Abstract
Objectives
Acute myelogenous leukemia (AML) largely affects older adults. Few interventions have sought to improve functional status and health-related quality of life (HRQL) during treatment. The objective of this study is to examine the feasibility of an exercise intervention among older adults with AML undergoing induction chemotherapy.
Materials and Methods
Pilot study of adults ≥50 years of age hospitalized for AML chemotherapy. The four week exercise intervention included stretching, walking, and strength exercises. Feasibility measures included recruitment, retention, number of exercise sessions completed, and barriers to participation. Physical function, HRQL, depression, and distress were assessed at baseline (week 1), upon completion of intervention (week 5), and during follow-up (weeks 9–13). Exploratory analyses used repeated measures ANCOVA to model changes over time.
Results
Among 55 eligible inpatients, 24 enrolled (43.6%). Mean age was 65.1 years (SD 7.8). 87.5% of participants completed baseline measures; 70.8% attended ≥1 exercise sessions, and 50.0% completed post-intervention assessment. Among baseline characteristics, only higher physical performance was associated with greater number of exercise sessions attended (p=0.001). Post intervention, HRQL and depressive symptoms improved (p <0.05).
Conclusion(s)
Recruitment to an exercise intervention was feasible. Exercise shows promise to maintain physical function and enhance HRQL. Strategies to enhance adherence to exercise are needed to maximize benefit.
Keywords: Acute myelogenous leukemia, elderly, physical activity, exercise, physical function, quality of life
Introduction
Acute myelogenous leukemia (AML) is a disease of older adults, and survival is lower with increasing age. Optimal therapy for this population remains controversial due to poor treatment outcomes in clinical trials. While selected older adults tolerate standard therapies well, older adults are generally more likely to experience treatment-associated toxicity and less benefit from treatment when undergoing standard induction and post-remission therapies.
Multiple factors contribute to poor outcomes experienced by older adults treated for AML. First, tumor biology has been identified as a strong predictor of prognosis. Second, patient-specific characteristics (e.g., increased comorbidity, performance status) also influence outcomes. Finally, even the most fit older adults remain at high risk for developing functional decline during induction chemotherapy. While there has been a concerted effort to investigate the impact of tumor biology and comorbidity on treatment outcomes in older AML patients, few studies have investigated interventions to minimize physical deconditioning during treatment.
Functional decline is a major concern for older adults with AML. Prolonged hospitalization alone has been consistently associated with deconditioning and disability in older adults. For AML patients, the prolonged hospitalization is typically complicated by infections related to cytopenias, further increasing the risk of deconditioning. Functional decline during initial therapy may affect health-related quality of life (HRQL) and decrease tolerance for additional treatments required for cure. Maintaining physical function during induction therapy could ultimately increase the number of older adults who are candidates for post-remission therapies and improve HRQL.
Exercise can improve physical function and decrease the risk of disability in older adults. A few studies have specifically reported the benefit of exercise during hospitalization on physical function in older non-cancer populations. Specific to cancer patients, an increasing body of literature supports the benefit of exercise both during and after cancer treatment. There is increased interest in promoting exercise early in treatment to maximize functional status during and after receipt of chemotherapy. The underlying premise of this approach is that it may be easier to prevent or minimize functional decline than to recover from more severe decline.
Specific to hematologic malignancies, a recent comprehensive review identified 24 exercise intervention studies that provide evidence for improvements in body composition, physical function, symptoms, and quality of life. Two of these studies investigated exercise for patients with AML receiving induction chemotherapy. Chang et al. randomized 22 inpatients with AML (mean age<55) to a 3-week walking intervention or usual care and demonstrated benefits in fitness, fatigue and distress in the walking group. Battaglini et al. reported on the feasibility of an aerobic and strength training intervention in a non-randomized study of 10 subjects with AML <50 years of age. No studies to date have evaluated the feasibility of an exercise intervention specifically in older AML patients, who are at highest risk for functional decline.
The primary objective of this study was to assess the feasibility of conducting a hospital-based exercise intervention for older adults undergoing induction chemotherapy for AML. The secondary objective was to obtain preliminary data on the efficacy of the intervention on physical function, HRQL, depression, distress.
Materials and Methods
Design and Setting
This prospective, non-randomized study was conducted between June 2007 and December 2008. All participants were recruited from the inpatient Leukemia Service of the Wake Forest University Baptist Medical Center (WFUBMC). This study was approved by the Institutional Review Board and all participants provided written informed consent.
