Abstract
BACKGROUND
Gastroesophageal reflux disease (GERD) and gastric acid hypersecretion respond well to suppression of gastric acid secretion. However, clinical management and research in diseases of acid secretion have been hindered by the lack of a non-invasive, accurate and reproducible tool to measure gastric acid output (GAO). Thus, symptoms or, in refractory cases, invasive testing may guide acid suppression therapy.
AIM
To present and validate a novel, non-invasive method of GAO analysis in healthy subjects using a wireless pH sensor, SmartPill® (SP) (SmartPill® Corporation, Buffalo, NY).
METHODS
Twenty healthy subjects underwent conventional GAO studies with a nasogastric tube. Variables impacting liquid meal-stimulated GAO analysis were assessed by modeling and in vitro verification. Buffering capacity of Ensure Plus® was empirically determined. SP GAO was calculated using the rate of acidification of the Ensure Plus® meal. Gastric emptying scintigraphy and GAO studies with radiolabeled Ensure Plus® and SP assessed emptying time, acidification rate and mixing. Twelve subjects had a second SP GAO study to assess reproducibility.
RESULTS
Meal stimulated SP GAO analysis was dependent on acid secretion rate and meal buffering capacity but not on gastric emptying time. On repeated studies, SP GAO strongly correlated with conventional BAO (r=0.51, P=0.02), MAO (r=0.72, P=0.0004) and PAO; (r=0.60, P=0.006). The SP sampled the stomach well during meal acidification.
CONCLUSIONS
SP GAO analysis is a non-invasive, accurate and reproducible method for the quantitative measurement of GAO in healthy subjects. SP GAO analysis could facilitate research and clinical management of GERD and other disorders of gastric acid secretion.
Keywords: acid output, wireless capsule, SmartPill®, gastroesophageal reflux disease
INTRODUCTION
Acid-related diseases are often chronic, under-investigated and over-treated.1 They range from common, such as gastroesophageal reflux disease (GERD), to less common, such as Barrett’s esophagus and Zollinger-Ellison syndrome (ZES). Worldwide, 10 to 20% of people have GERD and the prevalence has increased over the last two decades.2 In the United States, the rate of outpatient visits for GERD increased 2000 percent between 1975 and 2004.3 The most effective therapy is proton pump inhibitors (PPIs). Omeprazole was the sixth most commonly dispensed US prescription in 2011 and a PPI ranked fifth in global sales at $7.9 billion.4 In addition, PPIs are inappropriately prescribed one-third to three-quarters of the time.5, 6 One important area of overuse is in refractory GERD, which accounts for approximately 30% of all GERD patients.7 Unfortunately, there is no non-invasive, accurate and reproducible method to manage gastric acid secretion and to assist the provider in PPI management. Therefore, the provider relies only on symptomatic response, a poor indicator since more than 85% of acid reflux episodes are symptom-free and 30% of symptomatic episodes are unrelated to reflux.8 These patients not only represent a clinical challenge, but also constitute the group most at risk of PPI adverse effects.9 Given this need for better management of acid-related disorders, gastric acid output measurement has recently seen a resurgence in interest.10-12
Measurement of GAO has largely been abandoned outside of large academic centers. However, data support that acid output is important in the pathophysiology of GERD13 and that GAO analysis is underutilized.14, 15 GERD patients secrete significantly more acid than healthy control patients and refractory GERD patients secrete significantly more acid than responsive patients.12, 16-18 The current test for management of refractory GERD, ambulatory pH/impedance testing, is poorly tolerated, altering patients daily habits and reducing reliability. Ambulatory pH/impedance testing is also dependent on symptoms and reflux occurring during a limited time window and in patients with low numbers of events, symptom indices have been shown to be unreliable and misleading.19 Measurement of stimulated gastric acid output may be an alternative, non-invasive means to determine if GERD symptoms could be related to acid without reliance on patient reporting.
Diverse methods are available for GAO analysis; these are described in detail elsewhere.10 Briefly, invasive tube tests are uncomfortable, inefficient and often inaccurate when used with meals, while current tubeless tests are not validated, quantitative or readily available.20-28 We present and validate a new method of GAO analysis using the SmartPill® liquid-meal stimulated GAO (SP GAO) which overcomes most of the weaknesses of previous methods. Our studies in healthy subjects indicate that SP GAO is a non-invasive, well-tolerated and reproducible way to measure GAO that highly correlates with measurements made by the conventional nasogastric tube test. SP GAO may therefore be useful in the assessment and therapeutic management of patients with diseases of gastric acid secretion.
MATERIALS & METHODS
Overview of comprehensive assessment
To accurately assess GAO, both the pH and volume of secretion must be known. To assess pH we used the SmartPill® capsule (SmartPill® Corporation, Buffalo, NY). To quantitate the volume of acid secretion in the stomach, we measured the acidification of a standard meal with a known buffering capacity— Ensure Plus® (creamy milk chocolate flavor, Abbot Laboratories, Abbott Park, Illinois). The ideal method should be able to 1) measure a wide range of secretion, 2) have adequate mixing and sampling of gastric contents, 3) account for residual acid in the fasting stomach, 4) sample the stomach for sufficient time, 5) apply to diverse populations and 6) be reproducible. Some of these performance characteristics have already been validated (Table 1); we further confirmed and enhanced validation of these characteristics in our method as described below.
Table 1.
Necessary conditions for successful SP measurement of GAO.
| Conditions | Supporting Evidence |
|---|---|
| Sufficient gastric residence time | |
| Accounts for pre-test residual gastric acid |
|
| Wide secretion range measurement | |
| Accuracy in diverse populations | |
| Reproducibility |
Mathematical Modeling and In vitro study
A mathematical model was constructed to determine the feasibility of measuring GAO by a meal stimulated wireless pill method. This model helped to clarify the contribution of variables that impact the gastric acid analysis of a buffered meal. To model the buffering reaction within the stomach, we considered the reaction, B ⇔ H+ + A−, where B is a weak acid, H is a hydrogen ion, and A is the conjugate base. We considered the stomach to be a well-mixed medium. The conjugate base (buffering agent) is added to the stomach, which increases the volume. This induces the addition of hydrogen ions through acid secretion. The entire well-mixed components within the stomach empty simultaneously. We modeled the kinetics of this reaction with the following system
where H+, A-, and B are measured in milliequivalents (mEq) and W is water measured in milliliters; kb is the backward rate of the buffer reaction measured in min-1 and kf is the forward rate measured in mL-mEq-1-min-1; d is the emptying rate of the stomach measured in min-1, with d=log(2)/(emptying half life in min); Hsec is the gastric secretion rate of acid in mEq-min-1; Abuf is the amount of added buffer in mEq and Wbuf is the volume of water of the added buffer in mL; g(t) is an impulse function of area one that indicates that the buffer is added as a single bolus; W0 is the baseline amount of water in the stomach measured in mL.
