Summary
Potassium channels play an important role in the regulation of arterial tone and decreased activity of these ion channels has been linked to pial artery vasospasm after subarachnoid hemorrhage (SAH). Our previous work has shown that acute application of a blood component, oxyhemoglobin, caused suppression of voltage-gated K+ (KV) channels through heparin-binding epidermal growth factor-like growth factor (HB-EGF) mediated activation of epidermal growth factor receptor (EGFR). Using patch clamp electrophysiology, we have now examined whether this pathway of KV channel suppression is activated in parenchymal arteriolar myocytes following long-term in vivo exposure to subarachnoid blood. We have found that KV currents, but not large conductance Ca2+ activated or inwardly rectifying K+ channel currents, were decreased in parenchymal arteriolar myocytes freshly isolated from Day-5 SAH model rabbits. Interestingly, parenchymal arteriolar myocytes from control animals were more sensitive to exogenous HB-EGF (IC50: 0.2 ± 0.4 ng/mL) compared to pial arterial myocytes (IC50: 2.4 ±1.3 ng/mL). However, HB-EGF and oxyhemoglobin failed to decrease KV currents in parenchymal arteriolar myocytes from SAH animals, consistent with EGFR activation and KV current suppression by SAH. These data suggest that HB-EGF/EGFR pathway activation contributes to KV current suppression and enhanced parenchymal arteriolar constriction after SAH.
Keywords: K+ channels, heparin-binding EGF-like growth factor (HB-EGF), parenchymal arteriole, patch clam, vascular smooth muscle, vasospasm
Introduction
Potassium-selective ion channels represent a family of ubiquitously expressed pore-forming proteins which have diverse physiological functions. Several types of K+ channels including voltage-gated K+ (KV) channels, large conductance Ca2+-activated K+ (BK) channels, inward rectifier K+ (Kir) channels and ATP-sensitive K+ (KATP) channels are expressed in cerebral arterial myocytes and play an important role in the regulation of arterial tone [11]. In arterial myocytes, decreased K+ channel activity promotes membrane potential depolarization leading to activation of voltage-dependent Ca2+ channels, increased Ca2+ influx and vasoconstriction [5].
Previous studies have demonstrated that decreased KV channel currents contribute to enhanced arteriolar tone in pial cerebral arteries following SAH [2, 13, 14]. With respect to potential mechanisms of decreased KV channel activity, our laboratory has demonstrated that acute exposure of cerebral artery myocytes to the blood component, oxyhemoglobin, caused KV current suppression via a pathway involving activation of the tyrosine kinase epidermal growth factor receptor (EGFR) by its ligand heparin-binding epidermal growth factor-like growth factor (HB-EGF) [2, 8]. These studies provided evidence that oxyhemoglobin increased matrix metalloprotease activity in cerebral arteries leading to the cleavage and release of HB-EGF from membrane-bound pro-HB-EGF. Binding of HB-EGF to EGFR may lead to KV channel endocytosis and vasoconstriction [8]. Presently, it is unclear whether the HB-EGF/EGFR pathway is activated in the cerebral vasculature after SAH.
Parenchymal arterioles, located in brain parenchyma downstream of the Virchow-Robin space, play an important role in the regulation of both local and global cerebral blood flow. Compared to brain surface arteries, relatively little is currently known regarding the impact of SAH on parenchymal arteriolar function. Recently, Nystoriak et al. [12] demonstrated that parenchymal arterioles isolated from SAH model rats exhibit enhanced myogenic tone and vascular smooth muscle membrane potential depolarization. Here, we have examined the hypothesis that decreased KV currents contribute to enhanced tone in parenchymal arterioles isolated from SAH model rabbits via a pathway involving HB-EGF-induced activation of EGFR.
Materials and Methods
Rabbit SAH model
This study used male New Zealand White rabbits (3.0–3.5 kg; Charles River) in accordance with the Guidelines for the Care and Use of Laboratory Animals (NIH publication 85–23, revised 1996) and followed protocols approved by the Institutional Animal Use and Care Committee of the University of Vermont. Under isoflurane anesthesia, animals received two injections of unheparinized autologous arterial blood (2.5 mL) into the cisterna magna at an interval of 48 hours [3, 6, 7, 10]. Immediately prior to each injection of blood, an equivalent volume of cerebral spinal fluid was removed. Buprenophine (0.01 mg/kg) was administrated as an analgesic every 12 hours for 36 hours after each surgery. Five days after the initial surgery, animals were euthanized by exsanguination and decapitation under deep pentobarbital anesthesia (60 mg/kg, iv). Parenchymal arterioles from the middle cerebral artery territory were dissected in ice-cold physiological saline solution (in mM: 118.5 NaCl, 4.7 KCl, 24 NaHCO3, 1.18KH2PO4, 2.5 CaCl2, 1.2 MgCl2, 0.023 EDTA and 11 glucose; pH7.4) aerated with 20% O2/5% CO2/ 75% N2.
