk JA, Birch LL. Validity of dietary restraint among 5- to 9-year old girls. Appetite. 2004;42:241–247. doi: 10.1016/j.appet.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Drewnowski A. Taste preferences and food intake. Annu Rev Nutr. 1997;17:237–253. doi: 10.1146/annurev.nutr.17.1.237. [DOI] [PubMed] [Google Scholar]
- 4.Furst T, Connors M, Bisogni CA, Sobal J, Falk LW. Food choice: a conceptual model of the process. Appetite. 1996;26:247–265. doi: 10.1006/appe.1996.0019. [DOI] [PubMed] [Google Scholar]
- 5.Rozin P. Preference and affect in food selection. In: Kroeze JHA, editor. Preference Behavior and Chemoreception. London: Information Retrieval; 1979. pp. 411–431. [Google Scholar]
- 6.Striegel-Moore RH, Silberstein LR, Rodin J. Toward an understanding of risk factors for bulimia. Am Psychol. 1986;41:246–263. doi: 10.1037//0003-066x.41.3.246. [DOI] [PubMed] [Google Scholar]
- 7.Raffaelli M, Crockett LJ, Shen YL. Developmental stability and change in self-regulation from childhood to adolescence. J Genet Psychol. 2005;166:54–75. doi: 10.3200/GNTP.166.1.54-76. [DOI] [PubMed] [Google Scholar]
- 8.Abramovitz BA, Birch LL. Five-year-old girls’ ideas about dieting are predicted by their mothers’ dieting. J Am Diet Assoc. 2000;100:1157–1163. doi: 10.1016/S0002-8223(00)00339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carper JL, Orlet Fisher J, Birch LL. Young girls’ emerging dietary restraint and disinhibition are related to parental control in child feeding. Appetite. 2000;35:121–129. doi: 10.1006/appe.2000.0343. [DOI] [PubMed] [Google Scholar]
- 10.Bonato DP, Boland FJ. Delay of gratification in obese children. Addict Behav. 1983;8:71–74. doi: 10.1016/0306-4603(83)90059-x. [DOI] [PubMed] [Google Scholar]
- 11.Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 12.Fisher JO, Birch LL. Restricting access to foods and children’s eating. Appetite. 1999;32:405–419. doi: 10.1006/appe.1999.0231. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JO, Birch LL. Restricting access to palatable foods affects children’s behavioral response, food selection, and intake. Am J Clin Nutr. 1999;69:1264–1272. doi: 10.1093/ajcn/69.6.1264. [DOI] [PubMed] [Google Scholar]
- 14.Guthrie CA, Rapoport L, Wardle J. Young children’s food preferences: a comparison of three modalities of food stimuli. Appetite. 2000;35:73–77. doi: 10.1006/appe.2000.0329. [DOI] [PubMed] [Google Scholar]
- 15.Birch L. Dimensions of preschool children’s food preferences. J Nutr Educ. 1979;11:77–80. [Google Scholar]
- 16.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 17.Slaughter MH, Lohman TG, Boileau RA, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol. 1988;60:709–723. [PubMed] [Google Scholar]
- 18.Killen JD, Taylor CB, Hayward C, et al. Pursuit of thinness and onset of eating disorder symptoms in a community sample of adolescent girls: a three-year prospective analysis. Int J Eat Disord. 1994;16:227–238. doi: 10.1002/1098-108x(199411)16:3<227::aid-eat2260160303>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Gehrman CA, Hovell MF, Sallis JF, Keating K. The effects of a physical activity and nutrition intervention on body dissatisfaction, drive for thinness, and weight concerns in pre-adolescents. Body Image. 2006;3:345–351. doi: 10.1016/j.bodyim.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 20.van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5:295–315. [Google Scholar]
- 21.van Strien T, Frijters JER, Staveren WA, Defares PB, Deurenberg P. The predictive validity of the Dutch Restrained Eating Scale. Int J of Eat Disord. 1986;5:747–755. [Google Scholar]
- 22.Laessle RG, Tuschl RJ, Kotthaus BC, Pirke KM. A comparison of the validity of three scales for the assessment of dietary restraint. J Abnorm Psychol. 1989;98:504–507. doi: 10.1037//0021-843x.98.4.504. [DOI] [PubMed] [Google Scholar]
- 23.Allison DB, Kalinsky LB, Gorman BS. The comparative psychometric properties of three measures of dietary restraint. Psychol Assess. 1992;4:391–398. [Google Scholar]
- 24.Muthen LK, Muthen BO. Mplus: Statistical Analysis With Latent Variables User’s Guide. Los Angeles: Muthén & Muthén; 2004. [Google Scholar]
- 25.Vermunt JK, Magidson J. Latent class cluster analysis. In: Hagenaars JA, McCutcheon AL, editors. Applied Latent Class Analysis. Cambridge, UK: Cambridge University Press; 2002. pp. 89–106. [Google Scholar]
- 26.McLachlan G, Peel D. Finite Mixture Models. New York: Wiley; 2000. [Google Scholar]
- 27.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- 28.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 29.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 30.Pérez-Rodrigo C, Ribas L, Serra-Majem L, Aranceta J. Food preferences of Spanish children and young people: the enKid study. Eur J Clin Nutr. 2003;57(Suppl 1):S45–S48. doi: 10.1038/sj.ejcn.1601814. [DOI] [PubMed] [Google Scholar]
- 31.Jaramillo SJ, Yang SJ, Hughes SO, et al. Interactive computerized fruit and vegetable preference measure for African-American and Hispanic preschoolers. J Nutr Educ Behav. 2006;38:352–359. doi: 10.1016/j.jneb.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braet C, Wydhooge K. Dietary restraint in normal weight and overweight children. A cross-sectional study. Int J Obes Relat Metab Disord. 2000;24:314–318. doi: 10.1038/sj.ijo.0801129. [DOI] [PubMed] [Google Scholar]
- 33.Shunk JA, Birch LL. Girls at risk for overweight at age 5 are at risk for dietary restraint, disinhibited overeating, weight concerns, and greater weight gain from 5 to 9 years. J Am Diet Assoc. 2004;104:1120–1126. doi: 10.1016/j.jada.2004.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustafson-Larson AM, Terry RD. Weight-related behaviors and concerns of fourth-grade children. J Am Diet Assoc. 1992;92:818–822. [PubMed] [Google Scholar]
- 35.Birch LL, Marlin DW, Rotter J. Eating as the “means” activity in a contingency: Effects on young children’s food preference. Child Dev. 1984;55:432–439. [Google Scholar]
- 36.Birch LL, Zimmerman SI, Hind H. The influence of social-affective context on the formation of children’s food preferences. Child Dev. 1980;51:856–861. [Google Scholar]
- 37.Wardle J, Cooke LJ, Gibson EL, et al. Increasing children’s acceptance of vegetables, a randomized trial of parent-led exposure. Appetite. 2003;40:155–162. doi: 10.1016/s0195-6663(02)00135-6. [DOI] [PubMed] [Google Scholar]