Abstract
Background
Treatment of COPD requires multiple pharmacological and non-pharmacological intervention strategies. One target is physical inactivity because it leads to disability and contributes to poor physical and mental health. Unfortunately, less than one percent of eligible patients have access to gold-standard pulmonary rehabilitation.
Methods
A single-site parallel group randomized trial was designed to determine if a self-management lifestyle physical activity intervention would improve physical functioning and dyspnea. During the first six weeks after enrollment patients receive COPD self-management education delivered by a health coach using a workbook and weekly telephone calls. Patients are then randomized to usual care or the physical activity intervention. The 20 week physical activity intervention is delivered by the health coach using a workbook supported by alternating one-on-one telephone counseling and computer assisted telephone calls. Theoretical foundations include social cognitive theory and the transtheoretical model.
Results
Primary outcomes include change in Chronic Respiratory Questionnaire (CRQ) dyspnea domain and 6-minute walk distance measured at 6-, 12-, and 18-months after randomization. Secondary outcomes include other CRQ domains (fatigue, emotion, and mastery), SF-12, and health care utilization. Other measures include process outcomes and clinical characteristics.
Conclusions
This theory driven self-management lifestyle physical activity intervention is designed to reach patients unable to complete center-based pulmonary rehabilitation. Results will advance knowledge and methods for dissemination of a potentially cost-effective program for patients with COPD.
Keywords: chronic obstructive pulmonary disease, self-management, physical activity, lifestyle, pulmonary rehabilitation, randomized trial
1. Introduction and Rationale
1.1. Burden of COPD
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity, mortality, and economic burden worldwide [1]. The absolute number of years lost to disability attributed to COPD is higher than the years of life lost due to premature death [2]. The Institute of Medicine identified emphysema (i.e., COPD) among 15 “priority” conditions needing multiple intervention strategies to improve outcomes [3].
1.2. Gaps in evidence-based management
Because there is no cure, the goal of treating COPD is to improve or maintain patient quality-of-life and functional status. However, there remain large gaps in providing optimal care [4,5] despite improvements in pharmacological and non-pharmacological treatments [1]. These gaps appear to be the result of a complex array of factors at the community, health system, physician, and patient level of care [6]. Of these factors patients’ beliefs, health literacy, coping skills, motivation, co-morbid conditions (e.g., depression), and access to care affect level of engagement in health-related behaviors, which in-turn affect outcomes. Therefore, a critical link for closing the gap and improving outcomes is to enhance self-management support for patients defined as “the systematic provision of education and supportive interventions by health care staff to increase patients’ skills and confidence in managing their health problems, including regular assessment of progress and problems, goal setting, and problem-solving support” [7].
The evidence supporting self-management interventions has been examined in several recent reviews as part of stand-alone programs [8] and multi-component interventions [9,10]. Overall, there appears to be potential benefit. However, variation between the studies in content, methods of intervention, and outcomes prevent specific recommendations [8].
1.3 Rationale for targeting physical inactivity
A specific target for intervention through self-management support is physical inactivity, which is associated with disability due physical de-conditioning [11,12] and poor outcomes including systemic inflammation [13], lower quality of life [14], hospitalizations [15,16], and mortality [16,17]. Moreover, exercise rehabilitation among patients with COPD has been associated with improvements in dyspnea, physical and psychological functioning, quality of life, and marital adjustment [18,19]. However, few patients have access to pulmonary rehabilitation and less than one percent complete these programs [20].
1.4 COPD self-management activation research trial (COPD-SMART)
COPD-SMART was designed to examine the effectiveness of a home-based self-management intervention. The goal of the intervention is to improve patient functioning by enhancing COPD self-management and increase lifestyle physical activity among an underserved COPD population. Specific hypotheses include: 1) Patients with COPD who receive physical activity self-management (PASM) will have significant improvements in health status (Chronic Respiratory Questionnaire [CRQ]-dyspnea domain) and functional performance (6 minute walk distance) compared to usual care (UC). 2) The PASM program will be more cost-effective compared to UC.
2. Methods
2.1. Overview
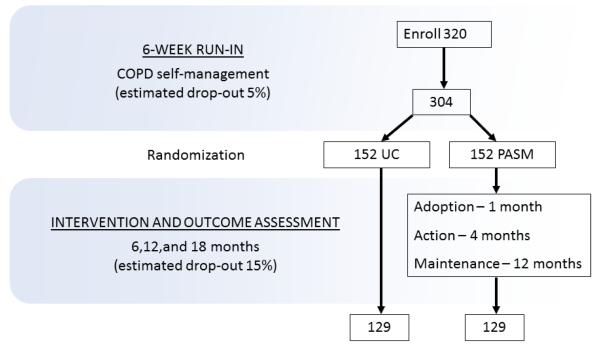
This is a single-site randomized-controlled trial with parallel-group design comparing COPD self-management education plus UC to a self-management plus PASM intervention (Figure). During the first six weeks after enrollment there is a run-in period when all patients are provided with COPD self-management education delivered by a trained health coach. Patients are then randomized to UC or PASM delivered over 20 weeks. Follow-up data are collected at 6, 12, and 18 months after randomization. The study was approved by the UTHSCT Institutional Review Board, and written informed consent was obtained prior to enrollment and data collection.
Proposed number and flow of patients with COPD in randomized trial comparing physical activity self-management (PASM) to usual care (UC).
2.2. Recruitment setting, eligibility, and enrollment
Patients are recruited from clinics of the University of Texas Health Science Center-Tyler (UTHSCT), which is one of five health systems in an eight county region in east Texas. The predominantly rural region has an area of about 6,139 square miles with a total population of 634,192 in 2009.