Electrophysiology
Arteriolar smooth muscle cell isolation
Individual myocytes were enzymatically dissociated from parenchymal arterioles as described previously [4, 7, 8]. Briefly, parenchymal arterioles were incubated at 37 °C for 17 min in glutamate-containing isolation solution (in mM; 55 NaCl, 5.6 KCl, 80 L-glutamic acid, 2.0 MgCl2, 10 HEPES and 10 glucose; pH 7.3) with 0.3 mg/mL papein and 0.7 mg/mL 1,4-dithioerythriol. Arterioles were then transferred into isolation solution containing collagenase (0.7 mg/mL collagenase type F and 0.3 mg/mL collagenase type H) and 0.1 mM CaCl2 for 10 min at 37 °C. After incubation in isolation solution with 2 mM CaCl2 on ice for 30 min, tissues were gently triturated using a small bore fire-polished Pasteur pipette.
Whole cell voltage-dependent K+ channel current recordings
Whole cell membrane K+ currents were measured at room temperature using the conventional whole cell configuration of the patch clamp technique. The bath solution consisted of (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 1.8 CaCl2, 10 glucose and 10 HEPES (pH 7.4). Except when specifically mentioned, bath solution also contained 1.8 mM CaCl2. Patch pipettes (8–10 MΩ) were filled with an (internal) solution containing (in mM): 87 K+ aspartate, 20 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 EGTA and 25 KOH (pH 7.2). Outward currents were elicited by a series of 500-msec depolarizing voltage steps from a holding potential of −70 mV to + 50mV, followed by a step to −40 mV for 300 msec. Voltage steps were made at 10 mV increments at intervals of 10 sec.
Inward rectifier K+ channel (Kir) current measurement
Kir currents were measured using conventional whole cell patch clamp electrophysiology. The bath solution contained (in mM): 140 KCl, 1MgCl2, 10 glucose and 10 HEPES (pH 7.4). Internal (pipette) solution contained (in mM): 87 K+ aspartate, 20 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, 10 EGTA and 25 KOH (pH 7.2). From a holding potential of −70 mV, voltage was stepped to −100 mV for 100 msec, then ramped from −100 mV to +40 mV over a period of 500 msec. Kir currents were defined as membrane currents sensitive to 100 µM BaCl2.
Statistical Analysis
Data are expressed as mean ± SEM with n representing the number of cells per group and N representing the number of animals per group. Student’s paired or unpaired t-test were used to determine statistical significance at the level of P < 0.05 (*) or P < 0.01 (**).
Results
Decreased KV currents in parenchymal arteriolar myocytes after SAH
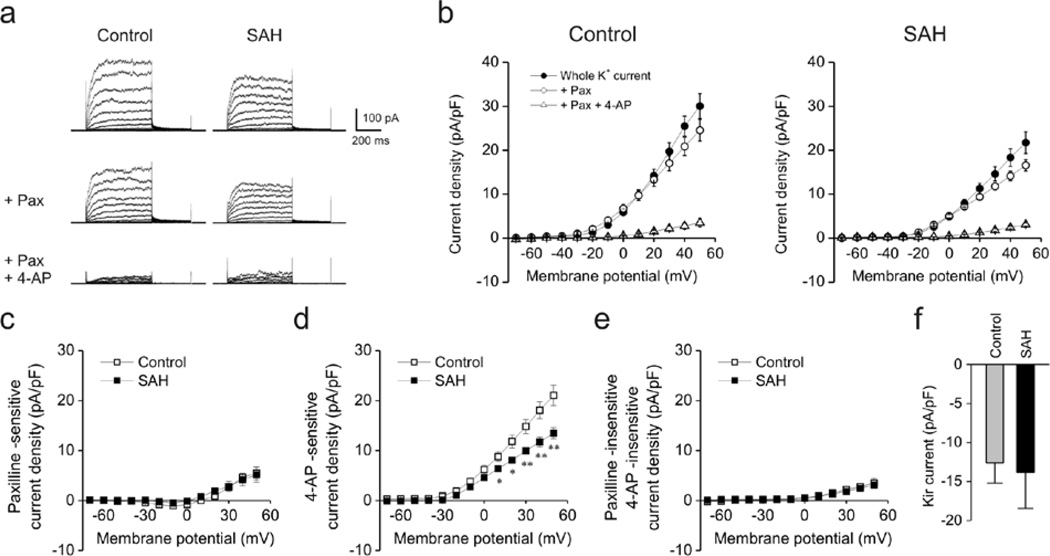