We assume at time zero that A- and B are both zero; W=W0, and H+ is the initial concentration of acid multiplied by W0. Then the buffer is introduced over a brief period of time specified by g(t). We assumed that gastric acid is secreted into the stomach at a constant rate. The pH of the stomach is given by −log(H+/W).
To validate this model we performed laboratory experiments using a beaker to simulate a stomach. The beaker had a residual volume of gastric secretions, similar to that of a stomach. A stir bar simulated gastric mixing and peristaltic pumps were used to simulate fixed rates of acid secretion and gastric emptying. Ensure Plus® at varying dilutions served as the meal and was rapidly poured into the beaker at time zero. The change in pH was measured over time with a Pinnacle 3-in-1 refillable pH electrode (Nova Analytics Corporation, Woburn, MA) and a Corning 430 pH meter (Corning Incorporated, Corning, NY).
Subjects
Twenty healthy subjects (13 men, 7 women, ages 21-53, 12 Caucasians, 4 African Americans and 4 Asians) were prospectively enrolled and studied between July 2008 and March 2009. Five subjects were excluded for failure to complete the studies, psychiatric instability and the presence of H. pylori. Subjects were excluded for a history of gastric or small bowel surgery, recent bowel motility medications, recent antacid, PPI or NSAID use, hypo- or hyper-secretory acid disease, nasogastric tube relative contraindications, allergy to sulfa-colloid, pentagastrin relative contraindications, SmartPill® contraindications29, pregnancy, lactose intolerance, Helicobacter pylori infection, documented gastroparesis, GERD, ulcer disease, inflammatory bowel disease, irritable bowel syndrome, major illness and unstable psychiatric condition. The study protocol was approved on April 30, 2008 by the National Institutes of Health Intramural NIDDK Institutional Review Board and informed consent was obtained from all participants.
Study Design
A history and physical exam, electrocardiogram, complete blood count, chemistry panel, coagulation panel, pregnancy test, stool Helicobacter pylori antigen and fasting serum gastrin was obtained at the screening visit. After eating a low residual diet the night before and fasting after midnight, the subject underwent a gastric emptying study the next morning with Technetium (Tc) 99m sulfur-colloid labeled Ensure Plus®. This was prepared by mixing 120 mL of Ensure Plus® at room temperature with 120 mL of water to which was added 1 millicurie (mCi) of Tc99m sulfur colloid. On a subsequent morning, the fasting subject underwent basal gastric acid output analysis (BAO) via a nasogastric tube. Accurate placement of the nasogastric tube was confirmed with air auscultation and the return of 50 mL of previously instilled water. At the start of the study, gastric secretions were suctioned and discarded. Next, every fifteen minutes for sixty minutes gastric secretions were aspirated and collected. At sixty minutes, a maximal acid output analysis (MAO) was performed by administering pentagastrin (Pentagastrin Injection BP, Cambridge Laboratories, Tyne and Wear, UK) 6 mcg/kg intramuscularly with aspiration of secretions every fifteen minutes for one hour. BAO, MAO and PAO were calculated as previously reported.30
On a separate visit within a week of the tube study and at approximately the same time of day, twelve of twenty subjects underwent a SmartPill® meal-stimulated acid output analysis. After an overnight fast, subjects ingested a SmartPill® with a cup of water during a live monitoring session using the MotiliGI software, version 1.4 (SmartPill® Corporation). Once a baseline pH was established, the subject ingested a mixture of 120 mL of Ensure Plus® and 120 mL of water. The resulting increase in pH to between approximately 6 and 7 was monitored. The subject was then instructed to fast for six hours so that gastric emptying and acidification times of the buffered meal could be established.
On a separate visit, nineteen of twenty subjects underwent a second gastric emptying study with Tc-99m labeled Ensure Plus® and an Indium-111 labeled SmartPill®. The SmartPill® was radio-labeled by aspirating 0.1 mL of oil from the pressure chamber and replacing it with an equal volume of 0.01 to 0.05 mCi of Indium-111 (In-111). As in the previous study, the fasting subject ingested the radio-labeled SmartPill® with water, drank the meal after establishing a pH baseline by live monitoring and then fasted for six hours. Using this method of SmartPill® ingestion, establishment of a pH baseline and then Ensure ingestion, only one of thirty-one (3%) studies had early egress of the SmartPill® into the duodenum before intragastric titration could occur.
Scintigraphy Imaging Methods
Subjects were placed between two detectors and planar, anterior and posterior views were obtained. Concurrent acquisitions with two spectral windows (for Tc-99m and In-111 respectively) provided two image sets: one for the meal and one for the SmartPill®. Immediately after drinking the meal, the subjects were scanned dynamically in the upright position every minute for the first ten minutes. Subjects were then scanned supine and statically for one minute every twenty minutes for the following 110 minutes except for the images at minutes 60 and 120 when subjects were scanned upright again to encourage pill motion. For alignment, two Barium-133 (Ba-133) markers were attached to the subjects at the level of the lower rib.