Patients ≥ 45 years of age with physician-diagnosed COPD are recruited from a registry (n=5582), which is comprised of an administrative data base and provider referrals. The administrative database includes all patients seen at the clinics of UTHSCT with a coded COPD diagnosis (ICD-9 491, 492, 496) (Figure). The goal is to randomly recruit a sample broadly representative of patients with COPD eligible for center-based pulmonary rehabilitation. Spirometry results and other exclusion criteria (Table 1) are reviewed in the medical record to determine initial eligibility. Potentially eligible patients are mailed a letter of invitation and contacted by telephone to schedule an enrollment visit. Final determination of eligibility is made at the enrollment visit. The registry will be continually sampled until the enrollment target is achieved (Figure).
Table 1.
Inclusion and Exclusion Criteria for Patients Interviewed with COPD
| Inclusion | Exclusion |
|---|---|
| Age ≥ 45 years | Participation in pulmonary rehabilitation program within 12 months |
| Physician diagnosis of COPD | Nursing home resident |
| FEV1/FVC* < 70% and FEV1 < 70% | Uncontrolled hypertension, angina, heart failure |
| MMRC+ dyspnea score ≥ 2 | Unstable EKG findings (e.g., uncontrolled dysrhythmia, active ischemia) |
| Dementia, uncontrolled psychiatric illness | |
| Life expectancy < 12 months | |
| Resting oxygen saturation < 90% and inability to obtain supplemental oxygen |
|
| 6 minute walk < 110 m | |
| Other safety concerns with participating in physical activity |
FEV1 = forced expiratory volume in one second, FVC = forced vital capacity,
MMRC dyspnea questionnaire = modified Medical Research Council [51]
2.3. Randomization
A list of randomized unique patient identification numbers with group assignment was completed before patient enrollment by the data coordinating center (DCC) at the University of Alabama using a permuted block design. Blocked randomization ensures that an equal number of subjects are randomized to each study arm within each block, while randomly permuting blocks minimizes investigator bias by randomly determining the size of each block. Patients were sequentially assigned unique patient identification numbers at the time of enrollment but group assignment is provided only to the study coordinator (RR) and concealed from other study personnel and patients until after completion of the six-week COPD self-management component of the intervention described below.
2.4. Data collection
Data collection is conducted at UTHSCT during the enrollment visit and at 6, 12, and 18 months after randomization as well as monthly using automated telephone calls (Table 2). Monthly baseline data collection is comprised of self-reported questionnaire items and physical measurements. The six month assessment is conducted to measure the short-term effects of the PASM intervention, and the 12- and 18-months assessments are intended to measure the intermediate and longer term maintenance of the intervention. In addition, automated telephone calls collect self-reported health care utilization (see section 2.5.5). While data collection instruments are self-report, trained interviewers review items for completeness and are available to answer questions. Trained respiratory therapists perform spirometry and 6-minute walk tests using standardized protocols. The interviewers and respiratory therapists are blinded to intervention group assignment. After completion of each data collection visit at 6, 12, and 18 months patients are paid $25, $50, and $75, respectively. A more detailed description of the study measures is provided in section 2.6.
Table 2.
Schedule of Measurements
| Measurements | Baseline | 6-months | 12-months | 18-months |
|---|---|---|---|---|
|
| ||||
| Primary Outcomes | ||||
| CRQ-dyspnea | X | X | X | X |
| 6-minute walk distance (m) | X | X | X | X |
|
| ||||
| Secondary Outcomes | ||||
| CRQ-other | X | X | X | X |
| SF-12 | X | X | X | X |
| Health care utilization* | X | X | X | X |
|
| ||||
| Process Outcomes | ||||
| MMRC | X | X | X | X |
| Self-efficacy | X | X | X | X |
| Activation | X | X | X | X |
| RAPA | X | X | X | X |
| Readiness to change physical activity | X | X | X | X |
|
| ||||
| Clinical Characteristics | ||||
| Demographics | X | |||
| Smoking status | X | X | X | X |
| Co-morbidity | ||||
| BMI | X | |||
| Charlson index | X | |||
| GDS | X | X | X | X |
| CAGE | X | |||
| Medications | X | X | X | X |
| Spirometry | X | |||
Also measured monthly using computer assisted telephone calls.
CRQ=Chronic Respiratory Questionnaire, BMI=body mass index, GDS=Geriatric Depression Scale, CAGE=alcohol screening questionnaire, MMRC=modified Medical Research Council dyspnea questionnaire, RAPA=Rapid assessment of physical activity questionnaire.
2.5. Interventions
After baseline data collection all patients are interviewed by the health coach using a semi-structured self-management needs assessment. In addition, patients are given an overview of the program, their first assignment is reviewed, and a follow-up telephone call is scheduled.
2.5.1. Self-management needs assessment
The purpose of the needs assessment is to build rapport, foster study buy-in, and obtain personal information, which will be used to assist the health coach tailor telephone counseling. The needs assessment is comprised of ten open-ended questions and four sets of structured questions. Open-ended questions are used to determine each patient’s general COPD and physical activity self-management needs. COPD related topics include concerns and fears, learning needs, previous experiences, social support, goals, and barriers to self-management. Physical activity related topics include identification of enjoyable physical activities, perceived barriers to physical activity, and social support for physical activity.
2.5.2. COPD self-management education (Weeks 1-6)
To enhance enrollment and retention all patients receive COPD self-management education comprised of a COPD self-management manual and a weekly telephone call from the health coach delivered over approximately six weeks. During calls the health coach reviews readings, answers questions, and reinforces learning. Topics covered in the manual include: 1) understanding COPD and its impact; 2) communicating with physicians; 3) understanding medications; 4) non-pharmacologic strategies for controlling symptoms; 5) exacerbation action plans; 6) enhancing physical activity; 7) healthy eating; 8) smoking cessation; and 9) mood management. The manual was adapted from Living Well with COPD [21], which has been a component of efficacious interventions [22]. After the sixth week of self-management education patients are randomized to UC or the PASM intervention.