Study Sample
Eligibility criteria included the following: (a) age ≥50 years; (b) histologically documented AML; (c) inpatient status; (d) planned induction chemotherapy during hospitalization; (e) ambulatory (Eastern Cooperative Group Oncology Performance Score 0–3); (f) absence of acute medical instability (i.e., acute thrombosis, active ischemia, hemodynamic instability, uncontrolled pain); (g) absence of probable cognitive impairment (defined as <3 incorrect responses on the Pfeiffer Mental Status Scale) ; (h) ability to understand English; and (i) physician’s approval.
Recruitment and Procedures
A research assistant screened potentially eligible patients and obtained informed consent within 7 days of AML diagnosis or hospital admission (for patients admitted with a confirmed diagnosis). Research staff confirmed medical eligibility with the attending physician.
Study participants completed a baseline evaluation that included demographic, self-report, and physical performance assessments during week 1 of the study (Time 1). Next, an orientation to the exercise sessions was conducted (detailed below) followed by the four week intervention. At intervention completion (Weeks 5–6; Time 2) and at readmission for consolidation therapy or in clinic (Weeks 9–13; Time 3), study participants repeated the physical performance and self-report assessments.
Intervention
Each participant had an orientation session led by a trained exercise interventionist to introduce the exercise protocol and ensure proper technique and safety. During weeks 2–5, a total of twelve exercise sessions were offered three times per week on the inpatient ward. Each of the 30–45 minute sessions focused on strength, flexibility and walking, and were tailored to each participant’s level of energy and treatment-related symptoms. Prior to sessions, the interventionist checked the participant’s medical chart and discussed current status and potential exercise contraindications with the nurse. If a participant was too sick to exercise during the intervention period, (s)he was monitored daily to determine when the intervention could be resumed.
Each session consisted of a warm-up; walking, strength and flexibility exercises; a second walking phase; and a cool-down period. The 5-minute warm-up included walking in place and mild stretching. Next, the participant began the first (mild intensity) walking phase, which was gradually lengthened, if possible, up to 15 minutes. Participants then performed a tailored 15-minute strength and flexibility program with resistance bands to target the upper and lower body. Next, each participant completed a second walking phase, following the same progression as the first walking phase. Last, a 5 minute cool-down was completed. Post-activity heart rate and ratings of perceived exertion (RPE) were recorded after each phase. All participants received two resistance bands and written guidelines for continuing exercises after the intervention was completed.
Data Collection
All study participants were asked to complete self-report questionnaires at Times 1, 2 and 3. If assistance was needed, the research assistant administered them via interview. Physical performance testing [Short Physical Performance Battery (SPPB) and grip strength] was also obtained at these timepoints. All data were collected at these timepoints regardless of level of intervention compliance. Before and after each exercise session, patients rated current level of fatigue, nausea, distress, and pain from 0 to 10 (i.e., 0=no fatigue to 10=extreme fatigue). The following information was abstracted from the medical record: sociodemographics, AML diagnosis and treatment, cytogenetic risk group, height, weight, comorbid conditions for classification into a modified Charlson Comorbidity Index (excluding leukemia), age, and serologic parameters at admission.
Outcome Measures
Feasibility
The primary outcome for this pilot study was feasibility, assessed by: (1) percentage of eligible patients recruited; (2) number of exercise sessions completed; (3) percentage of participants completing assessments; and (4) barriers to recruitment. Qualitative intervention feedback from participants was also collected.
Objective Physical Performance Assessment
The Short Physical Performance Battery (SPPB) was used to evaluate lower extremity physical function. This validated measure is comprised of a short walk, repeated chair stands, and balance test. Each of the 3 performance measures was scored ranging from 0–4 (0 = unable to complete the test; 4 = highest performance level), with total summed score range from 0–12. To test static upper body strength, a grip-strength dynamometer was used. Two trials were performed for each hand and the best score (recorded in kilograms) was analyzed.
Self-Report Measures
Health-related quality of life (HRQL) was assessed using the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu). The FACT-Leu is made up of the Functional Assessment of Cancer Therapy (FACT-G) assessment and a 17-item leukemia-specific subscale. The FACT-G is a 27-item self-report questionnaire that consists of 4 subscales: physical well-being, social/family well-being, emotional well-being, and functional well-being.
Additional self-report measures included depression, distress, and physical activity level. The 11-item Center for Epidemiologic Studies Depression Scale – Short Form (CES-D) was used to measure depressive symptoms in the past week on a 3-point scale, yielding total scores from 0 to 22 (higher scores reflect greater depressive symptoms). We used the Distress Thermometer, a single-item rating from 0 (no distress) to 10 (extreme distress) to evaluate distress. Physical activity was recorded as days per week of mild, moderate or strenuous activity performed during the month prior to diagnosis.