Whole cell voltage-dependent K+ currents, representing a combination of BK and KV channel activity, were measured in freshly isolated parenchymal arteriolar myocytes from control and SAH model animals. Whole cell currents were significantly decreased in cells from SAH model animals (Figure 1a–b). For example, current density at +50 mV was 30.0 ± 2.8 pA/pF (n = 6, N = 2 animals) and 21.7 ± 2.5 pA/pF (n = 5, N = 2) in myocytes from control and SAH animals, respectively. Cell capacitance was not different between groups (control: 11.2 ± 1.4 pF, n = 6; SAH: 10.7 ± 0.8 pF, n = 5). To examine BK channel currents, cells were treated with paxilline, a blocker of BK channels. Paxilline-sensitive currents were similar at + 50 mV in myocytes from control (5.5 ± 1.3 pA/pF, n = 6) and SAH (5.2 ± 1.5 pA/pF,n = 5) animals, indicating that BK currents were unaltered after SAH (Figure 1c). In contrast, currents sensitive to 4-aminopyridine (4-AP, 10 mM), a KV channel blocker, were markedly decreased in parenchymal arteriolar myocytes from SAH model animals (Figure 1d). At +50 mV, 4-AP-sensitive current density was 21.1 ± 2.0 pA/pF at +50 mV in myocytes from control animals (n = 6) compared to 13.5 ± 1.1 pA/pF in myocytes from SAH animals (n=5). Outward K+ currents insensitive to both Paxilline and 4-AP were similar between groups (Figure 1e), as were Kir currents (Figure 1f). These data demonstrate that KV currents are selectively decreased in parenchymal arteriolar myocytes from SAH model animals.
Figure 1. Decreased voltage-gated K+ (KV) currents in rabbit parenchymal arteriolar myocytes following SAH.
a) Representative traces of voltage-dependent K+ channel currents obtained using conventional whole cell patch clamp electrophysiology. Blockers of large conductance Ca2+-activated K+ (BK) channels (paxilline, Pax, 1 µM) and voltage-gated K+ (KV) channels (4-aminopyridine, 4-AP, 10 mM) were cumulatively added to cells at an interval of 10 min. Cell capacitance in control and SAH myoctes were 11.1 pF and 10.9 pF, respectively. b) Summary of current densities in the presence and absence of paxilline and 4-AP. Current density was calculated by dividing membrane currents by cell capacitance for each myocyte. Whole K+ currents (in the absence of paxilline and 4-AP, closed circles) were significantly smaller in parenchymal arteriolar myocytes from SAH model animals. Control: n = 6 cells from 2 animals, SAH: n = 5cells from 2 animals. c–e) Summary of K+ currents obtained in the presence of paxilline and 4-AP. 4-AP-sensitive (Kv) currents were decreased in myocytes obtained from SAH model animals (panel d). Paxilline-sensitive (panel c) and Paxilline-insensitive/4-AP-insensitive (panel e) currents were similar between groups. f) Inward-rectifier K+ (Kir) currents obtained at −100 mV were similar between Control (n = 15 cells from 8 animals) and SAH (n = 12 cells from 5 animals) myocytes.