Scintigraphy Image Analysis
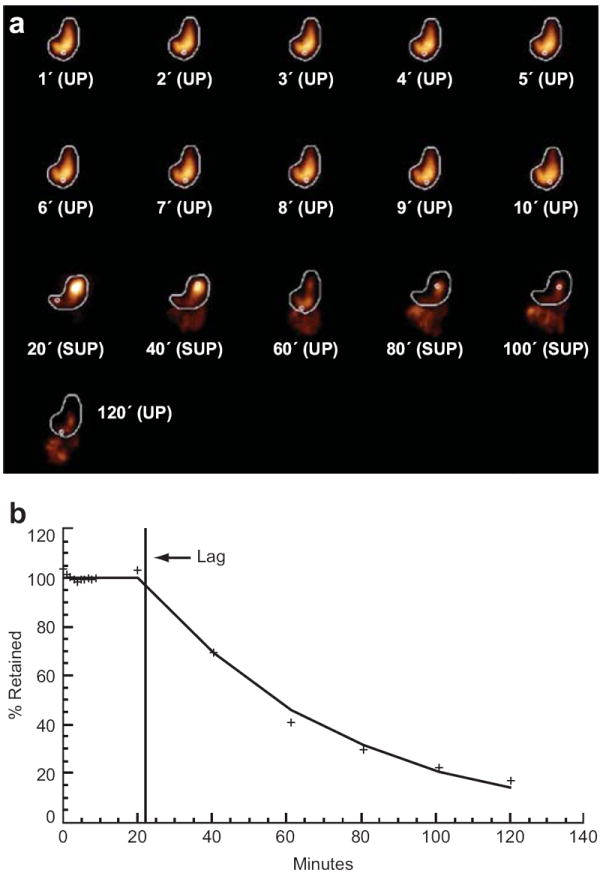
To compensate for the attenuation of the gamma rays by the body, the anterior and posterior views for each acquisition time were converted into a single geometric mean (GM) image. The Ba-133 markers in the In-111 GM images were used as fiduciary landmarks for alignment. One image in the set was selected as reference and the rest were aligned to it in the x-z plane. The same alignment operation was applied to the concurrently acquired Tc-99m image so that all images (gastric or SP) were aligned with each other regardless of the position of the subject. Regions of interest were drawn around the stomach (Tc-99m set) and around the SP (In-111 set). Outlines of these regions superimposed on the corresponding Tc-99m (gastric) image showed the location of the SP with respect to the contents of the stomach, indicating, for example, whether the pill had left the stomach. The activity on the stomach ROI was summed in each image to generate a time-activity curve (TAC). The first 10-20 minutes of TAC data were fitted to two straight lines in an attempt to identify a lag phase and its duration. A model, consisting of a zero-slope line followed by another with a negative slope, was fitted to the data using a nonlinear optimization routine. The objective was to optimize both the slope of the second line and the location of the inflection point between the two lines. The latter became the estimate for the duration of the lag phase. The data following the lag phase (i.e. the emptying phase) were fitted to a simple exponential curve to provide estimates for the emptying half time.
SmartPill® movement was calculated by counting the number of times the traced outline of the radio-labeled capsule changed position from the study start time to the time pH 2 was reached.
Data Analysis of SmartPill® Meal-stimulated Acid Output
To calculate the gastric acid output from the meal-stimulated SmartPill® study, we determined in vitro the quantity of 1 N HCl required to titrate a 120 mL mixture of Ensure Plus® and 120 mL of water from a pH of 5 to 2. The mixture was added to a beaker, continuously mixed on a stir plate and the pH continuously measured during the addition of 1 mL aliquots of 1 N HCl. Twenty-two mEq of 1 N HCl were required to titrate the 50% Ensure Plus® from a pH of 5 to 2.
The time and pH data from the in vivo SmartPill® studies were plotted in Prism 4 (GraphPad, La Jolla, CA) in order to perform linear regression curve fitting of the primary data and to smooth the second-to-second variability in pH. This curve fitting permitted a more accurate calculation of the time required to acidify the meal from pH 5 to 2. The SmartPill® meal-stimulated acid output (in mEq/hr) was calculated by multiplying the amount of acid required for in vitro titration of the Ensure Plus® meal, 22 mEq, by sixty over the time (in minutes) required to acidify the meal in vivo from pH 5 to 2.
Statistical Analysis
Assuming that correlation would range between 0.5 and 0.7 between methods, a sample size of 20 subjects would allow for acceptable confidence intervals. Correlations were calculated using Pearson’s correlation analysis and ANOVA except in the case of SP emptying time vs. GES and SP GAO vs. BAO, where Spearman’s ranked correlation and ANOVA were used. Wilcoxon sign-rank was used to compare emptying times. Paired t-test was used to compare the labeled and unlabeled SP GAO means and SP location with subject position during GES. All statistical analyses were performed using JMP 8.0 (SAS Institute, Cary, NC).
RESULTS
Mathematical modeling of gastric acid secretion and in vitro validation
Mathematical modeling of gastric acid secretion indicated that the two most influential variables on GAO analysis were meal buffering capacity (Figure 1a) and gastric acid secretion rate (Figure 1b). Significant variation in the gastric emptying time had little influence on GAO analysis because the contents empty in the acidified state under the assumed conditions of rapid mixing and fast forward rate constant, kf (Figure 1c).
Figure 1.
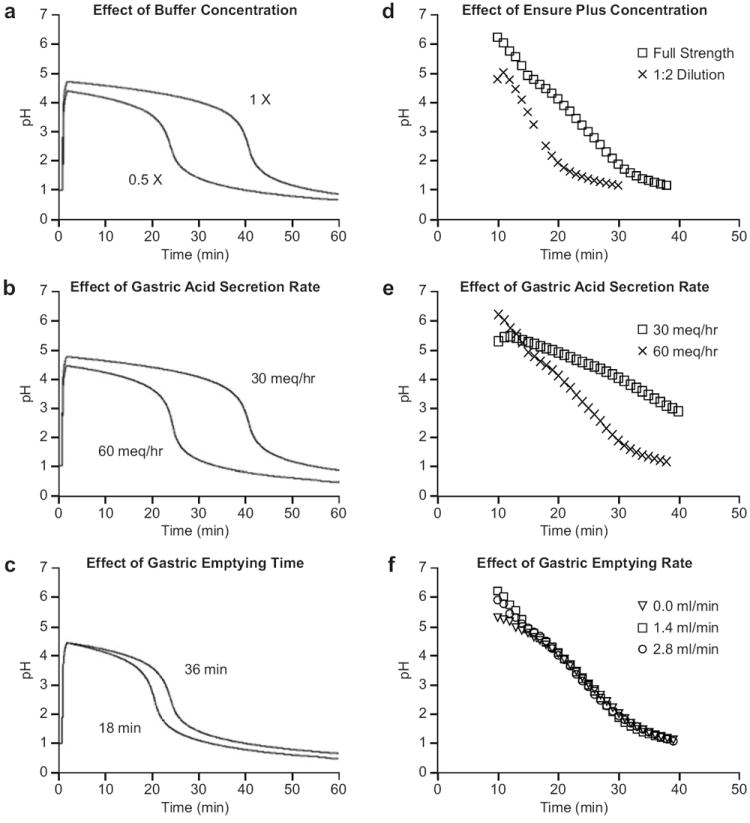
Mathematical and in vitro modeling of gastric acid secretion: Modeling predicts and in vitro data confirm that a two-fold increase in the buffering capacity of a meal (a and d, respectively) or acid secretion rate (b and e, respectively) result in a nearly linear increase or decrease in acidification time, respectively, while a two-fold increase in gastric emptying time (c and f, respectively) would have a minimal effect.
