Summary
Many species of bacteria form surface-attached communities known as biofilms. Surrounded in secreted polymers, these aggregates are difficult to both prevent and eradicate, posing problems for medicine and industry [1, 2]. Humans play host to hundreds of trillions of microbes that live adjacent to our epithelia and we are typically able to prevent harmful colonization. Mucus, the hydrogel overlying all wet epithelia in the body, can prevent bacterial contact with the underlying tissue. The digestive tract, for example, is lined by a firmly adherent mucus layer that is typically devoid of bacteria, followed by a second, loosely adherent layer that contains numerous bacteria [3]. Here, we investigate mucus's role as a principle arena for host-microbe interactions. Using defined in vitro assays, we found that mucin biopolymers, the main functional constituents of mucus, promote the motility of planktonic bacteria, and prevent their adhesion to underlying surfaces. The deletion of motility genes, however, allows Pseudomonas aeruginosa to overcome the dispersive effects of mucus and form suspended antibiotic-resistant flocs, which mirror the clustered morphology of immotile natural isolates found in the cystic fibrosis lung mucus [4, 5]. Mucus may offer new strategies to target bacterial virulence, such as the design of anti-biofilm coatings for implants.
Keywords: Pseudomonas aeruginosa, mucins, motility, extracellular polymeric substances, cystic fibrosis
Results and Discussion
Mucins reduce surface adhesion and biofilm formation of P. aeruginosa
To begin to dissect mucin-bacterial interactions, we developed an in vitro assay that uses defined concentrations of native mucins. As a source of mucins we purified native porcine gastric mucus to obtain an extract composed predominantly of MUC5AC, which is one of the major gel-forming components in the lungs and stomach [6]. The use of natively purified mucins is decisive for the utility of this assay, as commercially available mucins are processed and have lost the ability to form viscoelastic hydrogels, as are generated by the native polymers [7, 8]. The second critical feature for this assay is the presentation of mucins in solution, as they exist in the secreted lung mucus, instead of depositing them onto a surface. This detail is important as the surface deposition of mucins is likely to adsorb functional groups, thereby partially dehydrating and altering the biochemical activity of the polymer.
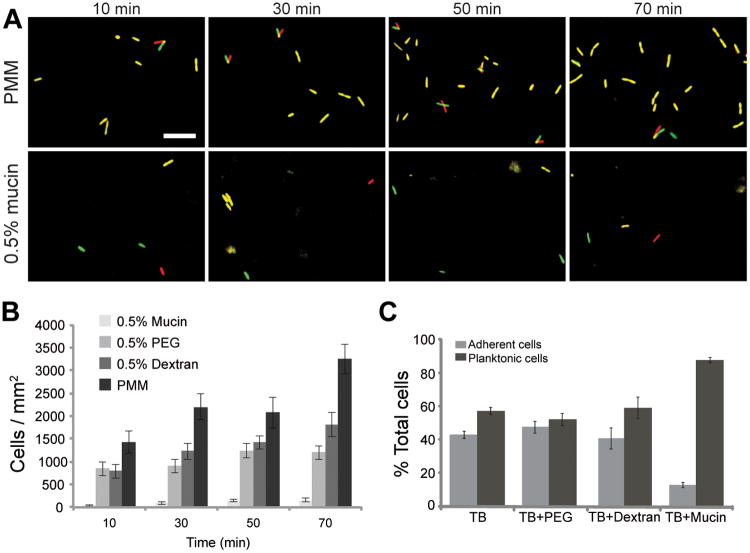
First, we tested the effect of mucins on the ability of bacteria to colonize an immersed surface. A glass coverslip was suspended in culture medium that contained physiological concentrations of mucins [9]. Using the motile, opportunistic pathogen Pseudomonas aeruginosa, we quantified firm attachment by placing exponential-phase cells in contact with the coverslip and imaging using phase contrast microscopy. Cells that adhered to the surface and fully arrested (based on overlaying pairs of images separated by 2 s) were considered firmly attached, and were counted at 20-minute intervals (fig. 1a).
Figure 1. Mucins block P. aeruginosa attachment to surfaces.