HB-EGF causes KV current suppression in parenchymal arteriolar myocytes from control animals
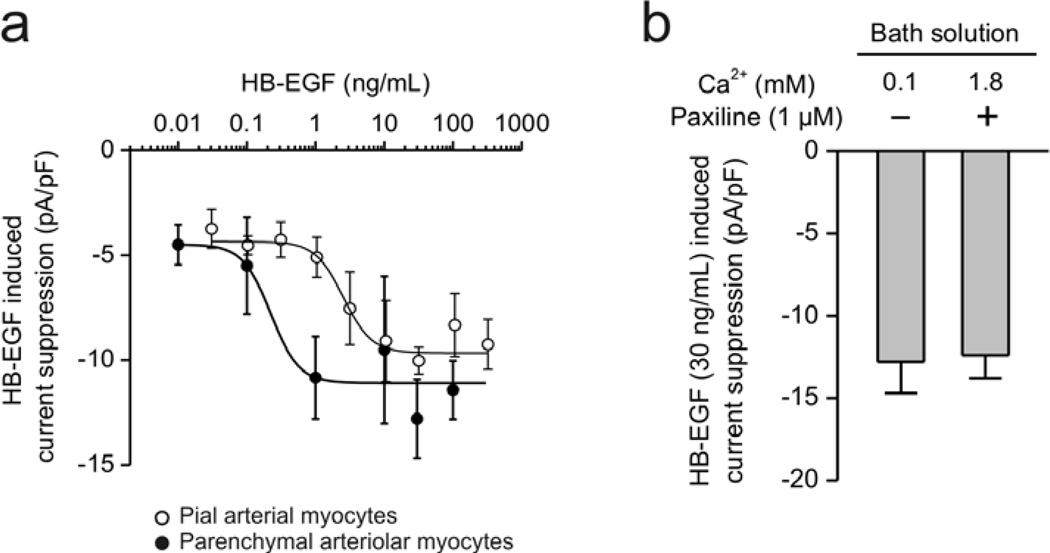
We have previously shown that acute application of oxyhemoglobin suppresses KV currents in pial artery myocytes from control animals via a pathway involving HB-EGF and EGFR activation [8]. We therefore hypothesized that activation of the HB-EGF/EGFR pathway contributes to KV current suppression in parenchymal arteriolar myocytes after SAH. To examine this hypothesis, we first examined the ability of exogenous HB-EGF to suppress KV currents in parenchymal arteriolar myocytes from control animals. In this experimental series, Ca2+ in the bath solution was lowered from 2 mM to 0.1 mM to minimize BK currents. Treatment of exogeneous HB-EGF decreased KV currents in a concentration-dependent manner in parenchymal arteriolar myocytes from control animals (Figure 2a). Interestingly, control parenchymal arteriolar myocytes (IC50: 0.2 ± 0.4 ng/mL) were more sensitive to HB-EGF compared to pial artery myocytes (IC50: 2.4 ± 1.3 ng/mL). In control parenchymal arteriolar myocytes, 30 ng/mL HB-EGF suppressed whole cell K+ currents by approximately 45 %, or 12.8 ± 1.9 pA/pF (n = 4, N = 3). In a second experimental series, the effect of HB-EGF on KV current was also examined using bath solution containing 1.8 mM Ca2+ and the BK channel blocker, paxilline (figure 2b). Using these conditions, HB-EGF (30 ng/mL)-induced current suppression was 12.4 ± 1.4 pA/pF, n=5, N=3, which was similar to that observed using 0.1 mM Ca2+ in the bath solution. These data demonstrate that HB-EGF can potently suppress KV currents in parenchymal arteriolar myocytes in the absence of SAH.
Figure 2. Heparin-binding EGF-like growth factor (HB-EGF) induced KV current suppression in parenchymal and cerebral arterial myocytes isolated from control rabbits.
a) Concentration-response curve of HB-EGF-induced K+ current suppression. K+ currents were obtained from rabbit parenchymal arteriolar (closed circle) and cerebral arterial myocytes (opened circle) using 800-msec of voltage steps to +50 mV from a holding potential of −70 mV. To minimize BK current, Ca2+ in the bath solution was lowered to 0.1 mM. Decreased current densities after HB-10 min EGF treatment were plotted (n = 4–6 cells) for each HB-EGF concentration. Data from pial arterial myocytes was modified from Koide et al., Am J Physiol, 2007 [8]. b) Suppression of KV currents by HB-EGF (30 ng/mL) in parenchymal arteriolar myocytes at + 50 mV. Prior to treatment with HB-EGF, BK currents were minimized either by lowering Ca2+ in the extracellular solution from 1.8 mM to 0.1 mM or the addition of paxilline (1 µM), suggesting HB-EGF suppresses KV current, not BK current (n = 4–5).