Plan of Analysis
Descriptive statistics were used to characterize baseline characteristics of the sample and report feasibility outcomes. The association between baseline variables and total number of exercise sessions completed was analyzed using Pearson correlation coefficients for continuous measures and analysis of variance (ANOVA) for categorical measures. A mixed model ANCOVA was used to model changes in physical function, HRQL, and depression over time. All analyses were conducted with SAS statistical software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
Sample Characteristics
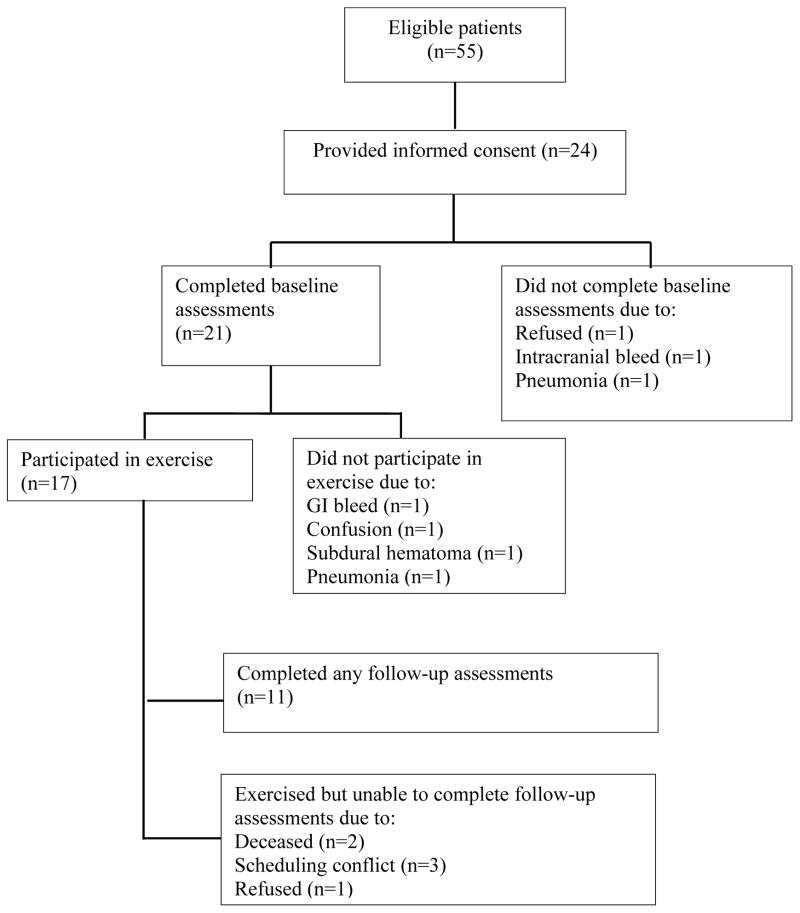
Among 55 eligible inpatients, 24 (43.6%) agreed to participate. Baseline characteristics are presented in Table 1. Mean age (SD) was 65.1±7.8 years and 62.5% were female. Significant comorbidity (Charlson score ≥1) was reported in 37.5%. In addition, the population was obese (mean BMI>30), had extremely poor physical performance (mean SPPB scores<9), and reported low levels of physical activity prior to diagnosis. Approximately 20% had been previously treated for AML, and 27.3% presented with unfavorable tumor biology. All participants received induction chemotherapy, with 62.5% receiving standard daunorubicin and cytarabine ± etoposide. More than one cycle of induction chemotherapy was administered in 29.2% of the sample. The average hospital stay (SD) was 37.6±13.2 days. Among those who declined enrollment, 61.4% were “not interested,” 22.5% felt “too sick,” and 16.1% were “overwhelmed”.
Table 1.
Baseline Patient Characteristics (N=24).
