Abstract
Type 2 Diabetes (T2DM) is the seventh leading cause of death in the United States, and is quickly becoming a global pandemic. T2DM results from reduced insulin sensitivity coupled with a relative failure of insulin secretion. Reduced insulin sensitivity has been associated with reduced nitric oxide synthase (NOS) activity and impaired glucose uptake in T2DM skeletal muscle. Upon insulin stimulation, NO synthesis increases in normal adult skeletal muscle, whereas no such increase is observed in T2DM adults. Endothelial NOS is activated by phosphorylation in the C-terminal tail in response to insulin. Neuronal NOS (nNOS), the primary NOS isoform in skeletal muscle, contains a homologous phosphorylation site, raising the possibility that nNOS, too, may undergo an activating phosphorylation event upon insulin treatment. Yet it remains unknown if or how nNOS is regulated by insulin in skeletal muscle. Data shown herein indicate that nNOS is phosphorylated in response to insulin in skeletal muscle and that this phosphorylation event occurs rapidly in C2C12 myotubes, resulting in increased NO production. In vivo phosphorylation of nNOS was also observed in response to insulin in mouse skeletal muscle. These results indicate, for the first time, that nNOS is phosphorylated in skeletal muscle in response to insulin and in association with increased NO production.
Keywords: Nitric oxide synthase, Type 2 Diabetes (T2DM), insulin signaling, myotubes, skeletal muscle
Introduction
Type 2 diabetes mellitus (T2DM) is characterized by insulin resistance and impaired insulin-stimulated nitric oxide (NO) generation in both endothelium and skeletal muscle. NO is an important gaseous regulatory molecule, produced by isoforms of nitric oxide synthase (NOS). NO acts variably as a vasodilator [1,2], neurotransmitter [3], or cytotoxic agent [4], depending upon its source and location of production. NOS enzymes synthesize NO from L-arginine and molecular oxygen [5], utilizing electrons donated by NADPH. The homeostatic regulation of NO synthesis is crucial in biological systems, as the generation of insufficient amounts of NO can result in deleterious outcomes, such as increased blood pressure due to uncontrolled vasoconstriction. In contast, overproduction of NO can yield toxic sequelae, such as endotoxic shock.
nNOS and eNOS knockout mice demonstrate insulin resistance, suggesting that NO produced by nNOS plays an important role in mediating insulin action [6,7]. However, the direct effect of insulin on skeletal muscle nNOS is unknown. One prior study by Kashyap/Roman et al. [8], using euglycemic/hyperinsulinemic clamps, showed that NO production in muscle biopsies is significantly reduced in obese, T2DM human subjects compared to obese, non-diabetic controls. Moreover, in the insulin-resistant subjects, insulin treatment failed to evoke increases in NO in skeletal muscle, whereas insulin stimulated a robust increase in NO in the control subjects [8]. These results suggest that insulin action in skeletal muscle involves NO production, thus implicating mechanisms regulating NO bioavailability. These mechanisms must be elucidated to understand the pathogenesis of muscle insulin resistance, which is an important component of the pathology of T2DM.
Phosphorylation plays a critical role in the potentiation of NO generation by vascular endothelial NOS (eNOS), which is activated by insulin-dependent AKT-catalyzed phosphorylation at eNOS Ser1177 [9,10], potentially explaining the vasodilatory effects of insulin. While the role of eNOS in insulin action and its inactivation as a component of insulin resistance in the vasculature has been well-documented [2,11–13], the metabolic role of NOS in other tissues, such as skeletal muscle, remains elusive. The predominant NOS isoform in skeletal muscle is nNOS, several splice variants of which, i.e., nNOSμ, nNOSβ, and nNOSα [14,15], may be present. In this report, we do not distinguish among the nNOS variants, hereafter collectively referred to as skeletal muscle nNOS.