The predictions of the mathematical model were confirmed by the laboratory simulation of acidification of an Ensure Plus® meal. The GAO needed to acidify a meal was dependent on the buffering capacity of the meal (Figure 1d) and the rate of acid secretion (Figure 1e) but was relatively independent of the gastric emptying rate (Figure 1f). Therefore, the in vitro simulation predicted that meal emptying time should not confound the accuracy of the SP GAO method within a wide range of normal gastric emptying.
Gastric Emptying Time of Liquid Meal
According to our model and lab simulation, a widely variable gastric emptying time would not be expected to affect GAO analysis performed with a liquid meal. Confirmation of this in vitro result was assessed by in vivo measurement of emptying time for a 50% Ensure Plus® meal. We also assessed whether the presence of the SmartPill® itself altered liquid gastric emptying time, which could potentially lead to falsely high or low GAO measurements. The median scintigraphic half-emptying time of the 50% Ensure Plus® meal alone was 27 minutes for 19 subjects (range, 128 to 15 min). In the presence of the SmartPill®, the median scintigraphic half-emptying time was 30 minutes for the same 19 subjects (range 115 to 19 min). There was no significant difference between these emptying times (P=0.82), confirming that the presence of the SmartPill® does not significantly alter liquid gastric emptying time (Figure 2a). Moreover, the SmartPill®-computed emptying time correlated well with liquid gastric emptying scintigraphy (P=0.01, r=0.78). The median emptying time calculated by SmartPill® was 162 minutes (range 110 to 270 min).
Figure 2.
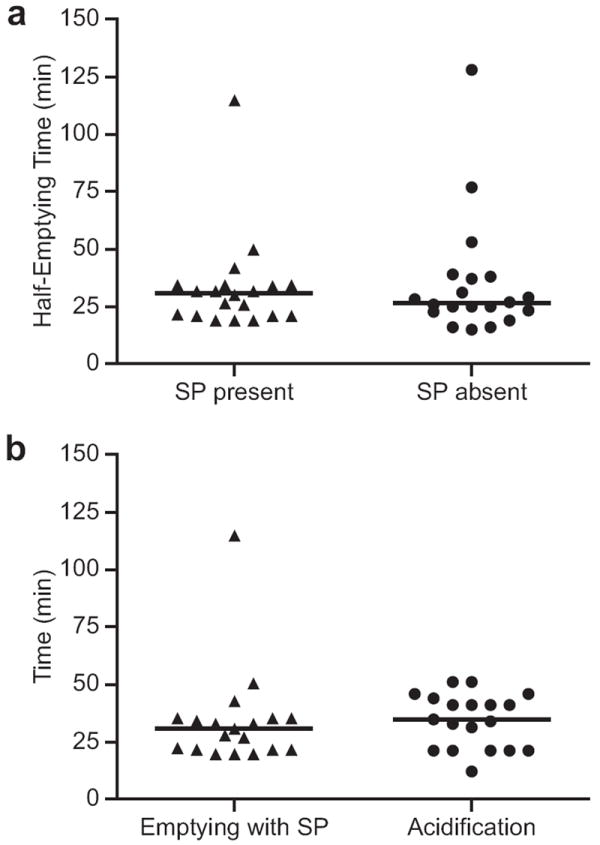
Assessment of Gastric Emptying: a) Comparison of median half empyting times by scintigraphy in the presence (30 min) and absence (27 min) of the SP. Emptying times were not signficantly different (P=0.82). b) Comparison of half-emptying time measured by scintigraphy in the presence of the SP to the time needed to acidify the meal from pH 5 to 2. The median acidification time (34 min) was not significantly different from the median emptying time of the meal (30 min) (P=0.21).
Time needed for Intragastric Titration
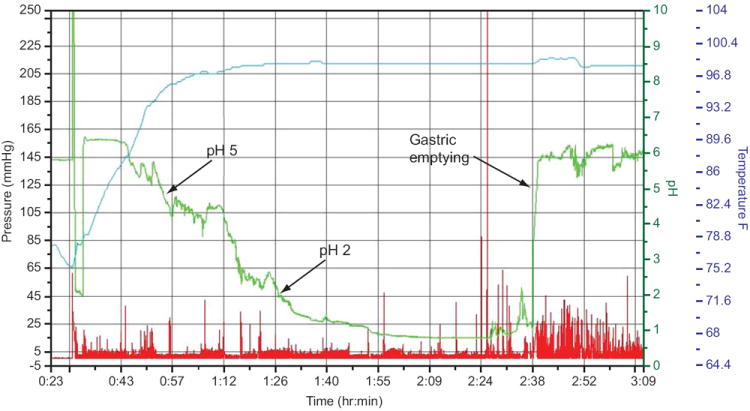
As gastric residence time of the meal relative to acid secretion can be an important factor in meal-stimulated SP GAO analysis, especially at the extremes of rapid gastric emptying, the time needed for acidification of the meal from pH 5 to pH 2 (Figure 3) was studied. The median time for intragastric titration was 34 minutes (range, 50 to 11 minutes) for the 19 subjects who had gastric emptying scintigraphy with the SmartPill® present. The half-emptying time by GES was not significantly different from the time required for acidification of the meal (P=0.21) (Figure 2b).
Figure 3.
Screen shot from a representative SmartPill® tracing illustrating the rate of acidification of an Ensure Plus® meal and gastric emptying time: Arrows indicate the 34 minute time period elapsed between pH 5 and pH 2 (equivalent to 22 mEq of HCl buffering capacity) resulting in a calculated GAO of 38.8 mEq/hour. The rightmost arrow denotes a rapid rise in pH corresponding to egress of the SP from the acidic stomach to the alkaline duodenum and is recorded as the gastric emptying time (110 min).
Assessment of Adequate Mixing
Homogenous mixing of a meal with gastric acid and sampling of the pH throughout the stomach is necessary for accurate and consistent measurement of GAO. To assess whether the SP adequately sampled the stomach, it was radiolabeled with In-111 so its movements could be measured during a Tc-99 gastric emptying study. For 19 subjects, there was an average of 3.5 distinct gastric locations sampled and 3.6 subject position changes during acidification of the meal to pH 2 (r=0.95, P<0.0001) (Figure 4a). Therefore, the frequent SmartPill® movement during the period of acidification indicates that there is adequate mixing and adequate sampling of gastric contents by the SmartPill®.
Figure 4.