HB-EGF and oxyhemoglobin do not cause KV current suppression in parenchymal arteriolar myocytes from SAH model animals
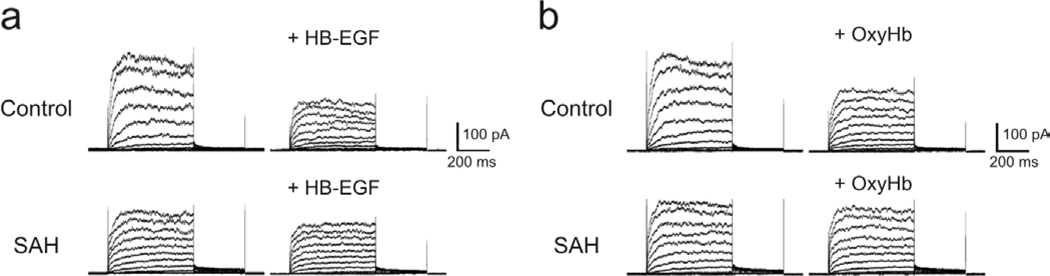
Considering that SAH may cause KV current suppression via EGFR activation, it is conceivable that the actions of exogenous activators of EGFR may be diminished when applied to parenchymal arteriolar myocytes from SAH model animals. Consistent with this possibility, KV current suppression induced by HB-EGF (Figure 3a) and oxyhemoglobin (Figure 3b) were markedly decreased in parenchymal arteriolar myocytes from SAH animals compared to similar cells from control animals. Although further study is needed, these data strongly suggest that activation of the HB-EGF/EGFR pathway contributes to KV current suppression in parenchymal arteriolar myocytes after SAH.
Figure 3. Suppression of KV currents by HB-EGF and oxyhemoglobin in parenchymal arteriolar myocytes is reduced in SAH compared to control animals.
a) Representative traces of the effects HB-EGF treatment (30 ng/mL) on KV currents in parenchymal arteriolar myocytes from control and SAH animals. Cell capacitance was 10.3 pF (control) and 9.7 pF (SAH). b) Oxyhemoglobin (OxyHb, 10 µM) decreased KV currents in parenchymal arteriolar myocytes from control, but not SAH animals. Cell capacitance was 10.8 pF (control) and 11.1 pF (SAH). Recordings were obtained in the presence of the BK channel blocker, paxilline (1 µM).
Discussion
Here we demonstrate that KV currents are suppressed in parenchymal arteriolar myocytes after SAH. KV current suppression would cause membrane potential depolarization [12], increased voltage-dependent Ca2+ channel activity [12] and enhanced arteriolar tone [3, 6, 12]. Our findings are in agreement with previous studies demonstrating SAH-induced KV current suppression in pial arteries [2, 13, 14]. Further, we provide evidence of a novel mechanism linking SAH to KV current suppression involving HB-EGF mediated activation of EGFR. Consistent with activation of this pathway after SAH, suppression of KV currents by HB-EGF and oxyhemoglobin in parenchymal arteriolar myocytes was reduced in SAH compared to control animals. Considering that the blood component oxyhemoglobin is able to activate the HB-EGF/EGFR pathway [8], release of oxyhemoglobin from subarachnoid blood may contribute to SAH-induced KV current suppression in parenchymal arterioles. However, parenchymal arterioles are downstream of the Virchow-Robin space and are tightly encased by astrocyte endfeet. Further research is required to determine whether blood components such as oxyhemoglobin directly interact with parenchymal arteriolar myocytes to cause KV channel suppression.
Interestingly, we found that HB-EGF caused KV current suppression at lower concentrations in parenchymal arteriolar myocytes compared to myocytes from pial arteries. This finding suggests that activation of the HB-EGF/EGFR pathway during pathological conditions (e.g. SAH) may have a greater impact on parenchymal arterioles than brain surface arteries. Further, the HB-EGF/EGFR pathway may play an important role in the physiological regulation of parenchymal arteriolar tone. Consistent with this possibility, the IC50 value determined for HB-EGF-induced KV current suppression (approximately 0.2 ng/mL, figure 2) is close to serum levels of HB-EGF (0.05–0.15 ng/mL) reported in humans [9]. It is also possible the enhanced HB-EGF/EGFR mediated KV channel suppression may contribute to enhanced pressure-induced constrictions reported in parenchymal arterioles isolated from healthy animals [1, 12].
In summary, diminished KV currents were observed in parenchymal arteriolar myocytes from SAH model rabbits. Suppression of KV currents promoting enhanced arteriolar tone and decreased cerebral blood flow may contribute to the development of neurological deficits following SAH. Further, our findings suggest involvement of the HB-EGF/EGFR pathway in KV current suppression following SAH. This work describing a novel pathway of KV current suppression should provide new insights for the development of treatments not only for patients after cerebral aneurysm rupture, but also brain trauma, where SAH is a common but understudied occurrence.