The rapid increase in NO production observed in the human insulin clamp studies suggests that post-translational modification of skeletal muscle nNOS occurs in direct response to insulin rather than as insulin-regulated transcription [8]. Since skeletal muscle nNOS contains a homologous residue to the AKT-phosphorylated serine in eNOS (Ser1177), it was possible that nNOS could be phosphorylated in response to insulin in skeletal muscle. There is evidence of nNOS phosphorylation in response to insulin in brain: prior immunoblot studies in rat hypothalamus demonstrated an increase in nNOS Ser1416 phosphorylation [16], the rat equivalent of Ser1412 in mouse nNOS. In vitro kinase assays in rat brain nucleus tractus solitarii additionally showed nNOS is phosphorylated at this residue in an insulin-dependent manner [17].
To examine nNOS phosphorylation by insulin treatment, the murine muscle C2C12 cell line, which can be differentiated from myoblasts to myotubes, was used to probe putative Ser1412 phosphorylation on the C-terminal tail of skeletal muscle nNOS in response to stimulation by insulin. Our results show that insulin stimulation resulted in significantly increased phosphorylation of skeletal muscle nNOS in C2C12 myotubes, as well as a concomitant increase in NO levels. In vivo, insulin-stimulated nNOS phosphorylation was also observed in WT mouse C57BL/6J muscle tissue, further validating that insulin-mediated phosphorylation of nNOS is an important component of the insulin-signaling cascade.
Materials and Methods
Cell Culture and Treatment
Murine-derived muscle cell line C2C12 (Cell Biology Labs) myoblasts were grown to near 100% confluency in high glucose (25 mM) DMEM (Sigma-Aldrich) with 10% fetal bovine serum (Gibco), hereafter called complete media. Myoblasts were then switched to high glucose DMEM media, 2% horse serum (Gibco), hereafter called horse serum media. After four days in serum starvation, cells were completely differentiated to myotubes, and were treated with either vehicle (3 µM HCl) or 100 nM insulin (Sigma Aldrich) from 0–60 min.
Animals
Wild-type male C57BL/6J mice were fed ad libitum a standard lab chow (Harlan, 11.5 kcal% fat) and maintained in micro-isolator cages, 5 to a cage, on a 12-hour dark/light cycle. Mice (4–6 months of age) were given intraperitoneal injections of either vehicle (PBS) or 5 mU per gram body weight insulin (Novo Nordisk). Ten minutes after insulin injection, mice were sacrificed and quadriceps femoris muscles were collected for analysis.
All animal studies have been approved by Institutional Animal Care and Use Committee (IACUC), University of Texas Health Science Center at San Antonio, San Antonio, TX, USA. Animals are housed in an Association for Assessment and Accreditation of Laboratory Animal Care, International (AAALAC) accredited facility with full veterinary support. The facility is operated in compliance with the Public Law 89-544 (Animal Welfare Act) and amendments, Public Health Services Policy on Humane Care and Use of Laboratory Animals (PHS Policy), and The Guide for the Care and Use of Laboratory Animals.
NO Detection
The NO-specific fluorescent dye 4,5-diaminofluorescein diacetate (DAF-2 DA; Sigma) was used to measure NOS activity indirectly. C2C12 myoblasts were seeded onto 6-well plates, maintained in complete media and differentiated into myotubes with horse serum-containing media. For fluorescent microscopy imaging, myotubes were treated with either vehicle or 100 nM insulin in horse serum media for 60 min, rinsed 2× with PBS, then treated with 5 µM DAF-2 DA in PBS+glucose for 30 min. After rinsing, clear α-MEM (Sigma-Aldrich) was applied to the living cells, which were then imaged with at 10× using a Zeiss Axioscope 2 HBO 100 with excitation wavelength at 495 nm and emission at 515 nm. For quantitative analysis of DAF-2 DA fluorescence, myotubes were treated with 5 µM DAF-2 DA in PBS+glucose for 30 min, rinsed 2× with PBS, then treated with either vehicle or 100 nM insulin in horse serum media for 60 min. As a positive control, cells treated with 450 µM S-Nitroso-N-Acetyl-D,L-Penicillamine (SNAP, Cayman Chemical). After rinsing, PBS was applied to the cells, and measurements were made in a Tecan plate reader with excitation wavelength at 475 nm and emission at 550 nm.