(A) Images of coverglass surfaces at the indicated time points, depicting cell adhesion. Cells in PMM or PMM plus mucins were photographed at 2 s intervals at each time point. Images from these intervals were false-colored red and green, respectively, and overlaid, allowing visualization of active cell motility or Brownian motion, versus firm adhesion. Scale bar is 10 μm. (B) Number of wild-type cells firmly adherent to coverglass in PMM, or PMM supplemented with PEG, dextran or mucins after the indicated incubation periods. Error bars indicate SEM of 8-11 different data points. (C) PAO1 wild-type bacteria were grown in polypropylene tubes containing TB or TB plus 1% (w/v) PEG, dextran, or mucin. After 6 h, the relative amount of planktonic versus surface-attached cells was quantified using MTT staining. Error bars represent the standard deviation.
We found that mucins reduced bacterial surface adhesion by 20-fold over a 70 minute period (fig. 1b). To test if this inhibitory effect was specific to the mucins, or a generic result of the presence of polymers, we compared the effects of mucins to the effects of solutions of polyethylene glycol (PEG), a polymer often used as an antiadhesive coating [10], and dextran, a branched, high-molecular-weight polysaccharide. In comparison to mucins, PEG and dextran demonstrated only mild reductions in bacterial adhesion at these early time points, indicating that mucins have singular effects that cannot be attributed to their polysaccharide or soluble polymeric attributes alone. At 6 h, a time at which biofilms have begun to form, approximately 90% of P. aeruginosa cells remained planktonic in the presence of mucins, compared with 50-60% in tryptone broth (TB) alone or TB plus PEG or dextran (fig. 1c).
Mucin gels maintain or augment bacterial swimming motility
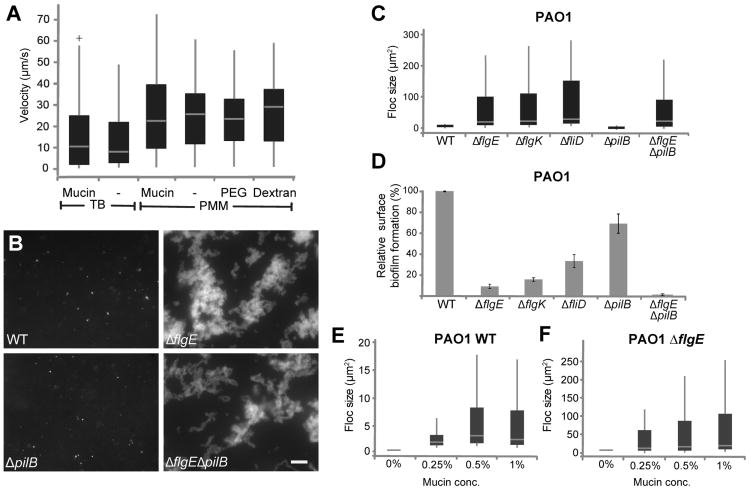
It is tempting to speculate that bacteria failed to access the underlying surface because they were trapped within the mucin network. If this is true, we should expect to see a measurable decrease of motility within the mucin hydrogel. First, to test if motion was hindered in the presence of mucins we tracked the movements of P. aeruginosa cells that carried a deletion in the flagellar hook gene (flgE), and were thus deficient in self-propulsion. These cells demonstrated a significant decrease in diffusivity (p<0.001) in mucin environments, from 2.4 ± 0.2 × 10−9 cm2/s to 1.0 ± 0.1 × 10−9 cm2/s (n ≥ 96 cells), reflecting a higher apparent viscosity of mucin-containing gels, and suggesting that geometric hindrance was present. However, the wild-type cells remained highly motile in the presence of the mucins (supp. mov. S1-5). The distribution of velocities of swimming cells in mucins is similar to that in liquid medium, despite the differences in apparent viscosity (fig. 2a, S1a). This effect was apparent when we compared cells in Pseudomonas minimal medium (PMM) as well as in tryptone broth (TB) with or without mucins. To test whether this effect is specific for Pseudomonas, or if it is a more general phenomenon that affects other swimming bacteria, we tracked a different motile bacterium, Escherichia coli. Despite a significant decrease in diffusivity (p<0.001) of non-flagellated cells (ΔfliC) in mucins, from 2.2 ± 0.2 × 10−9 cm2/s to 0.7 ± 0.1 × 10−9 cm2/s (n ≥ 92 cells), the wild-type cells had significantly increased swimming velocities in mucins compared with medium-only (fig. S1b,c).