2.5.3. Usual care (UC)
Patients randomized to usual care are directed to continue regular follow-up with their physician and to call the health coach using a toll-free number if they have any questions. Study-related contact occurs through monthly automated telephone calls, which collect health care utilization data (see section 2.2.5), and follow-up visits for data collection at 6, 12, and 18 months (see section 2.6).
2.5.4. Physical activity self-management (weeks 7-36)
The PASM intervention is a lifestyle physical activity program designed to increase daily physical activity among patients with COPD. The intervention was adapted from the Active Living Everyday (ALED) program [23,24]. Theoretical foundations for the intervention include social cognitive theory [25] and the transtheoretical model [26]. Social cognitive theory is a comprehensive model positing that the initiation and maintenance of health behaviors such as physical activity are determined by a complex interaction of intrapersonal (e.g., self-efficacy), social (e.g., reinforcement), and physical environment (e.g., community access) factors. The transtheoretical model often referred to as the stages of change also incorporates multiple theories including decisional balance theory, social learning theory, and the theory of planned behavior. The transtheoretical model describes the steps individuals progress through when initiating and maintaining a health behavior and proposes that individuals differ in their readiness to change. Each stage is marked by the different cognitive and behavioral processes individuals use in the context of changing behavior. The stages include precontemplation, contemplation, preparation, action, and maintenance. Each workbook chapter is designed to teach a specific cognitive or behavioral skill (e.g., goal setting, problem solving, identifying pros of change, social support, cognitive restructuring), which in theory increases the likelihood of behavior change. In addition, the health coach assesses stage of change and tailors the call accordingly. The program is delivered using a structured workbook supported by one-on-one telephone counseling every other week by the health coach with computer assisted telephone calls on alternating weeks. Coach and computer assisted calls follow standardized scripts developed for this intervention.
The core behavioral intervention is delivered in workbook format, which includes 25 chapters. These chapters were adapted from the ALED workbook and integrate anecdotes and real-life examples from patients with COPD. Chapters 1-20, the activation phase are completed weekly (weeks 7-26) and intended to engage patients in accumulating moderate intensity physical activity over the course of the day. The maintenance phase, chapters 21-25 (weeks 27- 66), are completed every other month and focus on the maintenance of regular physical activity.
Activation phase (weeks 7-26)
The main purpose of the activation phase is to help patients change their beliefs and attitudes towards physical activity as well as introduce them to key behavioral and cognitive strategies known to help individuals initiate and maintain physical activity. Patients are mailed the PASM workbook after completion of the COPD self-management education intervention component. The PASM intervention includes weekly workbook activities and every-other-week telephone calls from the health coach. The health coach follows standardized scripts, which target understanding of key concepts, self-efficacy, problem solving, goal setting, adherence, and identification of barriers. Patient safety with participation in physical activity is addressed during the first week. In addition, the health coach uses information obtained during the needs assessment to tailor subsequent calls and help assess patient’s readiness to change. Examples of tailoring include identifying enjoyable physical activities (e.g., gardening, dancing) and barriers (e.g., unsafe walking conditions), and then encouraging more enjoyable activities and exploring options to overcome barriers. On alternating weeks automated telephone calls reinforce the week’s topic and collect data (e.g., stage of change, health care utilization). The health coach is available using a toll-free “help-line” for questions during standard working hours and patients are able to leave messages after hours.
The ultimate goal of the PASM arm of the intervention is to have patients accumulate at least 30 minutes of moderate intensity physical activity per day defined as a dyspnea level of 4-5 on the Borg scale and taking 1-2 minutes to recover. Patients unable to meet recommendations are instructed to strive for multiple intervals of moderate intensity physical activity and are repeatedly reminded that “some activity is better than none and more is better than some”.
Maintenance phase (weeks 27-66)
During this 10-month period calls initiated by the health coach end. These calls are replaced with five reading assignments and once monthly automated telephone messages. The goals during this phase are to review and reinforce major principles of the activation phase with a focus on maintenance of regular lifestyle physical activity by prompting, setting new goals, problem solving, and encouraging further development of self-efficacy. Patients are able to initiate calls to ask questions from their health coach as needed using the toll-free number.
Health coach training and monitoring intervention fidelity
The fidelity of the intervention is optimized through training and monitoring of the health coach (JP). The health coach has an undergraduate degree in psychology and received standardized training for this project that included education about COPD self-management and certification in the principles used in the Active Living Everyday program [27]. ALED training was included to ensure understanding of the basic principles of lifestyle physical activity and the theoretical underpinnings of the PASM behavioral intervention. The health coach was trained by the principal investigator (DBC) in COPD self-management and the health psychologist (JA) in practical skills of rapport building, active listening, and problem solving. Total time for training was approximately 100 hours and included on-line training, reading assignments, discussions, and role-playing of intervention delivery.
Quality of the health coach intervention calls are monitored by in-person observations and audio-taping phone calls. All baseline sessions and a 10% random sample of telephone counseling sessions were audio-taped and evaluated by the health psychologist (JA). A standardized rating scale is used to assess health coach performance and adherence to study protocol. Feedback is provided to the health coach monthly.
2.5.5. Computer assisted telephone system (CAT)
The CAT system is used to deliver components of the intervention and for data collection. This system used in this study is commercially available (TeleMinder™, Los Altos, CA) and used in health care settings for patient reminders and surveys [28]. The CAT system provides automated messages from the health coach beginning during the third week of the activation phase of the intervention. The goals of these automated calls are to provide ongoing contact with patients while decreasing the call burden on the health coach. Data collection using the CAT system was limited to monthly self-report of health care utilization for all patients (see section 2.4.2.).