Immunoblot Analyses
Cell lysates from C2C12 cells were prepared by collecting cells in PBS with phosphatase and protease inhibitors (Thermo-Fisher) and Benzonase, and then homogenized. For mouse quadriceps muscle lysates, after an overnight fast, mice were given an intraperitoneal injection of 5 mU per gram body weight of insulin (Novo Nordisk) or an equivalent volume of sterile saline. Ten min post injection, quadriceps muscle was collected and frozen in liquid nitrogen, then whole tissue homogenates were prepared. For all immunoblots, thirty micrograms of lysates were electrophoresed in gradient SDS-PAGE (2–15%; Bio-Rad) and proteins were transferred onto PVDF membrane (Millipore). Membranes were probed with antibodies to phospho-S1417 nNOS (recognizes mouse nNOS at Ser1412, Abcam), total nNOS (BD Biosciences), phospho-S473 AKT (Cell Signaling Technology), total AKT (Cell Signaling Technology), and/or GAPDH (Cell Signaling Technology), as indicated, followed by a 1-hr incubation in horseradish-peroxidase linked secondary antibody (Santa Cruz). Detection was by Immobilon Western Chemiluminescent HRP Substrate (Millipore). Immunoblots were analyzed using ImageJ [18].
Results/Discussion
nNOS is phosphorylated in response to insulin in C2C12 myotubes
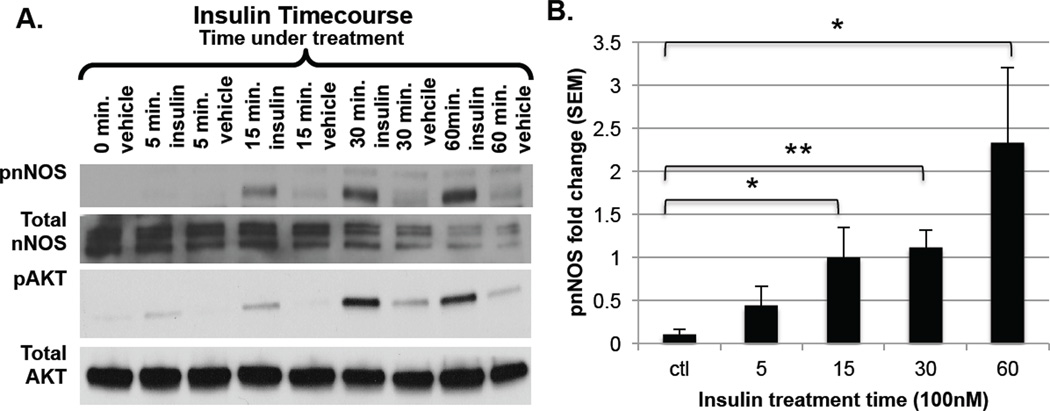
To determine whether nNOS is phosphorylated in skeletal muscle in response to insulin, the murine-derived in vitro muscle cell line C2C12 was used as a model system. Control and insulin-treated C2C12 cell lysates were probed for nNOS phosphorylated on residue S1412 via immunoblot analysis. As shown in Fig. 1A, insulin treatment resulted in increased nNOS phosphorylation at Ser1412 in a time-dependent manner, with significant (P<0.05) phosphorylation occurring at 15, 30, and 60 min post-insulin addition. Fig. 1B shows the ratio of phosphorylated nNOS to total nNOS, clearly demonstrating the significant increase of this event, particularly at the 30 min time point. Phosphorylation of nNOS occurred concomitantly with activation of the insulin-signaling pathway, shown by the increase in phospho-AKT at Ser473 by insulin treatment (Fig. 1A). This result suggests that nNOS phosphorylation is a consequence of activated insulin signaling in C2C12 myotubes.