Figure 2. Non-motile flagella mutants, but not their motile counterparts, form flocs in mucin environments.
(A) Boxplots depicting swimming velocities of P. aeruginosa in various conditions. Cells were grown in the media indicated, but swimming experiments were in 50%-strength media. Velocities were obtained from particle tracking analyses of 20-s swimming videos obtained at 20 frames per second. See also Movies S1-S5. (B) Floc formation of wild-type cells, flagella mutant (ΔflgE), a pili mutant (ΔpilB), and double flagella and pili mutant (ΔflgEΔpilB)in PMM with 1% mucins after 20 h of incubation. Images are of cells in suspension only. Scale bar is 20 μm. Boxplots (C, E, F) quantifying floc size of wild type, flagella, pili and matrix and motility mutants for the strains indicated in μm2, after 20 h of growth in 1% mucin (unless otherwise indicated). For details on the quantification method see experimental procedures. For all boxplots, boxes extend from the 25th to the 75th percentile, the central line is the median, and whiskers extend to the data point nearest to 1.5 times the interquartile range above and below the box. Outliers are plotted as plus signs. (D) Surface attached biofilm formation was quantified by crystal violet (CV): liquid cultures of the strains indicated were inoculated in 96-well plates at an OD600 of 0.01, and incubated for 7 h at 37°C. The biofilms that formed were quantified by staining with 0.1% CV as described previously [30]. After staining, each plate was rinsed, and the remaining CV was destained with 33% acetic acid for 15 min. and measured using a plate reader (OD595). Data are presented as percent biofilm formation relative to wild type. The error bars represent standard deviation. See also Fig. S1.
Immotile P. aeurginosa cells can form suspended flocs in mucin gels
If mucins can prevent surface colonization by maintaining cellular motility, we speculated that cells lacking motility may be able to overcome this dispersion effect and succeed in adhesion and biofilm formation in mucin environments. This line of inquiry may have direct physiological relevance, as isolates of P. aeruginosa from cystic fibrosis (CF) mucus are often non-motile [5]. As with the wild-type, mucins detectably reduced surface adhesion of non-motile cells (ΔflgE), which are already poorly adherent (fig. S1d; compare to fig. 1b). To look beyond surface adhesion in the presence of mucin, we observed the bacteria in the volume of the mucin gel after 20 h of incubation. The wild-type cells remained largely as individual cells or small, suspended colonies (fig. 2b, c) of up to 20 μm2 (this corresponds roughly to clusters of 10-20 cells) distributed throughout the volume of the mucin medium. Increasing mucin concentration did not visibly increase cellular cluster size (fig. 2e). However, when observing the ΔflgE mutant, we noticed a striking difference compared to the behavior of wild-type cells. The flagella mutant formed large aggregated flocs of up to 250 μm2 (fig. 2b, c). These differences are not likely due to variations in cellular populations in the mucin medium, as PAO1 displayed similar growth rates in the presence and absence of mucins (fig. S1e). A similar behavior was found for two additional flagella mutants, ΔflgK, which lack a hook filament junction protein, and ΔfliD, which lack an adhesive protein at the tip of the flagellar filament (figs 2b, c and S1f) but not for ΔpilB which lack pilus-mediated adhesion and twitching motility (fig. 2b, c). The ability of cells to form suspended flocs was inversely correlated with their ability to form surface biofilms in mucin-free environments (fig. 2d). For example, wild-type and ΔpilB cells formed substantial surface biofilms, but failed to form large suspended flocs in the presence of mucins. Conversely, the various flagellar mutants formed large flocs, but had reduced surface biofilms in the absence of mucins. All mutants tested displayed similar growth rates (fig. S1g). The flocs formed by ΔflgE strains increased in maximum size with increasing mucin concentration (fig. 2f).