2.5.6. Safety monitoring
Several methods are used to identify adverse events. All patients have access to study personnel at UTHSCT to report adverse events 24/7 using a toll-free number. In addition, regular monitoring of patients is conducted through monthly health care utilization calls using the CAT system and review of hospitalization records at UTHSCT. These data are reviewed by the principal investigator (DBC) to determine whether an adverse event was study related. Serious adverse events are defined as any hospitalization or death. All adverse events are reported to the local IRB and a local Data Safety and Monitoring Board.
2.6. Study measures
The study measures are comprised of primary and secondary health-related outcomes, process outcomes, and patient clinical characteristics. An overview of the measures and schedule for data collection is summarized in Table 2.
2.6.1. Primary outcome measures
The two primary outcome measures include the dyspnea domain of the self-administered Chronic Respiratory Questionnaire (CRQ-SA) [29] and the 6-minute walk distance [30].
CRQ-SA
This is a disease-specific instrument with 20 items and four domains (dyspnea, fatigue, emotional function, and mastery). For each domain patients rank their responses on a 7- point scale (1=maximum impairment, 7=no impairment). The CRQ has demonstrated reliability and validity [31]. Intra-class correlation coefficients for test-retest reliability have consistently ranged from 0.73 to 0.95, and internal reliability (Cronbach’s α) has ranged from 0.81 to 0.90. The CRQ is responsive to change among patients with COPD, and the minimal clinically significant difference for each CRQ domain is 0.5 [29,32].
6-minute walk
This is performed by trained respiratory therapists according to the ATS standardized protocol [30]. The test is self-paced with patients choosing their own intensity of walking, and they are allowed to stop and rest during the test. Total distance walked in 6-minutes is the outcome. Average differences between baseline and follow-up walk distance are used to determine treatment effect. Compared to the CRQ, the minimal clinically significant difference for the 6-minute walk is not as well established and is discussed in more detail in section 2.7.2. The approach for categorizing patients as “responders” and “non-responders” requires using the coefficient of repeatability [33]. The minimal clinically significant difference will be calculated using the coefficient of repeatability for individual patients with a wider confidence interval (e.g. 90%). The coefficient of repeatability is defined as twice the standard deviation of the difference between baseline and subsequent 6-minute walk distances.
2.6.2. Secondary outcome measures
CRQ-SA
In addition to the CRQ-dyspnea domain other CRQ domains including fatigue, emotion, and mastery will be used as secondary outcome measures [29].
SF-12
This is a generic health-related quality of life instrument comprised of twelve questions, which provide physical and mental health composite scores (PCS and MCS). Scores for the PCS and MCS range from 0 to 100 where lower scores suggest poorer quality of life [34]. Normalized scores are calculated with an average of 50 where scores above 50 suggests health-related quality of life higher than the general population. The minimal clinically significant difference in PCS and MCS is 3 and 3.5, respectively [35].
Health-care utilization
Self-reported health-care utilization for the 6 months prior to the follow-up interviews is determined separately for physician office visits, emergency department visits, and hospitalizations for lung disease and other conditions [36]. The health care utilization data along with the SF-12 will be used to derive quality adjusted life years (QALY) for cost-utility analysis [37] described in greater detail in section 2.8.
2.6.3. Process outcomes
In addition to health-related outcomes described above four process measures will include self-efficacy, patient activation, self-reported physical activity, and readiness to change physical activity (Table 2). These are intermediate variables that may be affected by the intervention and influence the primary and secondary outcomes.
COPD self-efficacy scale
This is assessed using a 34-item questionnaire specifically developed for patients with COPD [38]. This scale measures confidence in managing or avoiding breathing difficulty across five domains: 1) negative affect (e.g., when feeling down or depressed); 2) intense emotional arousal (e.g., when becoming angry); 3) physical exertion (e.g., when going up stairs too fast); 4) weather/ environment (e.g., with humidity); and 5) behavioral (e.g. when overeating). The average number of responses pertaining to each domain is calculated where higher scores indicate lower confidence in ability to cope and manage dyspnea.
Patient activation
This is categorized based on patient confidence in self-management (“How confident are you that you can identify when it is necessary for you to get medical care?”) and motivation to participate in their care (“How often do you take a list of all your prescribed medicines to your doctor visit?) [39]. From these questions four categories of activation [39] include: 1) Active-very confident and usually/always take medicines to visit; 2) Passive-less than very confident and never/sometimes take medicines to visit; 3) High effort-not very confident and usually/always take medicines to visit; 4) Complacent-very confident and never/sometimes take medicines to visit.
Rapid Assessment of Physical Activity
This questionnaire measures readiness to meet CDC physical activity goals of accumulating at least 30 minutes of moderate-intensity physical activity five or more days per week [40]. The 9-item questionnaire asks respondents to rate the amount of physical activity, strength training, and stretching activities performed weekly and has been validated among adults 50 years of age and older. Scores are calculated based on the highest degree of physical activity with an affirmative response, and activity level is categorized as sedentary, underactive, regular underactive (light activities), regular underactive, and regular active [40].
Readiness to change physical activity level
This is assessed according to the transtheoretical model [41–44] using an algorithm used in previous interventions [23,24,45]. For example, “Are you accumulating at least 30 minutes of moderate-intensity physical activity more than 4 days a week?” Affirmative responders are then asked about how long they have been this physically active, and negative responders are queried about their level of activity and whether or not they intend to increase their activity level. Based on these questions, responders are categorized into one of the following stages of change: pre-contemplation, contemplation, preparation, action, and maintenance [41–44].
2.6.4. Patient clinical characteristics
In addition to standardized questions on demographics and lifestyle factors (e.g., smoking) other measures to characterize patients include co-morbid illnesses, medications, and physical measurements (Table 2).