Figure 1. Immunoblot analysis of nNOS phosphorylation in C2C12 cells at varying times of insulin treatment.
A. C2C12 myoblasts were grown in DMEM with 10% FBS. After differentiation to myotubes using DMEM with 2% horse serum, cells were treated with either vehicle (3 µM HCl) or 100 nM insulin from 0–60 min. Blots were probed with polyclonal antibodies recognizing phospho-S1412 nNOS, total nNOS, phospho-S473 AKT, and total AKT; B. Phospho-nNOS results are expressed relative to total nNOS. Data shown are mean ± SEM for 4 separate experiments. *P<0.05, **P<0.01 compared with control.
NO production increases in skeletal muscle with insulin treatment
Having demonstrated nNOS phosphorylation by insulin treatment in C2C12 myotubes, it was important to investigate what changes this post-translational modification conveyed to nNOS activity, particularly given the documented increase in eNOS activity by insulin-stimulated phosphorylation at a homologous residue [8,9]. Furthermore, the observation that, in human skeletal muscle, there was an increase in NO production in insulin-treated subjects [8], absent in those with T2DM, indicated that insulin-induced phosphorylation of nNOS likely results in increased NO synthesis. To detect changes in NO levels in the cells, the NO-specific cell permeable compound DAF-2 DA was used. DAF-2 DA interacts with NO and NO-derived nitrosating species to produce a fluorescent by-product [19], which can be used as an indicator of NO synthesis in living cells [20]. This method has been used successfully in endothelial cells [21] and has been adapted herein for use in C2C12 cells.
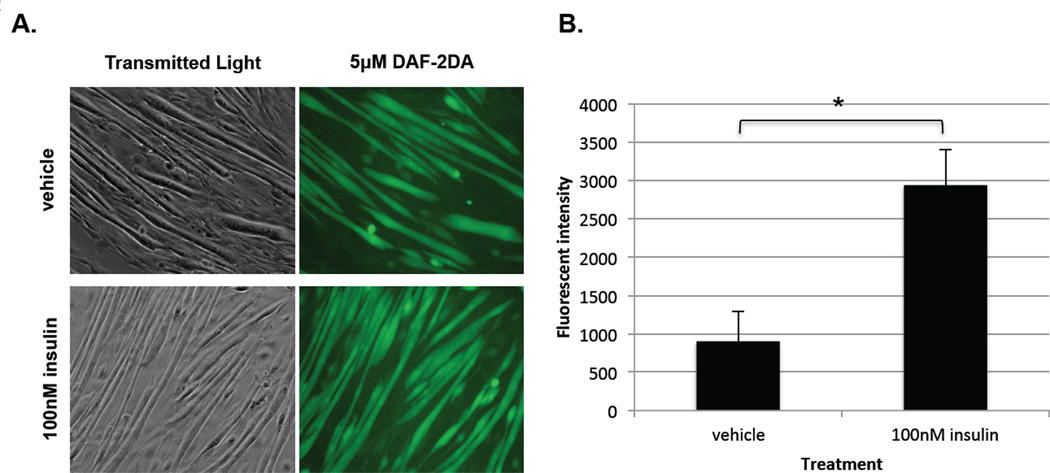
Fig. 2A shows the transmitted light and fluorescent micrographs of C2C12 cells treated with vehicle (top) or insulin (bottom). The transmitted light micrograph demonstrates that the number and quality of myotubes is equivalent in either case. The cells treated with insulin, however, demonstrate a clear increase in fluorescence intensity over those treated with vehicle. Similar experiments in which cell lysates were isolated and fluorescence measured were used to quantitate the increase in NO production upon nNOS phosphorylation (Fig. 2B), and demonstrated a significant increase in fluorescent intensity and thus NO production in the insulin-treated cells. These results strongly support the idea that insulin signaling leads to phosphorylation of nNOS S1412, causing activation of the enzyme and thus increased NO production.