We hypothesized that loss of flagellar motility (rather than other properties of flagella, such as adhesion) was the dominant contributor to the observed aggregation. To test this, we measured mucin-dependent flocculation by a PA14 strain that carries a fully assembled flagellum, but is paralyzed due to deletions in all four stators in the motor complex (ΔmotABΔmotCD). This mutant formed substantially larger flocs (up to 60 μm2) than the wild-type (S1h,i), but the structures were smaller than those formed by the ΔflgK strain. Again, floc-forming ability in mucins tended to be negatively correlated with surface biofilm formation in medium-only environments (fig. S1j). Both a loss of motility and loss of the flagella itself, therefore, appear to contribute to mucus colonization. Complementing the flgE deletion in PAO1 ΔflgE restored swimming motility and diminished the capacity of the bacteria to form flocs in mucin, indicating that it is indeed the lack of flagella that caused the formation of flocs (fig. S2).
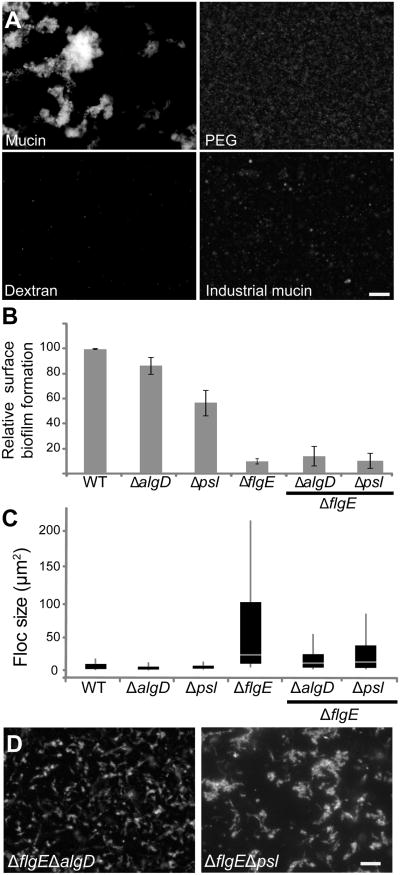
Our data suggest that mucins are highly effective at preventing swimming cells from surface attachment and forming suspended aggregates. Previous work has indicated that fliD is an adhesin for mucin [11], yet it does not appear to be required for the aggregative phenotype (Fig 2c). How then do the flagella mutants achieve aggregate formation? It appears that their lack of motility enables cells to form clonal outgrowths of individual cells within the mucus. This was supported by culturing mixtures of fluorescent and non-fluorescent immotile cells in mucin medium. Over the course of 20 h small homogeneous patches of ten to twenty cells emerged and further expanded (fig. S2). Notably, floc formation did not occur in PEG, dextran or industrially purified mucins (fig. 3a). It appears that this phenomenon depends on specific features unique to native mucins.
Figure 3. Flocs formed in mucin environments are exopolysaccharide-dependent.
(A) ΔflgE strains were grown for 20 h in PMM containing 1% (w/v) either PEG, dextran or industrially purified mucins (NBS Biologicals). Only in the presence of native mucins is floc formation observed. Scale bar is 20 μm. (B) Liquid cultures of EPS secretion mutants and motility mutants were quantified by CV, as described in Figure 2. The experiments were performed in triplicate. The error bars represent the standard deviations. (C) Boxplots of floc size of wild-type cells and the indicated motility and matrix mutants in PMM with 1% mucins after 20 h of incubation. Boxplots are drawn as described in Fig. 2. See also Fig. S2
P. aeruginosa floc formation is dependent on the production of Psl-and alginate
Flagella loss appears to allow bacteria to effectively colonize mucus in a manner reminiscent of surface attached biofilms. Just how similar are these two forms of bacterial aggregation? To address this, we tested if floc formation by non-motile cells required extracellular matrix, a hallmark of biofilms. Specifically, we looked at Psl, which plays a structural role in the maturation of surface-attached biofilms [12] and alginate, which appears to play only a minor role in biofilm formation (fig. 3b, [13]) but is overexpressed in colonies adapted to growth in CF lung mucus [14, 15]. Using previously characterized single algD and psl mutant strains [12, 16], we introduced additional flgE mutations to study the importance of the extracellular matrix on the immotile flocs. Complementation of the double mutants with flgE was able to restore motility (Fig. S2). We found that both polymers, particularly alginate, were important for floc formation (fig. 3c, d). This phenotype may be relevant to CF pathology, where the formation of P. aeruginosa flocs inside the lung mucus is associated with the rise of antibiotic resistance [17]. In sum, our data suggest that mucin-based flocs and biofilms have the same broad reliance on extracellular matrix, but the mechanistic details differ in important ways. Specifically, flocs rely on alginate and flagella loss in a manner not seen in surface attached biofilms.