Co-morbidities
The Charlson index [46], alcohol abuse screening questionnaire [47], and Geriatric Depression Scale (GDS) [48] are used to assess co-morbid conditions. The Charlson index is based on patient self-reports and assesses 22 different conditions [46]. Scores are assigned to each condition and totaled. Depressive symptoms are assessed using the GDS-short form, a 15-item self-report instrument [48]. A score of 6 or greater suggests clinically significant depression.
Medications
Current medications including prescribed and over-the-counter are determined from patient self-report and medication lists obtained from an electronic prescribing system. Medication use and concordance with management guidelines is defined according to severity of spirometric impairment [49]. Guideline concordance for an FEV1 60%-80% is defined as use of any short-acting inhaled bronchodilators (i.e., anticholinergic, B-agonist, combination, or nebulizer). In addition to use of short-acting bronchodilators concordance for an FEV1 <60% also required use of a long-acting bronchodilator (i.e., anti-cholinergic, B-agonist, or B-agonist/corticosteroid) or inhaled corticosteroid.
Physical measurements
In addition to questionnaires several physical measurements are conducted at baseline including body mass index (BMI=weight [kg]/height [m2]), blood pressure, electrocardiogram, post-bronchodilator spirometry, and oximetry. Spirometry is performed by trained respiratory therapists according to ATS standards [50]. Disease severity is categorized using FEV1 percent predicted as described by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification [1]. The BODE index ([B] body-mass index (weight [kg]/height [meters2]), [O] degree of airflow obstruction (i.e., spirometry), [D] dyspnea level (i.e., Medical Research Council dyspnea questionnaire), and [E] exercise capacity (i.e., 6- minute walk), provides a measure of severity and prognosis in patients with COPD and is a better predictor of mortality than spirometry alone [51].
2.7. Statistical methods
2.7.1. Statistical methods to compare groups
Baseline comparisons will be made using independent sample t-test for continuous outcomes and Fisher’s exact test for categorical outcomes. An intention-to-treat approach will be used for all analyses. In order to examine improvements in CRQ-dyspnea and 6 -minute walk, changes over time will be analyzed with mixed model analysis of variance (ANOVA) models to compare mean differences between the PASM and UC groups. The models will include the following terms: treatment group, gender, time point, and treatment group by time point interaction. The statistical significance of the treatment group time point interaction will help guide planned contrasts to be performed in order to examine differences in the mean dyspnea domain score between the groups by each time point. Since our interests are both CRQ-dyspnea and 6 -minute walk, a multivariate mixed model approach will be used to determine which factors affect both outcomes together and each one separately. Similar analyses will be performed to examine the secondary outcomes including other domains of the CRQ, SF-12, and frequency of healthcare utilization. The level of statistical significance for the incremental differences between the groups will be two-tailed (p <0.05). Bonferroni adjustments will be made for analyses involving multiple comparisons.
2.7.2. Sample size estimates
The minimal clinically significant differences for the primary outcome measures, CRQ-dyspnea and 6-minute walk distance, were used to estimate sample size for the trial. The sample size determination was also based on an estimated overall attrition of approximately 20% over the 18 month duration of the study.
CRQ
The minimal clinically significant difference for each CRQ domain is 0.5 [29,32]. With the assumptions of a mean value of 3.3 and standard deviation of 1.1 a total of 129 subjects in each group is estimated to achieve power of 95% at a significance level of 0.05. This combined with estimated 20% overall attrition results in a total sample size of 320 targeted for enrollment.
6 minute walk distance
At the time this study was originally designed in 2009, the available evidence suggested that a 54m (SD 60m) increase in walking distance was the minimal clinically significant difference [30]. Based on this assumption 34 subjects in each group provide 95% power at a significance level of 0.05. Since the start of patient enrollment in 2010, a number of studies have been published that suggest the minimal clinically significant difference is lower ranging from 25m [52] to 35m [53].
2.7.3. Interim analyses, stopping rules
Interim analyses of study outcomes and for data safety monitoring are conducted using stringent p-values in order to evaluate each group’s risks and benefits. Evaluation of the risks and benefits requires examination of rates of adverse outcomes and main measures of benefit comparing the intervention and control groups. For assessment of risk, the rates of all and serious adverse events is calculated and compared between the two groups using p<0.001 as the level of statistical significance according to the Peto approach [54] for defining interim stopping levels. Interim analysis of benefit compares results for the CRQ and 6-minute walk for the two groups using the same level of significance.
2.7.4. Data management
A number of quality control procedures are used to minimize errors associated with data collection and entry. Standardized data collection instruments are used and trained interviewers review all instruments for missing items before submitting for data entry. An electronic data entry system developed by the DCC is used that includes logic checks to prevent entry of invalid data combined with independent double data entry and a reconciliation process. Initial data entry is conducted at UTHSCT and secondary entry is conducted at the DCC.
2.8. Cost-utility analysis
If outcomes among the PASM intervention group are significantly better compared to UC a cost-utility analysis will be conducted using SF-12 to calculate quality-adjusted life years (QALY). The societal perspective will be used and all significant costs considered including costs associated with delivering the intervention and health care utilization. The cost-utility of the PASM program will be described by the cost per QALY.
2.9. Study management
The study is comprised of three major components including: 1) patient recruitment, enrollment, data collection, and intervention; 2) computer-assisted telephone system; and 3) data management and analyses. These components are managed by three organizations including the UTHSCT, University of Texas at Tyler, and University of Alabama, respectively. Oversight and coordination of all activities is provided by the principal investigator (DBC) and project coordinator (RR).