Figure 2. Fluorescent imaging of C2C12 myotubes with DAF-2DA shows increase in NO under insulin treatment.
A. DAF-2DA detects NO, as fluorescence increases as NO levels increase, giving an indirect means to measure NOS activity. C2C12 myotubes were treated with either vehicle (3 µM HCl) or 100 nM insulin for 60 min, then incubated in 5 µM DAF-2DA for 30 min. Living cells were then imaged using fluorescence microscopy, with 450 nm excitation and 550 emission. (n=3); B. C2C12 myotubes were preloaded with 5 µM DAF-2DA for 30 min, then treated with either vehicle (3 µM HCl) or 100 nM insulin for 60 min. Living cells’ fluorescence intensity was measured by Tecan plate reader, with 450 nm excitation and 550 emission. Insulin treatment fluorescence results are expressed relative to vehicle control fluorescence as means ± SEM for 3 individual experiments. *P<0.05 compared with control.
nNOS is phosphorylated in response to insulin in mouse skeletal muscle in vivo
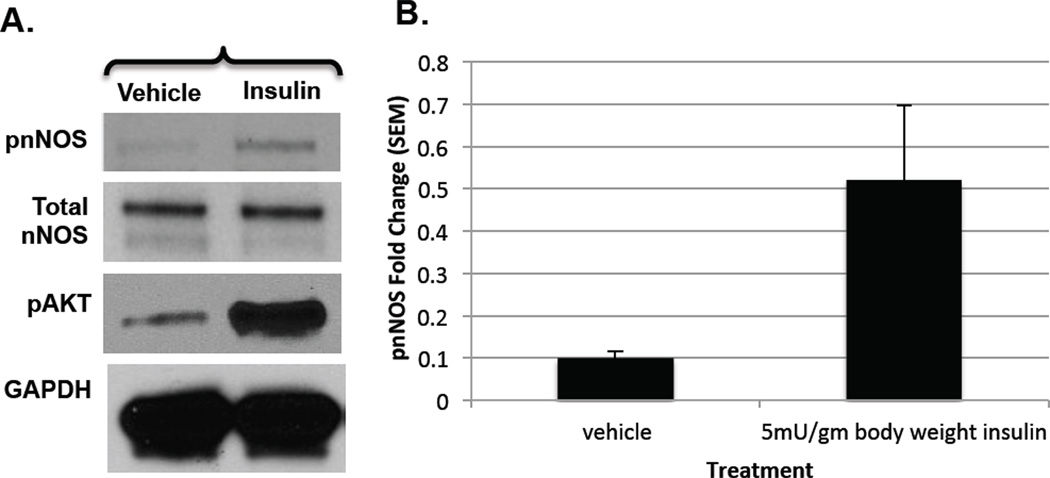
Having observed an increase in Ser1412 nNOS phosphorylation in C2C12 myotubes by insulin treatment, the relevance of this phosphorylation event was tested in C57BL/6J mouse skeletal muscle tissue lysates to confirm that nNOS phosphorylation was indeed occurring in the tissue concomitant with insulin signaling. As shown in Fig. 3A,B, insulin significantly increased nNOS phosphorylation in mouse skeletal muscle, as compared to the vehicle control, while total nNOS protein levels remained constant. Phosphorylation of Akt was also stimulated in the muscles of insulin-treated animals, as expected. These results recapitulate in vivo the insulin-stimulated skeletal muscle nNOS phosphorylation we observed in C2C12 cells in culture, demonstrating the physiological relevance of our results in skeletal muscle tissue.
Figure 3. Immunoblots of nNOS phosphorylation by insulin treatment in mice.