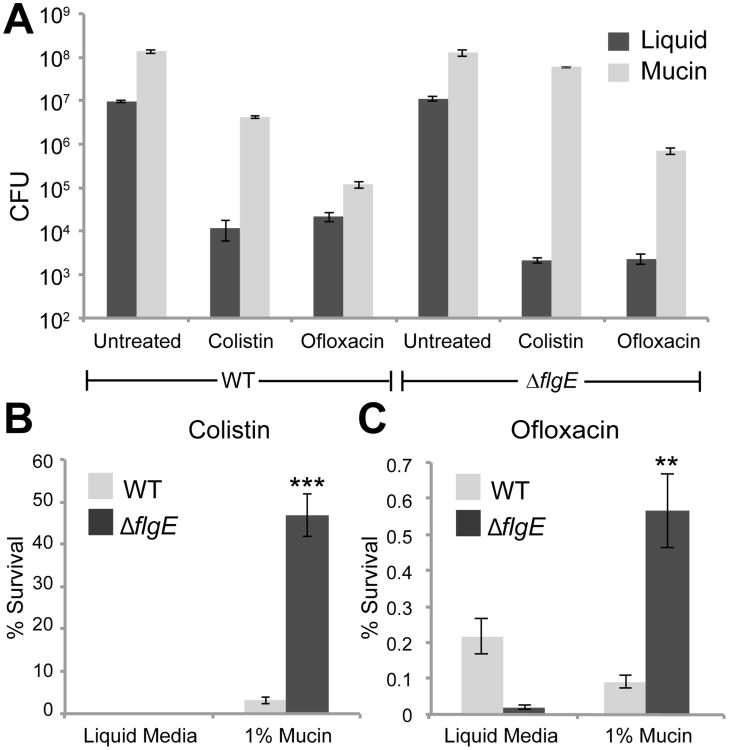
P. aeruginosa flocs that emerge in mucin gels are antibiotic resistant
Last, we asked whether floc formation can provide bacteria with a selective advantage. Again by analogy with biofilms, we hypothesized that the immotile cellular aggregates that emerge in the presence of mucins also have a higher resistance toward antibiotics. We grew wild-type and the non-motile ΔflgE cells in mucin media for 20 h, and then subjected both strains to two clinically relevant antibiotics that differ in their mode of action (Fig. 4). This experiment revealed two points: first, both wild-type and ΔflgE bacteria were systematically more resistant to colistin in the presence of mucins as compared to liquid culture without mucins. This suggests that the mucins themselves have the capacity to reduce the efficacy of colistin, regardless of whether cells are planktonic (wild-type) or form flocs (ΔflgE). Second, it appeared that the floc-forming ΔflgE cells were more resistant to both antibiotics in the mucin medium than the motile wild type cells. To test for this possibility we determined the percent survival of the bacteria in either condition, by normalizing to the cell numbers in the untreated samples in liquid and mucin. Inside the mucin medium, the non-motile flagella mutants were on average 14 times more resistant to colistin (fig. 4b) and approximately 6 times more resistant to ofloxacin (fig. 4c) than wild-type cells, both of which are statistically significant differences. We conclude that the aggregates that emerge upon loss of motility indeed have an increased resistance compared to motile wild-type cells, possibly due to the presence of an altered composition or quantity of extracellular matrix components, or due to a protective effect of increased cell density [18].
Figure 4. Flocs grown in mucin environments are antibiotic resistant.
(A) Wild-type and ΔflgE cells were grown in liquid culture or in 1% mucin for 20h and then exposed to colistin and ofloxacin. After 3h of antibiotic exposure, the cells were plated to determine the number of surviving cells. (B, C) Data from (A) replotted as survival of antibiotic treated cells as a percentage of CFUs of untreated cells. Each trial was repeated at least 3 times. Error bars represent SEM. **, p<0.01; ***, p<0.001, comparing survival of ΔflgE to wild type in 1% mucins.