Acknowledgement
This work was supported by the Totman Trust for Medical Research, the Peter Martin Brain Aneurysm Endowment and the NIH (P01 HL095488, R01 HL078983 and R01 HL078983-05S1).
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1.Cipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol. 2004;44:1–8. doi: 10.1097/00005344-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Ishiguro M, Morielli AD, Zvarova K, Tranmer BI, Penar PL, Wellman GC. Oxyhemoglobin-induced suppression of voltage-dependent K+ channels in cerebral arteries by enhanced tyrosine kinase activity. Circ Res. 2006;99:1252–1260. doi: 10.1161/01.RES.0000250821.32324.e1. [DOI] [PubMed] [Google Scholar]
- 3.Ishiguro M, Puryear CB, Bisson E, Saundry CM, Nathan DJ, Russell SR, Tranmer BI, Wellman GC. Enhanced myogenic tone in cerebral arteries from a rabbit model of subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol. 2002;283:H2217–H2225. doi: 10.1152/ajpheart.00629.2002. [DOI] [PubMed] [Google Scholar]
- 4.Ishiguro M, Wellman TL, Honda A, Russell SR, Tranmer BI, Wellman GC. Emergence of a R-type Ca2+ channel (CaV 2.3) contributes to cerebral artery constriction after subarachnoid hemorrhage. Circ Res. 2005;96:419–426. doi: 10.1161/01.RES.0000157670.49936.da. [DOI] [PubMed] [Google Scholar]
- 5.Knot HJ, Nelson MT. Regulation of membrane potential and diameter by voltage-dependent K+ channels in rabbit myogenic cerebral arteries. Am J Physiol. 1995;269:H348–H355. doi: 10.1152/ajpheart.1995.269.1.H348. [DOI] [PubMed] [Google Scholar]
- 6.Koide M, Nystoriak MA, Brayden JE, Wellman GC. Impact of subarachnoid hemorrhage on local and global calcium signaling in cerebral artery myocytes. Acta Neurochir Suppl. 2011;110:1–50. doi: 10.1007/978-3-7091-0353-1_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koide M, Nystoriak MA, Krishnamoorthy G, O'Connor KP, Bonev AD, Nelson MT, Wellman GC. Reduced Ca2+ spark activity after subarachnoid hemorrhage disables BK channel control of cerebral artery tone. J Cereb Blood Flow Metab. 2011;31:3–16. doi: 10.1038/jcbfm.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koide M, Penar PL, Tranmer BI, Wellman GC. Heparin-binding EGF-like growth factor mediates oxyhemoglobin-induced suppression of voltage-dependent potassium channels in rabbit cerebral artery myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1750–H1759. doi: 10.1152/ajpheart.00443.2007. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto S, Kishida K, Shimomura I, Maeda N, Nagaretani H, Matsuda M, Nishizawa H, Kihara S, Funahashi T, Matsuzawa Y, Yamada A, Yamashita S, Tamura S, Kawata S. Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochem Biophys Res Commun. 2002;292:781–786. doi: 10.1006/bbrc.2002.6720. [DOI] [PubMed] [Google Scholar]
- 10.Murakami K, Koide M, Dumont TM, Russell SR, Tranmer BI, Wellman GC. Subarachnoid Hemorrhage Induces Gliosis and Increased Expression of the Pro-inflammatory Cytokine High Mobility Group Box 1 Protein. Transl Stroke Res. 2011;2:72–79. doi: 10.1007/s12975-010-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–C8. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 12.Nystoriak MA, O'Connor KP, Sonkusare SK, Brayden JE, Nelson MT, Wellman GC. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol. 2011;300:H803–H812. doi: 10.1152/ajpheart.00760.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quan L, Sobey CG. Selective effects of subarachnoid hemorrhage on cerebral vascular responses to 4-aminopyridine in rats. Stroke. 2000;31:2460–2465. doi: 10.1161/01.str.31.10.2460. [DOI] [PubMed] [Google Scholar]
- 14.Wellman GC. Ion channels and calcium signaling in cerebral arteries following subarachnoid hemorrhage. Neurol Res. 2000;28:690–702. doi: 10.1179/016164106X151972. [DOI] [PubMed] [Google Scholar]