3. Discussion
3.1 Need for interventions to address gaps in COPD management
Chronic obstructive pulmonary disease is progressively disabling and incurable. To lessen the burden of the disease multiple simultaneous interventions are necessary [1]. Optimal treatment focuses on pharmacologic and non-pharmacologic interventions, which include medication management, patient education, action plans for exacerbations, and pulmonary rehabilitation. An essential component of pulmonary rehabilitation is exercise. Treatment becomes complex and requires patient self-management. Unfortunately little attention is given to the development of patients’ skills and confidence in self-management. Lack of attention to self-management is a result, in part, from limited research evidence and methodological limitations including lack of theory-based methods, inconsistent delivery methods, and variation in content [8,55]. Moreover, patients have limited access to comprehensive care including exercise-based pulmonary rehabilitation. This trial was designed to enable widespread dissemination of a self-management physical activity intervention and to provide access to key components of pulmonary rehabilitation to underserved patients with COPD.
3.2 Innovative behavioral intervention to enhance self-management and physical activity
COPD self-management interventions have been examined as stand-alone programs [8] and as components of pulmonary rehabilitation programs [11]. Effing and co-workers [8] found reductions in hospitalizations (odd ratio 0.64 [95% confidence interval 0.47-0.89]) and improvements in quality of life compared to usual care in a recent meta-analysis of COPD self-management trials. However, there were no differences in exacerbations or emergency room visits, and change in quality of life was not clinically significant. A few studies of self-management have included therapist directed home exercise protocols [22], but none have examined behavioral interventions designed to enhance sustained regular lifestyle physical activity among patients with COPD. Of importance is the distinction between exercise and lifestyle physical activity [56]. Exercise is planned repetitive body movement with the intent of improving fitness (e.g., walking on a treadmill). Lifestyle physical activity is any body movement requiring large muscle contractions (e.g., taking the stairs, gardening, parking further away). “Lifestyle” programs, as we define it, encourage the accumulation of moderate-intensity physical activity by changing beliefs and attitudes towards physical activity as well as teaching cognitive and behavioral skills associated with increased activity versus prescribing an exercise plan. While exercise education is a necessary component it is insufficient in changing physical activity behavior.
Social cognitive theory and the transtheoretical model provided theoretical foundations of the behavioral intervention. Standardized materials along with standardized training and monitoring of the health coach are used to ensure internal validity. Patients are provided an intervention manual, scripted telephone counseling from a health coach, and automated telephone calls. This correspondence approach was modeled after other trials and intended to facilitate learning and behavioral activation [57–60].
The initiation and maintenance of moderate intensity physical activity is the target of the intervention because of the adverse consequences associated with physical inactivity among patients with COPD [13–17] and the benefits to physical and mental health from exercise rehabilitation [11]. Rather than prescribing and monitoring an exercise routine the intervention was designed to teach patients skills and techniques known to increase the likelihood of physical activity. The goals on type, intensity, frequency, and duration of physical activity were derived from pulmonary rehabilitation research [11], which suggests that benefits of exercise may be achieved from regular aerobic activity in short intervals at moderate intensity with monitoring over at least 8-12 weeks.
3.3 Assessment of clinically relevant outcomes
Effectiveness of the intervention will be assessed with clinically relevant primary outcome measures used in previous studies of pulmonary rehabilitation [18]. Dyspnea (CRQ-D) and functional capacity (6-minute walk distance) were targeted for several reasons including: 1) COPD is associated with low levels of physical activity, which leads to de-conditioning and worsening dyspnea associated with airflow obstruction, 2) physical inactivity among patients with COPD is associated with poorer physical and mental health outcomes, and 3) exercise training is probably the critical component that contributes to benefits and preserving the ability to walk 400m. Walking 400m is a proxy for community ambulation and is key to maintaining quality of life and independence [61]. Secondary outcomes and process measures are obtained to tailor counseling, advance understanding of the mechanisms of change (e.g., self-efficacy, readiness to change), and potentially guide development of future interventions.
3.4 Potential limitations and methodological considerations
Several factors that may affect external and internal validity will need to be considered. First, the trial is being conducted at one institution, which may threaten external validity. However, the study site is not a referral center for patients with COPD. Recruitment is from primary and secondary care, and enrollment criteria designed to provide a representative sample of the COPD population. Second, because both groups receive six weeks of COPD self-management education before randomization, the control group may not represent usual care. However, in the design of the study this education period was an attempt to enhance patient enrollment and to establish similar baseline characteristics between groups (e.g., knowledge, medical management, commitment to study), with the recognition that education alone is unlikely to be effective in changing behavior and outcomes [36]. Third, assessment of non-adherence to the intervention is limited to detection by the health coach during her calls, but becomes an integral part of coaching to promote behavior change. Fourth, measurement of physical activity is limited to self-report instead of objective measures due to feasibility concerns. The RAPA has been validated among elderly persons [40] and will serve as a proxy.
3.5 Conclusions and implications
In conclusion, the PASM intervention in this trial targets physical inactivity, which is a modifiable risk factor associated with morbidity and mortality among patients with COPD. Results from this trial have the potential to advance knowledge and methods that will contribute to the dissemination of a cost-effective program for patients with COPD unable to attend comprehensive pulmonary rehabilitation. It may also provide a method to help those who do participate in center-based rehabilitation maintain gains once rehabilitation is completed.
Acknowledgements
This study is funded by National Institutes of Health-National Heart, Lung, and Blood Institute R18 HL092955. Clinicaltrials.gov identifier NCT1108991. We gratefully acknowledge the study participants and research staff Toyua Akers, Ginny Harleston, and staff of UTHSCT Cardiopulmonary Services. Health Kinetics, publisher of Active Living Everyday, offered their support and permission to adapt their materials for this research. Special acknowledgement goes to Dr. Steven Blair whose previous work on lifestyle physical activity is the cornerstone of this intervention.