Representative images of gastric emptying and SmartPill® movement during a liquid meal GES: a) The time lapse images represent projections of an In-111 labeled SP (small white circle) and Tc-99m labeled Ensure Plus® meal (gold) during a representative GES. First two rows: Imaging of the first ten minutes following ingestion of the radio-labeled SP and Ensure Plus® meal. Second two rows: Imaging at twenty minute intervals, with interval patient movement between supine and upright (SUP, UP) positions. b) Graphical representation of gastric emptying from a) expressed as percent meal retention over time. For the whole cohort, the SP sampled 3.5 distinct locations during acidification correlating with 3.6 subject position changes.
GAO Analysis
All twenty subjects had at least a BAO, MAO, PAO and SP GAO measured. To assess reproducibility, twelve of the twenty subjects had an SP GAO with an unlabeled SP and nineteen had an SP GAO with a labeled SP. Thus, twelve subjects had two independent SP GAO studies. The mean acid output calculation according to each method and each method’s respective variance is shown (Table 2). There was a strong correlation between SP GAO and both MAO and PAO regardless of whether the SmartPill® was radio-labeled (SP labeled MAO: r=0.72, P=0.0004 and PAO: r=0.60, P=0.006; SP unlabeled MAO: r=0.66, P=0.02 and PAO: r=0.62, P=0.03) (Figures 5a-d). We found no significant difference in the mean (±SD) measured GAO using labeled vs. unlabeled SP (56.0±16.6 vs. 43.2±15.5 mEq/hr, P=0.08). In addition, there was a strong correlation between MAO and BAO (r=0.45, P=0.048) and SP GAO and BAO (r=0.51, P=0.02) (Figures 5e and f).
Table 2.
Comparison of GAO measurements using conventional methods (BAO, MAO and PAO) versus the SmartPill (meal stimulated) method.
| GAO Method | Subjects (N) | Gastric Acid Output (mEq/hr) | ||
|---|---|---|---|---|
| Mean | Std. Dev | Range | ||
| Basal Acid Output (BAO) | 20 | 4.2 | N/A | 0.5 -16.6 |
| Maximal Acid Output (MAO) | 20 | 32.4 | 16.3 | 6.2 -79.2 |
| Peak Acid Output (PAO) | 20 | 41.9 | 22.2 | 7.6 -101.3 |
| SmartPill (unlabelled) | 12 | 54.2 | 16.8 | 30 -82.5 |
| SmartPill (labelled) | 19 | 46.7 | 23.3 | 26.4 – 120 |
Figure 5.
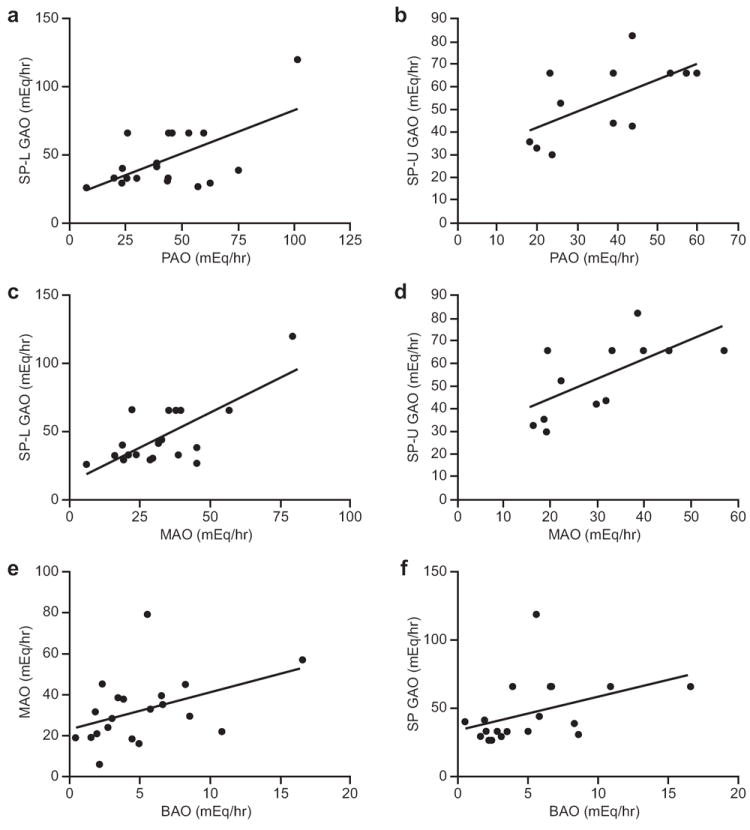
Correlation of conventional pentagastrin-stimulated GAO to meal-stimulated SP GAO and BAO to MAO and SP GAO: Correlations of PAO with SP GAO with (a, SP-L) and without (b, SP-U) labeling (n=19, r=0.60, P=0.006, and n=12, r=0.62, P=0.03, respectively) and correlations of MAO with SP GAO with (c, SP-L) and without (d, SP-U) labeling are shown (n=19, r=0.72, P=0.0004 and n=12, r=0.66, P=0.02, respectively). Correlations of BAO to MAO (e) (n=20, r=0.45, P=0.048) and BAO to SP GAO (f) (n=19, r=0.51, P=0.02) are also shown.
DISCUSSION
Increasing evidence suggests that gastric acid secretion plays an important and under-appreciated role in GERD pathophysiology.11-13, 16-18, 31 Current methods for assessment of gastric acid measure only gastric and esophageal pH and not gastric volume. Accurate GAO analysis is seldom used because of perceived obsolescence, patient intolerance, cost, lack of expertise and inaccuracy. Where still performed, GAO is helpful in the diagnosis and management of rare hypersecretory states, which can sometimes be missed due to PPI masking.14, 32, 33 If a method of GAO analysis were developed that was non-invasive, easily administered, accurate and reproducible, it is likely that its use would become more widespread for application in refractory GERD, acid hypersecretory states, antisecretory drug development,27 atrophic gastritis and gastric cancer screening.15, 34 This could have the potential of reducing PPI overuse. This study demonstrates that SP GAO is a validated, non-invasive analysis method. The technique correlated well with BAO, MAO and PAO and was reproducible, well tolerated and easily administered in healthy subjects.