Conclusions and Outlook
Here we have found that animals provide a candidate solution to inhibit biofilm formation, namely mucin polymers. Critically, our results demonstrate that mucins can limit bacterial surface attachment and biofilm formation without killing or trapping bacteria, which will help to limit selective pressure for resistance. Indeed, our only evidence for a resistance phenotype comes in the form of non-motile cells, which are likely to be strongly limited in other modes of virulence [5, 19]. Our observations of motility and reduced adhesion in mucin media are similar to findings for Campylobacter jejuni in mouse intestinal crypts. In a previous study, extracted epithelial scrapings from C. jejuni-colonized gnotobiotic mice demonstrated a lack of adhesion and unhindered motility within the crypts [20]. Similar to this, a recent study showed that when supplemented in agar plates, mucins appear to increase motility of P. aeruginosa[21]. At first sight these and our findings contrast with reports on surface-immobilized mucins, which arrest [11, 22] and can cause large aggregate formation of P. aeruginosa cells [23]. However, these findings can be reconciled if one considers that the effects of mucins on motility may depend on their native three dimensional structure and hence biophysical properties such as viscoelasticity and lubricity, which are preserved in native mucus and presumably inside agar gels, but not when adsorbed to a two-dimensional surface [21]. The gel-forming mucin MUC2 has an ordered repeating ring structure [24], and we speculate that also other gel-forming mucins, such as the MUC5AC used in our experiments, display three dimensional features that affect their interactions with bacteria. Indeed, Berg and Turner have observed that certain structured viscous solutions allow increased velocities of motile bacteria by providing a rigid framework for generating propulsive forces [25]. We anticipate that studying mucins in their native three-dimensional form will reveal valuable novel information about bacterial behavior that cannot be captured by collapsed mucin monolayers.
Experimental Procedures
Mucin purification
The source for purification of native MUC5AC was pig stomachs, which secrete MUC5AC, homologous to the human glycoprotein [26]. Porcine gastric mucins were purified as described previously, with the omission of the CsCl density gradient centrifugation [27]. Mass spectrometry analysis was used to determine the composition of the mucin preparation as described previously [28]. Briefly, the analysis was performed at the Harvard Microchemistry and Proteomics Analysis Facility by microcapillary reverse-phase HPLC nanoelectrospray tandem mass spectrometry on a Thermo LTQ-Orbitrap mass spectrometer. The spectra were analyzed using the algorithm Sequest [29]. The analysis showed that MUC5AC was the predominant mucin present in our purified extract, which also contained MUC2, MUC5B and MUC6 as well as other proteins including histones, actin and albumin. In addition, its quality was tested by rheology as described in [7, 27], which confirmed that the isolated mucins displayed viscoelastic properties similar to native mucus.
Microbial adhesion assays
For adhesion experiments, PAO1 wild-type and PAO1 ΔflgE were inoculated in LB and grown overnight at 37°C, shaking. Overnight cultures were diluted 1:100 into PMM and grown shaking at 37°C for 4 h. 1 ml of exponential phase cells (OD600 = 0.4 to 0.45) were centrifuged and cells were resuspended in 400 μL sterile PMM. These cells were diluted 1:10 in PMM and then further diluted 1:10 into the medium to be tested (PMM only, 0.5% mucin, 0.5% PEG 3350, or 0.5% dextran). 40 μL of this mixture was pipetted onto glass slides with shallow spherical depressions, covered with a glass coverslip and inverted. Pairs of images were taken 2 s apart in multiple fields for each sample at 10, 30, 50 and 70 min. Image pairs were compared to differentiate firmly attached cells from moving cells in each frame. Adherent cells were counted for each time point. Pairs of dividing cells were counted as single cells.