| Variable | Mean±SD or Percent |
|---|---|
| Demographics | |
| Age (years) | 65.1±7.8 |
| Race (% white) | 91.7 |
| Gender (% female) | 62.5 |
| Married | 61.9 |
| > High School Education | 60.0 |
| Clinical Characteristics | |
| BMI (kg/m2) | 31.0±6.5 |
| Charlson Comorbidity Index Score | 0.5±0.9 |
| White blood cell count | 52.6±61.3 |
| Hemoglobin (g/dl) | 9.1±1.5 |
| Relapsed disease | 20.8 |
| Unfavorable cytogenetic risk group | 27.3 |
| Objective physical function | |
| Grip strength (kg) | 24.7±8.9 |
| SPPB score (range 0–12) | 5.6±3.7 |
| Self-report Measures | |
| FACT-Leu (range 0–176) | 115.9±20.9 |
| CES-D (short form) (range 0–22) | 8.3±3.8 |
| Distress Thermometer (range 0–10) | 4.5±3.4 |
| Mild Physical Activity (days/week) | 4.3±2.4 |
Feasibility
Of those enrolled, 87.5% completed baseline measures; 70.8% attended ≥1 exercise sessions; and 50.0% completed at least one post-intervention assessment (Figure 1). The mean number of exercise sessions attended was 2.7 (range 0–8, SD 2.4). Among the exercise sessions performed, 23.4% included both walking phases and resistance band exercises, 31.3% consisted of one walking phase and resistance band exercises, and 45.3% consisted of resistance bands only. The primary reasons for missing individual sessions were because participants were too sick (71.0%) or were discharged during the 4-week intervention (21.0%). Four participants died from complications of disease during the study. Among baseline characteristics evaluated, the only variable that correlated with total number of exercise sessions attended was baseline SBBP score (r=0.71; p=0.0006). Better physical performance at baseline was associated with greater number of exercise sessions.
Figure 1.
Flow diagram of study.
Exercise was well tolerated with no adverse events during the intervention. Participants reported that the workload was “light,” with a mean RPE score of 10.5. Pre- and post-session symptom assessment revealed no significant improvements in fatigue, nausea or pain. However, there was a trend towards decrease in distress (p=0.09). Ten patients provided qualitative feedback at intervention completion. Overall, feedback indicated that most patients found exercise to be beneficial. Specific comments reflected improved motivation towards physical activity during hospitalization and perceived emotional support benefits.
Preliminary Efficacy Data
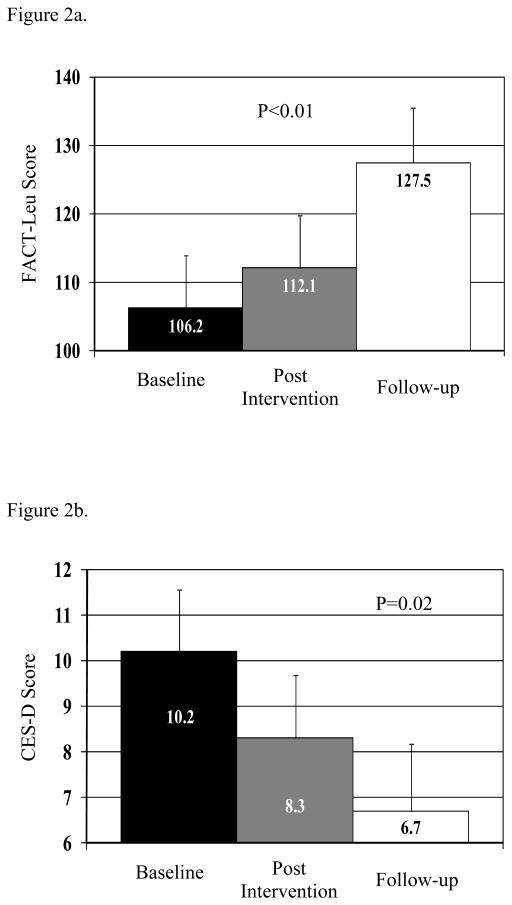
Preliminary efficacy data were analyzed in those participants who completed the baseline assessment, had at least 1 follow-up assessment, and completed at least one exercise session (n=11). There were no statistically significant differences in objectively tested physical performance during follow-up (SPPB or grip strength); however, SPPB increased from 7.3 to 8.6 from baseline to end of the intervention (p=0.19), which is considered a clinically meaningful increase (i.e. 0.5–1.5 range). Compared to baseline assessment, HRQL (FACT-Leu) significantly increased over time (p<0.01; Figure 2a). In particular, both the physical well-being subscale of the FACT-Leu (least square means: baseline 14.1, end of intervention 16.5, follow-up 20.4, overall test for trend p=0.03) and the leukemia subscale improved over time (least square means: baseline 38.6, end of intervention 42.2, follow-up 46.7, overall test for trend p=0.02); the other subscales of the FACT-Leu did not indicate a significant trend. There was also a significant decline in depression over time (p=0.02 for trend, Figure 2b).
Figure 2.
Figure 2a. Change in Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) score over time.
Figure 2b. Change in Center for Epidemiologic Studies Depression Scale–Short Form (CES-D) score over time.