Any meal-stimulated GAO analysis method must: 1) give a quantitative, wide ranging measure of gastric acid secretion, 2) have adequate mixing of gastric contents, 3) account for residual acid in the fasting stomach, 4) sample the stomach for sufficient time, 5) apply to diverse populations and 6) be reproducible.10 Before performing our study in subjects, we established whether our technique could satisfy some of these important parameters on the basis of previous studies (Table 1) and by predictive modeling of gastric acid secretion. Our mathematical model was consistent with the in vitro simulation as well as in vivo testing. Our model assumes that 1) gastric acid is secreted at a constant rate, 2) contents are rapidly and well-mixed and that 3) the liquid meal empties sufficiently slow to be in an acidified state. Evidence that gastric acid is secreted at an approximately constant rate after a short lag has been shown in the basal state and the liquid meal-stimulated state.30, 35 Adequate mixing was confirmed with the radio-labeling studies showing that the SP mixed with and sampled the meal well (Figure 4). The only previous study of a radio-labeled pH capsule did not capture sufficiently frequent gastric phase images to assess capsule mixing with the meal.36 Evidence that liquid meals enter the duodenum acidified was presented in studies of pancreatic insufficient and normal patients measuring pH along the GI tract after a liquid meal.37, 38
The most important variables affecting GAO analysis with a liquid meal-stimulated method were gastric acid secretion rate and meal buffering capacity; GAO was relatively independent of gastric emptying rate. The half-emptying time by GES compared to the time required for acidification of the meal was not significant, satisfying a criterion for accurate GAO. Given our in vitro simulation, our in vivo findings, and evidence that liquid meals empty acidified37, 38, it is unlikely that gastric emptying caused significant errors in our analysis. In contrast, the results of other meal-stimulated GAO methods such as intragastric titration and in vivo gastric autotitration of solid meals are significantly skewed by gastric emptying factors.10, 22, 23 Liquid nutrient meals, specifically Ensure Plus®, have been shown to be valid alternatives to the standard egg-white sandwich for GES.39, 40 While they may empty more rapidly than solid meals,41 liquid nutrient meals are superior for GAO analysis in homogeneity and in minimizing the sampling error that occurs with solid meals.22
The SmartPill® itself did not alter gastric emptying time (Figure 2a) and the SmartPill® emptying time correlated well with GES liquid emptying time. While solid GES is well-known to correlate strongly with SP solid emptying time, liquid emptying correlation had not been previously reported. Despite the Ensure Plus® being diluted 50%, our study also corroborated past studies that showed normal median liquid emptying time for the SmartPill® in Ensure Plus® is 150 to 162 minutes.39, 42
SP GAO also fulfilled the necessary criteria of yielding a quantitative, wide ranging measure of gastric acid secretion applicable to a diverse population. The most prevalent method of measuring acid output, BAO, correlated highly with MAO and SP GAO (Figures 5e,and f). Prior studies corroborated this result by showing a good correlation between BAO and MAO.43 This could allow the diagnosis of hypersecretory states by calculating a traditional BAO from a SP GAO value. SP GAO also correlated well with both conventional PAO and MAO. SP GAO measured outputs ranging from 30 to 120 mEq/hour with standard deviations similar to conventional methods (Table 2).
SP GAO can be viewed as a hybrid of two methods: the Heidelberg capsule and in vivo autotitration. SP GAO values for healthy subjects (46 to 54 mEq/hr) was similar to in vivo autotitration (47-60 mEq/hr) but higher than the Heidelberg capsule (14 to 30 mEq/hr), possibly because the Heidelberg method of stimulated intragastric titration with base increases gastric emptying time.16, 22, 24, 26 SP GAO has several advantages over Heidelberg capsule GAO. The use of a liquid meal is more physiologic as well as more relevant than alkaline pulses. Since the SmartPill® is larger, measuring 26 × 13 mm versus 20 × 7 mm, it rarely leaves the stomach prematurely; the smaller Heidelberg capsule requires an oral tether to maintain the capsule within the stomach. There was no significant difference in the very low rate of premature gastric egress of the SmartPill® in our study compared to the initial study of the SmartPill® for gastroparesis (3% vs. 3.8%, P=1).44 Thus, it is possible to measure a stable, reliable pH baseline in the vast majority of patients. While this information does not establish a BAO, it is still informative in diagnosing hypo- and hyper-secretory states such as atrophic gastritis and Zollinger-Ellison syndrome. The SmartPill® is widely used among providers for motility assessment due to its ease of use, portability and long battery life. The Heidelberg capsule requires that the patient stay in the physician’s office during a study, which may result in reduced mixing and sampling. Unlike in vivo autotitration, SP GAO samples the entire stomach and the liquid meal allows for better mixing and homogeneity than a solid meal.
Our study included diverse ethnic, gender and age groups and our method was reproducible. The main limitation of the study was a small sample size. Correlation coefficients and confidence intervals would be projected to be stronger with a larger study group. One potential weakness of our method is that in subjects with extremely rapid emptying, the meal would leave the stomach unacidified, causing a GAO overestimation. The fastest emptying time in our subjects was 15 minutes, a factor of two less than the median. On the basis of in vitro modeling and the strong in vivo correlations, this range is acceptable.
Despite these limitations, this study demonstrates that the SP GAO method is an accurate, easily administered, well tolerated, non-invasive and reproducible way to measure GAO in healthy subjects. Management of refractory GERD patients remains difficult, as evidenced by the continued development of new PPIs.45 The demonstrated value of the SP GAO method should help manage these difficult patients since symptoms alone are unreliable for therapy guidance.8 Future studies are warranted in the application of the SP GAO method to the assessment and management of refractory GERD and hypo- and hyper-secretory states. In conclusion, SP GAO overcomes most of the limitations of past non-invasive methods and offers an exciting new tool for the investigation and management of acid-related disease.
Acknowledgments
Financial Support
All work was funded by the NIDDK, NIH intramural program.
Abbreviations
- GAO
gastric acid output
- BAO
basal acid output
- PAO
peak acid output
- MAO
maximal acid output
- SP
SmartPill®
- GES
gastric emptying scintigraphy
- GERD
gastroesophageal reflux disease
- ZES
Zollinger-Ellison Syndrome
- PPI
proton pump inhibitor
Footnotes
Guarantor of article: Stephen A. Wank
Specific author contributions:
SdR and SAW contributed the research concept and design.
CCW performed the mathematical modeling.
DHW, SdR, LF, MW and XZ contributed to data acquisition.
DHW, LF, MW, ECW, ZD, RMM, MCC and SAW contributed to the analysis and interpretation of the data.
DHW and SAW drafted the manuscript.
All authors approved the final version of the article, including the authorship list.
Statement of Interests:
The authors declare no conflict of interest and specifically no relationship with the SmartPill® Corporation.
References
- 1.Majumdar SR, Soumerai SB, Farraye FA, et al. Chronic acid-related disorders are common and underinvestigated. Am J Gastroenterol. 2003;98(11):2409–14. doi: 10.1111/j.1572-0241.2003.07706.x. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Time trends of gastroesophageal reflux disease: a systematic review. Clin Gastroenterol Hepatol. 2007;5(1):17–26. doi: 10.1016/j.cgh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136(2):376–86. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 4. [December 23, 2012];Top-Line Market Data. 2011 Available at: www.imshealth.com.