Quantification of biofilm formation in mucin gels
Freshly growing cells at an OD600 of 0.01 were inoculated in polypropylene PCR tubes and incubated at 37°C in TB or in TB containing 0.5% (w/v) mucins. After 6 h the planktonic cells were removed for quantification, and the adherent cells in the tubes were washed 2 times with PBS to remove non-adherent cells. Planktonic and adherent cells were stained with 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for 2 h at 37°C, and subsequently destained with 20% sodium dodecyl sulfate in 50% dimethylformamide (adjusted to pH = 4.7) overnight at 37°C. The resulting solutions were quantified using a plate reader (OD595).
Particle tracking
For measurement of cell velocities, bacteria were grown to exponential phase as described above, stained with Syto9 live cell stain by adding Syto9 1:1000 into the culture, and incubated for 10 minutes at room temperature. The stained cells were diluted 1:10 into a 50% strength solution of growth medium (as indicated in figures 2A and S1A-C) or growth medium supplemented with mucin, dextran or PEG. These solutions were mixed and dispensed into chambers for visualization. Videos of cells were taken on an inverted fluorescent microscope at 20 frames per second to obtain trajectories (see SI for additional details). The trajectories obtained were processed using Matlab to determine velocities and diffusivities. Diffusivities were based upon mean squared displacement values for a range of lag times. Trajectories were also examined visually to ensure accuracy.
Antibiotic treatment
To determine the antibiotic resistance of flocs grown in mucin media, cells were grown in PMM with 1% (w/v) mucin. After 20 h, the number of cells was determined by counting CFU; this number was used as the reference number prior to treatment. The antibiotics ofloxacin and colistin were added to the cultures at final concentrations of 20 μg/ml, and the cultures were grown at 37°C for 3 h. After treatment, the number of survivors was estimated by measuring the CFU. To avoid aggregates, we bead-bashed each sample for 30 s before diluting and plating. Each experiment was carried out in triplicate. To determine the resistance of cells grown in the absence of mucins, an exponential phase culture was adjusted to contain the same number of cells as had grown in 1% mucin in 20 h, and challenged with antibiotics as described above.
Supplementary Material
Highlights.
Mucin biopolymers reduce bacterial adhesion to underlying substrates
Bacterial motility is maintained or increased in the presence of mucins
Mucins block aggregate formation by motile bacteria
Immotile Pseudomonas aeruginosa can form alginate and Psl-dependent flocs in mucus
Acknowledgments
This work was supported by the Cystic Fibrosis Foundation CFF grant number RIBBEC08I0 and MIT startup funds to KR. KRF is supported by European Research Council grant 242670. RSF is supported through the National Science Foundation Graduate Research Fellowship Program. We thank D.J. Wozniak for the EPS deletion strains, B. Berwin for providing the P. aeruginosa PA14 strains, W. Kim for the labeled conjugating strain, G.A. O'Toole for the complementation vector, and the lab of R. Kolter for the E. coli strain ZK2686.
References
- 1.Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis. 2001;33:1387–1392. doi: 10.1086/322972. [DOI] [PubMed] [Google Scholar]
- 2.Petrova OE, Sauer K. Sticky Situations - Key Components That Control Bacterial Surface Attachment. [Accessed May 29, 2012];J Bacteriol. 2012 doi: 10.1128/JB.00003-12. Available at: http://jb.asm.org/content/early/2012/02/27/JB.00003-12. [DOI] [PMC free article] [PubMed]
- 3.Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 5.Mahenthiralingam E, Campbell ME, Speert DP. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun. 1994;62:596–605. doi: 10.1128/iai.62.2.596-605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schade C, Flemström G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 1994;107:180–188. doi: 10.1016/0016-5085(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 7.Kocevar-Nared J, Kristl J, Smid-Korbar J. Comparative rheological investigation of crude gastric mucin and natural gastric mucus. Biomaterials. 1997;18:677–81. doi: 10.1016/s0142-9612(96)00180-9. [DOI] [PubMed] [Google Scholar]
- 8.Crater JS, Carrier RL. Barrier Properties of Gastrointestinal Mucus to Nanoparticle Transport. Macromolecular Bioscience. 2010;10:1473–1483. doi: 10.1002/mabi.201000137. [DOI] [PubMed] [Google Scholar]