A. WT C57BL/6J male mice, aged to 4–6 months, were given intraperitoneal injections of either vehicle (PBS) or 5 mU per gram body weight insulin. Quadriceps were collected for analysis. Muscle lysates were probed with phospho-S1417 nNOS, total nNOS, phospho-S473 AKT, and GAPDH polyclonal antibodies; B. Phospho-nNOS results are expressed as relative to total nNOS as means ± SEM for 2 individual experiments.
Overall, we find that nNOS, similar to eNOS, is phosphorylated at Ser1412 by insulin treatment in C2C12 myotubes as well as in mouse skeletal muscle, and that this phosphorylation event increases nNOS production of NO. Moreover, the phosphorylation events were observed to occur concomitantly with activated insulin signaling, suggesting that the NO produced by insulin-activated skeletal muscle nNOS may play a role in the insulin-signaling pathway. Indeed, it has been reported that NO contributes to insulin-stimulated glucose uptake in skeletal muscle [22–25]. In rat skeletal muscle tissue, NO had an additive effect on insulin-stimulated glucose transport [26], and in L6 myotubes, NO upregulated GLUT4 expression [23]. Additionally, pharmacological treatment with an NO donor, NONOate, increased glucose uptake in human skeletal muscle and L6 cells [27]. Our results suggest that, in T2DM subjects, the observed decrease in NO production is a result of decreased insulin-stimulated nNOS phosphorylation. Lower phospho-nNOS levels, resulting in attenuated nNOS activity, may then contribute to many T2DM sequelae, such as the observed decrease in NO-induced glucose uptake. The consequences for the loss of insulin-stimulated phosphorylation of the endothelial isoform, eNOS, are also quite detrimental as it results eventually in endothelial dysfunction and atherosclerosis. Thus it is clear that both nNOS and eNOS are phosphorylated as a result of insulin signaling and that NO plays an important role in the cellular response to insulin in a variety of tissues. Contrary to the action of NO in the endothelium, however, the mechanism of action of NO in skeletal muscle, particularly its involvement in glucose uptake, is less clear and remains to be elucidated.
Highlights.
nNOS is phosphorylated in response to insulin in C2C12 myotubes.
nNOS is phosphorylated in response to insulin in mouse skeletal muscle.
NO production increases in myotubes with insulin stimulation.
Acknowledgments
We would like to thank Srikanth Reddy Polusani, PhD, for his aid in all fluorescence experiments, as well as Satya P. Panda, PhD, for useful discussion. Supported by NIH GM052419 to LJR and BSM. BSM is the Robert A. Welch Distinguished Chair in Chemistry (AQ0012).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quyyumi AA, Dakak N, Andrews NP, et al. Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92(3):320–326. doi: 10.1161/01.cir.92.3.320. [DOI] [PubMed] [Google Scholar]
- 2.Huang PL. eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab. 2009;20(6):295–302. doi: 10.1016/j.tem.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rand MJ, Li CG. Nitric oxide as a neurotransmitter in peripheral nerves: Nature of transmitter and mechanism of transmission. Annu Rev Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- 4.Hibbs JB, Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: A cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 5.Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43(3):521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 6.Turini P, Thalmann S, Jayet PY, et al. Insulin resistance in mice lacking neuronal nitric oxide synthase is related to an alpha-adrenergic mechanism. Swiss Med Wkly. 2007;137(49–50):700–704. doi: 10.4414/smw.2007.11950. [DOI] [PubMed] [Google Scholar]
- 7.Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000;49(5):684–687. doi: 10.2337/diabetes.49.5.684. [DOI] [PubMed] [Google Scholar]
- 8.Kashyap SR, Roman LJ, Lamont J, et al. Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J Clin Endocrinol Metab. 2005;90(2):1100–1105. doi: 10.1210/jc.2004-0745. [DOI] [PubMed] [Google Scholar]