Abbreviations
- ALED
Active Living Everyday
- BODE index
[B]ody-mass index, degree of airflow [O]bstruction, [D]yspnea level, [E]xercise capacity
- BMI
Body mass index
- COPD
Chronic obstructive pulmonary disease
- CRQ
Chronic Respiratory Questionnaire
- CAT
Computer assisted telephone system
- COPD-SMART
COPD self-management activation research trial
- DCC
Data coordinating center
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- GDS
Geriatric Depression Scale
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- JA
Jamile Ashmore
- MCS
Mental health composite score
- PASM
Physical activity self-management
- PCS
Physical composite score
- DBC
David B. Coultas
- QALY
Quality adjusted life-years
- RR
Rennie Russo
- UTHSCT
University of Texas Health Science Center-Tyler
- UC
Usual care
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Global Initiative for Chronic Lung Disease [Last accessed 2/28/2013];Global Strategy for the Diagnosis Management and Prevention of COPD. 2011 :1–90. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2.
- [2].McKenna MT, Michaud CM, Murray CJL, Marks JS. Assessing the burden of disease in the United States using disability-adjusted life years. Am J Prev Med. 2005;28:415–23. doi: 10.1016/j.amepre.2005.02.009. [DOI] [PubMed] [Google Scholar]
- [3].IOM (Institue of Medicine) Crossing the Quality Chasm A New Health System for the 21st Century. National Academies Press; Wahington (DC): 2001. [Google Scholar]
- [4].IOM (Institue of Medicine) A Nationwide Framework for Surveillance of Cardiovascular and Chronic Lung Diseases. The National Academies Press; Washington (DC): 2011. [Google Scholar]
- [5].Make B, Dutro MP, Paulose-Ram R, Marton JP, Mapel DW. Undertreatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:1–9. doi: 10.2147/COPD.S27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].IOM (Institue of Medicine) Living Well with Chronic Illness: A Call for Public Health Action. National Academies Press; Washington (DC): 2012. [Google Scholar]
- [7].IOM (Institute of Medicine) In: Priority Areas for National Action: Transforming Health Care Quality. Adams K, Corrigan JM, editors. National Academies Press; Washington (DC): 2003. [Google Scholar]
- [8].Effing T, Monninkhof EM, Van der Valk PDLPM, Van der Palen J, Van Herwaarden CLA, Partidge MR, et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane database of systematic reviews (Online) 2007:CD002990. doi: 10.1002/14651858.CD002990.pub2. [DOI] [PubMed] [Google Scholar]
- [9].Adams SG, Smith PK, Allan PF, Anzueto A, Pugh J a, Cornell JE. Systematic review of the chronic care model in chronic obstructive pulmonary disease prevention and management. Arch Intern Med. 2007;167:551–61. doi: 10.1001/archinte.167.6.551. [DOI] [PubMed] [Google Scholar]
- [10].Peytremann-Bridevaux I, Staeger P, Bridevaux P-O, Ghali WA, Burnand B. Effectiveness of chronic obstructive pulmonary disease-management programs: systematic review and meta-analysis. Am J Med. 2008;121:433–443. doi: 10.1016/j.amjmed.2008.02.009. [DOI] [PubMed] [Google Scholar]
- [11].Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- [12].Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology. 2006;11:681–6. doi: 10.1111/j.1440-1843.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- [13].Watz H, Waschki B, Kirsten A, Müller K-C, Kretschmar G, Meyer T, et al. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest. 2009;136:1039–46. doi: 10.1378/chest.09-0393. [DOI] [PubMed] [Google Scholar]
- [14].Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, Pérez-Izquierdo J, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36:292–300. doi: 10.1183/09031936.00021409. [DOI] [PubMed] [Google Scholar]
- [15].Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–44. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- [16].Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–8. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Waschki B, Kirsten A, Holz O, Müller K-C, Meyer T, Watz H, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331–42. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- [18].Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev (Online) 2006;64 doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- [19].Emery CF, Green MR, Suh S. Neuropsychiatric function in chronic lung disease: the role of pulmonary rehabilitation. Respir Care. 2008;53:1208–16. [PubMed] [Google Scholar]
- [20].Johnston K, Grimmer-Somers K. Pulmonary rehabilitation: overwhelming evidence but lost in translation? Physiother Can. 2010;62:368–73. doi: 10.3138/physio.62.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McGill University Health Centre/Quebec Asthma and COPD Network Living Well with COPD [Internet] 2012 Available from: http://www.livingwellwithcopd.com/
- [22].Maltais F, Bourbeau J, Shapiro S, Lacasse Y, Perrault H, Baltzan M, et al. Effects of home-based pulmonary rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Ann of Intern Med. 2008;149:869–78. doi: 10.7326/0003-4819-149-12-200812160-00006. [DOI] [PubMed] [Google Scholar]
- [23].Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA. 1999;281:327–34. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- [24].Blair SH, Dunn AL, Marcus BH, Carpenter RA, Jaret P. Active Living Every Day: Get Active with a Proven 20-step Program. 2nd ed 2011. p. 194. [Google Scholar]
- [25].Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–64. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- [26].Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–5. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- [27].Human Kinetics [Last Accessed 2/28/2013];Activie Living Partners [Internet] http://www.activeliving.info/
- [28].Teleminder [Last Accessed 2/28/2013];TeleMinder Automated Appointment and Communications for Healthcare [Internet] 2012 http://www.teleminder.com/