- 9.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee I, Pangule RC, Kane RS. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Advanced Materials. 2011;23:690–718. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 11.Arora SK, Ritchings BW, Almira EC, Lory S, Ramphal R. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect Immun. 1998;66:1000–7. doi: 10.1128/iai.66.3.1000-1007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J Bacteriol. 2006;188:8213–21. doi: 10.1128/JB.01202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wozniak DJ, Wyckoff TJ, Starkey M, Keyser R, Azadi P, O'Toole GA, Parsek MR. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A. 2003;100:7907–12. doi: 10.1073/pnas.1231792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol. 2001;183:5395–401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stapper AP, Narasimhan G, Ohman DE, Barakat J, Hentzer M, Molin S, Kharazmi A, Hoiby N, Mathee K. Alginate production affects Pseudomonas aeruginosa biofilm development and architecture, but is not essential for biofilm formation. J Med Microbiol. 2004;53:679–90. doi: 10.1099/jmm.0.45539-0. [DOI] [PubMed] [Google Scholar]
- 16.Whitchurch CB, Erova TE, Emery JA, Sargent JL, Harris JM, Semmler ABT, Young MD, Mattick JS, Wozniak DJ. Phosphorylation of the Pseudomonas aeruginosa Response Regulator AlgR Is Essential for Type IV Fimbria-Mediated Twitching Motility. J Bacteriol. 2002;184:4544–4554. doi: 10.1128/JB.184.16.4544-4554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreau-Marquis S, Stanton BA, O'Toole GA. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pul Pharm. 2008;21:595–599. doi: 10.1016/j.pupt.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connell JL, Wessel AK, Parsek MR, Ellington AD, Whiteley M, Shear JB. Probing Prokaryotic Social Behaviors with Bacterial “Lobster Traps”. [Accessed September 16, 2012];mBio. 2010 1 doi: 10.1128/mBio.00202-10. Available at: http://mbio.asm.org/content/1/4/e00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josenhans C, Suerbaum S. The role of motility as a virulence factor in bacteria. Int J Med Microbiol. 2002;291:605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 20.Lee A, O'Rourke JL, Barrington PJ, Trust TJ. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect Immun. 1986;51:536–546. doi: 10.1128/iai.51.2.536-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeung ATY, Parayno A, Hancock REW. Mucin Promotes Rapid Surface Motility in Pseudomonas Aeruginosa. [Accessed May 11, 2012];mBio. 2012 3 doi: 10.1128/mBio.00073-12. Available at: http://mbio.asm.org/content/3/3/e00073-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vishwanath S, Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984;45:197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landry RM, An D, Hupp JT, Singh PK, Parsek MR. Mucin–Pseudomonas aeruginosa interactions promote biofilm formation and antibiotic resistance. Molecular Microbiology. 2006;59:142–151. doi: 10.1111/j.1365-2958.2005.04941.x. [DOI] [PubMed] [Google Scholar]
- 24.Ambort D, Johansson MEV, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, Koeck PJB, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berg HC, Turner L. Movement of microorganisms in viscous environments. Nature. 1979;278:349–351. doi: 10.1038/278349a0. [DOI] [PubMed] [Google Scholar]
- 26.Turner B, Bansil R, Afdhal NH. Expression of cysteine-rich C-terminal domains of Pig Gastric Mucin in Pichia pastoris. FASEB J. 2007;21 [Google Scholar]
- 27.Celli J, Gregor B, Turner B, Afdhal NH, Bansil R, Erramilli S. Viscoelastic properties and dynamics of porcine gastric mucin. Biomacromolecules. 2005;6:1329–33. doi: 10.1021/bm0493990. [DOI] [PubMed] [Google Scholar]
- 28.Lieleg O, Lieleg C, Bloom J, Buck CB, Ribbeck K. Mucin Biopolymers As Broad-Spectrum Antiviral Agents. [Accessed May 29, 2012];Biomacromolecules. 2012 doi: 10.1021/bm3001292. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22475261. [DOI] [PMC free article] [PubMed]
- 29.Yates JR, Eng JK, McCormack AL, Schieltz D. Method to Correlate Tandem Mass Spectra of Modified Peptides to Amino Acid Sequences in the Protein Database. Anal Chem. 1995;67:1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 30.Friedman L, Kolter R. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J Bacteriol. 2004;186:4457–65. doi: 10.1128/JB.186.14.4457-4465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.