- 9.Michell BJ, Griffiths JE, Mitchelhill KI, et al. The akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9(15):845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 10.Fulton D, Gratton JP, McCabe TJ, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg HO, Brechtel G, Johnson A, et al. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94(3):1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg HO, Chaker H, Leaming R, et al. Obesity/insulin resistance is associated with endothelial dysfunction. implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding H, Triggle CR. Endothelial dysfunction in diabetes: Multiple targets for treatment. Pflugers Arch. 2010;459(6):977–994. doi: 10.1007/s00424-010-0807-3. [DOI] [PubMed] [Google Scholar]
- 14.Silvagno F, Xia H, Bredt DS. Neuronal nitric-oxide synthase-mu, an alternatively spliced isoform expressed in differentiated skeletal muscle. J Biol Chem. 1996;271(19):11204–11208. doi: 10.1074/jbc.271.19.11204. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Newton DC, Robb G, Brett DS, et al. RNA diversity has profound effects on the translation of neuronal nitric oxide synthase. PNAS. 1999;96(21):12150–12155. doi: 10.1073/pnas.96.21.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canabal DD, Song Z, Potian JG, et al. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1418–R1428. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- 17.Chiang HT, Cheng WH, Lu PJ, et al. Neuronal nitric oxide synthase activation is involved in insulin-mediated cardiovascular effects in the nucleus tractus solitarii of rats. Neuroscience. 2009;159(2):727–734. doi: 10.1016/j.neuroscience.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 18.Rasband WS. bethesda, maryland, USA: 1997–2012. Image J, U. S. national institutes of health. http://Imagej.nih.gov/ij/ [Google Scholar]
- 19.Planchet E, Kaiser WM. Nitric oxide (NO) detection by DAF fluorescence and chemiluminescence: A comparison using abiotic and biotic NO sources. J Exp Bot. 2006;57(12):3043–3055. doi: 10.1093/jxb/erl070. [DOI] [PubMed] [Google Scholar]
- 20.Zielonka J, Zielonka M, Sikora A, et al. Global profiling of reactive oxygen and nitrogen species in biological systems: High-throughput real-time analyses. J Biol Chem. 2012;287(5):2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hien TT, Oh WK, Nguyen PH, et al. Nectandrin B activates endothelial nitric-oxide synthase phosphorylation in endothelial cells: Role of the AMP-activated protein kinase/estrogen receptor alpha/phosphatidylinositol 3-kinase/akt pathway. Mol Pharmacol. 2011;80(6):1166–1178. doi: 10.1124/mol.111.073502. [DOI] [PubMed] [Google Scholar]
- 22.Balon TW, Nadler JL. Evidence that nitric oxide increases glucose transport in skeletal muscle. J Appl Physiol. 1997;82(1):359–363. doi: 10.1152/jappl.1997.82.1.359. [DOI] [PubMed] [Google Scholar]
- 23.Lira VA, Soltow QA, Long JH, et al. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;293(4):E1062–E1068. doi: 10.1152/ajpendo.00045.2007. [DOI] [PubMed] [Google Scholar]
- 24.McConell GK, Rattigan S, Lee-Young RS, et al. Skeletal muscle nitric oxide signaling and exercise: A focus on glucose metabolism. Am J Physiol Endocrinol Metab. 2012;303(3):E301–E307. doi: 10.1152/ajpendo.00667.2011. [DOI] [PubMed] [Google Scholar]
- 25.de Castro Barbosa T, Jiang LQ, Zierath JR, Nunes MT. L-arginine enhances glucose and lipid metabolism in rat L6 myotubes via the NO/ c-GMP pathway. Metabolism. 2013;62(1):79–89. doi: 10.1016/j.metabol.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes. 2001;50(2):241–247. doi: 10.2337/diabetes.50.2.241. [DOI] [PubMed] [Google Scholar]
- 27.Deshmukh AS, Long YC, de Castro Barbosa T, et al. Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK)alpha1-specific activity and glucose transport in human skeletal muscle. Diabetologia. 2010;53(6):1142–1150. doi: 10.1007/s00125-010-1716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]