- 5.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228–34. [PubMed] [Google Scholar]
- 6.Pasina L, Nobili A, Tettamanti M, et al. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro-esophageal reflux disease in a cohort of hospitalized elderly. Eur J Intern Med. 2011;22(2):205–10. doi: 10.1016/j.ejim.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Gerson LB, Bonafede M, Princic N, Gregory C, Farr A, Balu S. Development of a refractory gastro-oesophageal reflux score using an administrative claims database. Aliment Pharmacol Ther. 2011;34(5):555–67. doi: 10.1111/j.1365-2036.2011.04755.x. [DOI] [PubMed] [Google Scholar]
- 8.Baldi F, Ferrarini F, Longanesi A, Ragazzini M, Barbara L. Acid gastroesophageal reflux and symptom occurrence. Analysis of some factors influencing their association. Dig Dis Sci. 1989;34(12):1890–3. doi: 10.1007/BF01536707. [DOI] [PubMed] [Google Scholar]
- 9.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139(4):1115–27. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh T, Lewis DI, Axon AT, Everett SM. Review article: methods of measuring gastric acid secretion. Aliment Pharmacol Ther. 2011;33(7):768–81. doi: 10.1111/j.1365-2036.2010.04573.x. [DOI] [PubMed] [Google Scholar]
- 11.Gardner JD. GERD: increased gastric acid secretion as a possible cause of GERD. Nat Rev Gastroenterol Hepatol. 2010;7(3):125–6. doi: 10.1038/nrgastro.2009.240. [DOI] [PubMed] [Google Scholar]
- 12.Kalach N, Badran AM, Jaffray P, Campeotto F, Benhamou PH, Dupont C. Correlation between gastric acid secretion and severity of acid reflux in children. Turk J Pediatr. 2003;45(1):6–10. [PubMed] [Google Scholar]
- 13.Cadiot G, Bruhat A, Rigaud D, et al. Multivariate analysis of pathophysiological factors in reflux oesophagitis. Gut. 1997;40(2):167–74. doi: 10.1136/gut.40.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metz DC, Starr JA. A retrospective study of the usefulness of acid secretory testing. Aliment Pharmacol Ther. 2000;14(1):103–11. doi: 10.1046/j.1365-2036.2000.00676.x. [DOI] [PubMed] [Google Scholar]
- 15.Hitchcock CR. Screening for gastric cancer. CA Cancer J Clin. 1957;7(5):159–61. doi: 10.3322/canjclin.7.5.159. [DOI] [PubMed] [Google Scholar]
- 16.Gardner JD, Sloan S, Miner PB, Robinson M. Meal-stimulated gastric acid secretion and integrated gastric acidity in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2003;17(7):945–53. doi: 10.1046/j.1365-2036.2003.01533.x. [DOI] [PubMed] [Google Scholar]
- 17.Collen MJ, Lewis JH, Benjamin SB. Gastric acid hypersecretion in refractory gastroesophageal reflux disease. Gastroenterology. 1990;98(3):654–61. doi: 10.1016/0016-5085(90)90285-9. [DOI] [PubMed] [Google Scholar]
- 18.Blonski WC, Shih GL, Brensinger CM, Katzka DA, Metz DC. Analysis of the acidity index and integrated intragastric acidity in 645 patients presenting with gastroesophageal reflux disease symptoms. Scand J Gastroenterol. 2006;41(4):382–9. doi: 10.1080/00365520500293002. [DOI] [PubMed] [Google Scholar]
- 19.Slaughter JC, Goutte M, Rymer JA, et al. Caution about overinterpretation of symptom indexes in reflux monitoring for refractory gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2011;9(10):868–74. doi: 10.1016/j.cgh.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Chang JH, Choi MG, Yim DS, et al. A novel placement method of the Bravo wireless pH monitoring capsule for measuring intragastric pH. Dig Dis Sci. 2009;54(3):578–85. doi: 10.1007/s10620-008-0399-3. [DOI] [PubMed] [Google Scholar]
- 21.Oh DS, Wang HS, Ohning GV, Pisegna JR. Validation of a new endoscopic technique to assess acid output in Zollinger-Ellison syndrome. Clin Gastroenterol Hepatol. 2006;4(12):1467–73. doi: 10.1016/j.cgh.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner JD, Ciociola AA, Robinson M. Measurement of meal-stimulated gastric acid secretion by in vivo gastric autotitration. J Appl Physiol. 2002;92(2):427–34. doi: 10.1152/japplphysiol.00956.2001. [DOI] [PubMed] [Google Scholar]
- 23.Halter F, Keller M. A comparison between intragastric titration and aspiration technique under basal conditions and after food or pentagastrin stimulation. Am J Dig Dis. 1978;23(8):723–9. doi: 10.1007/BF01072360. [DOI] [PubMed] [Google Scholar]
- 24.Andres MR, Jr, Bingham JR. Tubeless gastric analysis with a radiotelemetering pill (Heidelberg capsule) Can Med Assoc J. 1970;102(10):1087–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Connell AM, Waters TE. Assessment of Gastric Function by Ph Telemetering Capsule. Lancet. 1964;2(7353):227–30. doi: 10.1016/s0140-6736(64)90182-5. [DOI] [PubMed] [Google Scholar]
- 26.Yarbrough DR, 3rd, McAlhany JC, Weidner MG, Jr, Cooper N. Evaluation of the Heidelberg pH capsule. Method of tubeless gastric analysis. Am J Surg. 1969;117(2):185–92. doi: 10.1016/0002-9610(69)90303-1. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee R, Reddy DN, Guda NM, et al. Oral buffered esomeprazole is superior to i.v. pantoprazole for rapid rise of intragastric pH: a wireless pH metry analysis. J Gastroenterol Hepatol. 2010;25(1):43–7. doi: 10.1111/j.1440-1746.2009.05994.x. [DOI] [PubMed] [Google Scholar]
- 28.Clough MR, Axon AT. The Calcium Carbonate Breath Test, a noninvasive test of stimulated gastric acid secretion: preliminary communication. Eur J Gastroenterol Hepatol. 2009;21(3):266–72. doi: 10.1097/MEG.0b013e328321837e. [DOI] [PubMed] [Google Scholar]
- 29. [August 4, 2011];Smartpill Corporation: Contraindications. 2011 Available at: http://www.smartpillcorp.com/index.cfm?pagepath=Professionals/Contraindications&id=17819.