Discussion
This study is one of the few to investigate feasibility of an exercise intervention for hospitalized adults receiving aggressive induction chemotherapy for AML. To our knowledge, it is the first to focus on older adults exclusively. The baseline data for this cohort suggested that they were at high risk for disability-- they had low baseline physical performance, low levels of physical activity, high body mass index, and significant disease burden.
Our study demonstrated that recruitment to an exercise intervention among older adults hospitalized for AML treatment was feasible; 43.6% of eligible patients agreed to participate. This rate compares favorably to similar intervention studies for cancer patients actively receiving chemotherapy treatment. For example, Griffith et al. conducted a study of a walking intervention among patients undergoing treatment for solid tumors. Among 620 eligible patients, 138 (22.2%) enrolled on study. Even when comparing our recruitment to exercise studies done in other older adult inpatients without a cancer diagnosis, our recruitment rate remains favorable.
Adherence to the exercise program was a greater challenge in this population. Only 70.8% of enrolled subjects participated in exercise. Of those who participated, none completed all 12 prescribed sessions. The primary reason for declining any given session was “feeling poorly.” Adherence to exercise interventions in women with breast cancer has been reported at 70–80%. However, others have reported lower levels of participation or adherence when cancer patients were receiving active treatment and had a high symptom burden. For example, in a pilot study of structured exercise in patients with advanced non-small cell lung cancer, only 44% of participants completed the program as prescribed. Retention to exercise interventions is a particular challenge among older adults.
Our experience and that of others suggest multiple strategies that could improve adherence in this patient population. First, the intervention schedule should be flexible rather than fixed, and offered more frequently than we could do in our pilot study (3 fixed visits per week). Flexibility would maximize opportunities for patients to participate, recognizing that their conditions change frequently and demands of care during a hospitalization are abundant. The inpatient setting is uniquely suited to a higher frequency intervention, since patients do not need to travel to participate. Second, targeting the intervention to activities limited to the hospital room would likely increase participation. In our experience, participants were more likely to do the resistance exercises, which could be performed in their room. Third, developing a symptom-adapted program – with lower levels of prescribed activity during increased symptom burden and higher levels of activity during lower symptom burden – would likely maximize efficacy and potentially also participation. Tailored interventions in other frail elderly populations are being evaluated to a limited extent and appear promising. Finally, adding education to stress the benefits of regular exercise with the patient and family could further increase participation.
One important finding of this study is the safety of this type of intervention in acutely ill older adults receiving chemotherapy. No adverse events were reported, and post exercise symptom ratings were stable or improved. This finding is consistent with the two previous pilot studies in AML and provides further evidence for safety in an older and more frail patient population. Thus, our results support the safety of further trials in this population.
The longitudinal data in this study provide some preliminary information for evaluation of efficacy of the intervention. While efficacy cannot be evaluated directly in this non-randomized study design, we saw statistically significant improvements in HRQL, and a trend toward decreased depression after the intervention, suggesting that the measures used were sensitive to change over time and would be appropriate endpoints in future studies.
The improvement in HRQL, including self-reported physical well-being, is consistent with findings of other exercise interventions in this and other cancer populations. Decreased depressive symptomatology has been reported in previous pilot studies of exercise in AML patients. Finally, the non-significant improvement in physical performance is consistent with benefits in muscle strength and physical functioning reported in other studies of exercise in hematologic malignancy survivors. Exercise during active treatment may stabilize physical function by minimizing detrimental changes in body composition, strength and fitness.
This study has several strengths. We evaluated the exercise intervention in a novel population, older adults hospitalized for AML induction chemotherapy. The exercise intervention was practical in a hospital setting. If proven effective in future randomized trials, this type of intervention could be delivered by physical therapists as part of usual care. We also evaluated objective physical function in this study and piloted multiple HRQL and emotional outcome measures to be utilized in future trials.
Our project also had limitations. Despite being the largest study of exercise in elderly AML patients to date, our sample size was relatively small, limiting its generalizability. An important limitation is the lack of a control group. However, this study yielded valid preliminary data to inform a future randomized trial.
In conclusion, this study demonstrates the feasibility of recruiting older adult inpatients receiving induction chemotherapy to an exercise intervention. Future directions will include tailoring the intervention to maximize participation and designing a randomized trial to test effectiveness and generalizability. These preliminary data suggest that simple exercise programs initiated during treatment show promise in improving HRQL and physical well-being in older adults being treated for AML.
Acknowledgments
This work was funded by a Wake Forest University Cross Campus Pilot Grant. Dr. Klepin is supported by the American Society of Hematology, Atlantic Philanthropies, John A. Hartford Foundation, Association of Specialty Professors, and the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30 AG-021332).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.