- [29].Schünemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Austin P, et al. A comparison of the original chronic respiratory questionnaire with a standardized version. Chest. 2003;124:1421–9. doi: 10.1378/chest.124.4.1421. [DOI] [PubMed] [Google Scholar]
- [30].American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- [31].Schünemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ) COPD. 2005;2:81–9. doi: 10.1081/copd-200050651. [DOI] [PubMed] [Google Scholar]
- [32].Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10:407–15. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- [33].Dolmage TE, Hill K, Evans RA, Goldstein RS. Has my patient responded? Interpreting clinical measurements such as the 6-minute-walk test. Am J Respir Crit Care Med. 2011;184:642–6. doi: 10.1164/rccm.201103-0497CC. [DOI] [PubMed] [Google Scholar]
- [34].Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- [35].Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, et al. Health-related quality of life in patients by COPD severity within primary care in Europe. Respir Med. 2011;105:57–66. doi: 10.1016/j.rmed.2010.09.004. [DOI] [PubMed] [Google Scholar]
- [36].Coultas D, Frederick J, Barnett B, Singh G, Wludyka P. A randomized trial of two types of nurse-assisted home care for patients with COPD. Chest. 2005;128:2017–24. doi: 10.1378/chest.128.4.2017. [DOI] [PubMed] [Google Scholar]
- [37].Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–9. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- [38].Wigal JK, Creer TL, Kotses H. The COPD Self-Efficacy Scale. Chest. 1991;99:1193–6. doi: 10.1378/chest.99.5.1193. [DOI] [PubMed] [Google Scholar]
- [39].Heller A, Elliott MN, Haviland AM, Klein DJ, Kanouse DE. Patient activation status as a predictor of patient experience among Medicare beneficiaries. Med Care. 2009;47:850–7. doi: 10.1097/MLR.0b013e318197b661. [DOI] [PubMed] [Google Scholar]
- [40].Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Preventing Chronic Disease. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- [41].Marshall SJ, Biddle SJ. The transtheoretical model of behavior change: a meta-analysis of applications to physical activity and exercise. Ann Behav Med. 2001;23:229–46. doi: 10.1207/S15324796ABM2304_2. [DOI] [PubMed] [Google Scholar]
- [42].Hellsten L, Nigg C, Norman G, Burbank P, Braun L, Breger R, et al. Accumulation of behavioral validation evidence for physical activity stage of change. Health Psychol. 2008;27:S43–53. doi: 10.1037/0278-6133.27.1(Suppl.).S43. [DOI] [PubMed] [Google Scholar]
- [43].Astroth KS, Fish AF, Mitchell GL, Bachman JA, Hsueh K-H. Construct validity of four exercise stage of change measures in adults. Res Nurs Health. 2010;33:254–64. doi: 10.1002/nur.20380. [DOI] [PubMed] [Google Scholar]
- [44].Kosma M, Ellis R. Establishing construct validity of a stages-of-change algorithm for physical activity. Am J Health Promot. 2010;25:e11–20. doi: 10.4278/ajhp.090914-QUAN-296. [DOI] [PubMed] [Google Scholar]
- [45].Marcus BH, Forsyth L. Bahrke MS, Bernard K, Evans J, Hawkins S, Fritz KG, Fortney P, editors. Motivating People to Be Physically Active. (2nd ed) 2009:216. [Google Scholar]
- [46].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- [47].Bush B, Shaw S, Cleary P, Delbanco TL, Aronson MD. Screening for alcohol abuse using the CAGE questionnaire. Am J Med. 1987;82:231–5. doi: 10.1016/0002-9343(87)90061-1. [DOI] [PubMed] [Google Scholar]
- [48].Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15-item geriatric depression scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. Am J Geriatr Soc. 2005;53:1570–6. doi: 10.1111/j.1532-5415.2005.53461.x. [DOI] [PubMed] [Google Scholar]
- [49].Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–91. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- [50].Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- [51].Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- [52].Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91:221–5. doi: 10.1016/j.apmr.2009.10.017. [DOI] [PubMed] [Google Scholar]
- [53].Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schünemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J. 2008;32:637–43. doi: 10.1183/09031936.00140507. [DOI] [PubMed] [Google Scholar]
- [54].Schulz KF, Grimes DA. Multiplicity in randomised trials II: subgroup and interim analyses. Lancet. 2005;365:1657–61. doi: 10.1016/S0140-6736(05)66516-6. [DOI] [PubMed] [Google Scholar]
- [55].Effing TW, Bourbeau J, Vercoulen J, Apter AJ, Coultas D, Meek P, et al. Self-management programmes for COPD: moving forward. Chron Respir Dis. 2012;9:27–35. doi: 10.1177/1479972311433574. [DOI] [PubMed] [Google Scholar]
- [56].Ashmore JA, Frierson G, Blair SN. The Role of Physical Activity in Weight Loss and Weight Loss Maintenance. In: Akabas S, Lederman SA, Moore BJ, editors. Textbook of Obesity: Biological, Psychological and Cultural Influences. Wiley-Blackwell; 2012. pp. 344–54. [Google Scholar]
- [57].Dollard J, Smith J, Thompson DR, Stewart S. Broadening the reach of cardiac rehabilitation to rural and remote Australia. Eur J Cardiovasc Nurs. 2004;3:27–42. doi: 10.1016/j.ejcnurse.2003.10.002. [DOI] [PubMed] [Google Scholar]
- [58].Fries E, Edinboro P, McClish D, Manion L, Bowen D, Beresford S, et al. Randomized trial of a low-intensity dietary intervention in rural residents: the Rural Physician Cancer Prevention Project. Am J Prev Med. 2005;28:162–8. doi: 10.1016/j.amepre.2004.10.017. [DOI] [PubMed] [Google Scholar]
- [59].Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD. 2005;2:105–10. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- [60].Castro CM, King AC. Telephone-assisted counseling for physical activity. Exerc Sport Sci Rev. 2002;30:64–8. doi: 10.1097/00003677-200204000-00004. [DOI] [PubMed] [Google Scholar]
- [61].Fielding RA, Rejeski WJ, Blair S, Church T, Espeland MA, Gill TM, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–37. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]