- 30.Baron JH. Clinical tests of gastric secretion : history, methodology, and interpretation. New York: Oxford University Press; 1979. [Google Scholar]
- 31.Ravi K, Francis DL, See JA, Geno DM, Katzka DA. The effects of a weakly acidic meal on gastric buffering and postprandial gastro-oesophageal reflux. Aliment Pharmacol Ther. 2011;34(5):568–75. doi: 10.1111/j.1365-2036.2011.04761.x. [DOI] [PubMed] [Google Scholar]
- 32.Ellison EC, Sparks J. Zollinger-Ellison syndrome in the era of effective acid suppression: are we unknowingly growing tumors? Am J Surg. 2003;186(3):245–8. doi: 10.1016/s0002-9610(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 33.Wong H, Yau T, Chan P, et al. PPI-delayed diagnosis of gastrinoma: oncologic victim of pharmacologic success. Pathol Oncol Res. 2010;16(1):87–91. doi: 10.1007/s12253-009-9192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segal HL. Clinical measurement of gastric secretion: significance and limitations. Ann Intern Med. 1960;53:445–61. doi: 10.7326/0003-4819-53-3-445. [DOI] [PubMed] [Google Scholar]
- 35.Frislid K, Berstad A, Guldvog I. Simulated meal test. A new method for estimation of parietal and non-parietal secretion in response to food. Scand J Gastroenterol. 1985;20(1):115–22. doi: 10.3109/00365528509089642. [DOI] [PubMed] [Google Scholar]
- 36.Mojaverian P, Chan K, Desai A, John V. Gastrointestinal transit of a solid indigestible capsule as measured by radiotelemetry and dual gamma scintigraphy. Pharm Res. 1989;6(8):719–24. doi: 10.1023/a:1015998708560. [DOI] [PubMed] [Google Scholar]
- 37.Ovesen L, Bendtsen F, Tage-Jensen U, Pedersen NT, Gram BR, Rune SJ. Intraluminal pH in the stomach, duodenum, and proximal jejunum in normal subjects and patients with exocrine pancreatic insufficiency. Gastroenterology. 1986;90(4):958–62. doi: 10.1016/0016-5085(86)90873-5. [DOI] [PubMed] [Google Scholar]
- 38.Hannibal S, Rune SJ. Duodenal bulb pH in normal subjects. Eur J Clin Invest. 1983;13(6):455–60. doi: 10.1111/j.1365-2362.1983.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 39.Bahadur SSM, Kuo B, et al. Assessment of Upper GI Motility in Healthy Subjects Using Ambulant Capsule Technology. Gastroenterology. 2005;128(4):T1756. [Google Scholar]
- 40.Sachdeva P, Kantor S, Knight LC, Maurer AH, Fisher RS, Parkman HP. Use of a high caloric liquid meal (Ensure Plus) as a alternative meal for gastric emptying scintigraphy. Gastroenterology. 2010;138(5):S715–S716. doi: 10.1007/s10620-013-2665-2. [DOI] [PubMed] [Google Scholar]
- 41.Hasler W. The physiology of gastric motility and gastric emptying. In: Yamada T, Alpers DH, editors. Textbook of gastroenterology. 5. Chichester, West Sussex; Hoboken, NJ: Blackwell Pub.; 2009. p. 216. [Google Scholar]
- 42.Kuo B, Viazis N, Bahadur S, et al. Non-invasive simultaneous measurement of intra-luminal pH and pressure: assessment of gastric emptying and upper GI manometry in healthy subjects. Neurogastroent Motil. 2004;16:666. [Google Scholar]
- 43.Mozsik G, Venter E, Schmelczer M, Kutas J, Nagy L, Tarnok F. A critical analysis of the gastric secretory response of patients with duodenal ulcer in dependence of their age and duration of complaints. Acta Med Hung. 1981;38(2):117–28. [PubMed] [Google Scholar]
- 44.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27(2):186–96. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 45.Scarpignato C. Poor effectiveness of proton pump inhibitors in non-erosive reflux disease: the truth in the end! Neurogastroent Motil. 2012;24(8):697–704. doi: 10.1111/j.1365-2982.2012.01977.x. [DOI] [PubMed] [Google Scholar]
- 46.Malagelada JR, Longstreth GF, Summerskill WH, Go VL. Measurement of gastric functions during digestion of ordinary solid meals in man. Gastroenterology. 1976;70(2):203–10. [PubMed] [Google Scholar]
- 47.Phillips S, Hutchinson S, Davidson T. Preoperative drinking does not affect gastric contents. Br J Anaesth. 1993;70(1):6–9. doi: 10.1093/bja/70.1.6. [DOI] [PubMed] [Google Scholar]
- 48.Klein S, Butler J, Hempenstall JM, Reppas C, Dressman JB. Media to simulate the postprandial stomach I. Matching the physicochemical characteristics of standard breakfasts. J Pharm Pharmacol. 2004;56(5):605–10. doi: 10.1211/0022357023367. [DOI] [PubMed] [Google Scholar]
- 49.Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23(1):165–76. doi: 10.1007/s11095-005-8476-1. [DOI] [PubMed] [Google Scholar]
- 50.Horowitz M, Maddern GJ, Chatterton BE, Collins PJ, Harding PE, Shearman DJ. Changes in gastric emptying rates with age. Clin Sci (Lond) 1984;67(2):213–8. doi: 10.1042/cs0670213. [DOI] [PubMed] [Google Scholar]
- 51.Couturier O, Bodet-Milin C, Querellou S, Carlier T, Turzo A, Bizais Y. Gastric scintigraphy with a liquid-solid radiolabelled meal: performances of solid and liquid parameters. Nucl Med Commun. 2004;25(11):1143–50. doi: 10.1097/00006231-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Datz FL, Christian PE, Moore J. Gender-related differences in gastric emptying. J Nucl Med. 1987;28(7):1204–7. [PubMed] [Google Scholar]
- 53.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95(6):1456–62. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 54.White WD, Juniper K., Jr Repeatability of gastric analysis. Am J Dig Dis. 1973;18(1):7–13. doi: 10.1007/BF01072231. [DOI] [PubMed] [Google Scholar]
- 55.Hurlimann S, Dur S, Schwab P, et al. Effects of Helicobacter pylori on gastritis, pentagastrin-stimulated gastric acid secretion, and meal-stimulated plasma gastrin release in the absence of peptic ulcer disease. Am J Gastroenterol. 1998;93(8):1277–85. doi: 10.1111/j.1572-0241.1998.409_x.x. [DOI] [PubMed] [Google Scholar]


