Abstract
Mutations in MYO7A cause Usher syndrome type 1B, a disorder involving profound congenital deafness and progressive blindness. In the retina, most of MYO7A is localized in the apical region of the RPE (retinal pigmented epithelial) cells, and a small amount is associated with the ciliary and periciliary membrane of the photoreceptor cells. Its roles appear to be quite varied. Studies with MYO7A-null mice indicate that MYO7A participates in the apical localization of RPE melanosomes and in the removal of phagosomes from the apical RPE for their delivery to lysosomes in the basal RPE. In the first role, MYO7A competes with microtubule motors, but, in the second one, it may function cooperatively. An additional role of MYO7A in the RPE is indicated from its requirement for light-dependent translocation of the ER-associated, visual cycle enzyme, RPE65, and normal functioning of the visual retinoid cycle. In photoreceptor cells, lacking MYO7A, opsin accumulates abnormally in the transition zone of the cilium, suggesting that MYO7A functions in a selective barrier for membrane proteins at the distal end of the transition zone. It is likely that the progressive retinal degeneration that occurs in Usher 1B patients results from a combination of cellular defects in the RPE and photoreceptor cells.
Keywords: Myosin VIIa, retina, melanosome, phagosome, endoplasmic reticulum, cilium
INTRODUCTION
Myosin VIIa (MYO7A) is an unconventional myosin that is required for normal hearing and vision; mutations in the MYO7A gene result in Usher syndrome type 1B [1], which is characterized by congenital profound hearing loss and progressive retinal degeneration [2]. Actin-based motor activity was first demonstrated with MYO7A, using protein purified from mouse testes and retinas [3]. More recently, the mechanism of MYO7A motor activity has been the focus of biochemical and structural studies, which indicate MYO7A is primarily a monomer [4, 5]. As a monomer, MYO7A seems less likely to function in organelle or protein transport – a process better suited for a dimeric motor. However, some recent evidence suggests that MYO7A may be dimerized following interaction with cargo molecules [6].
Our interest concerns the function of MYO7A in the retina. A major approach of our studies has involved mutant phenotype analyses of shaker1 mice, which have mutations in the orthologue of the Usher 1B gene [7]. These studies indicate that MYO7A function includes organelle transport, and so they have particular relevance to the general capabilities of MYO7A. The present brief review summaries the results of these phenotypic studies.
LOCALIZATION OF MYO7A IN THE RETINA
Most of the MYO7A in the retina is present in the retinal pigment epithelium (RPE), as first shown by Hasson et al [8]. Some is also present in the photoreceptor cells, although there has been some disagreement about its localization in these cells. The one region that has been immunolabeled [9] and associated with a mutant phenotype [10] is the connecting cilium, which is structurally similar to the transition zone of other cilia [11, 12]. Support for restriction to this region has also come from immunolocalization studies that have included null mutant retinas for controls [13], or involved overexpression following gene therapy treatment (own unpublished data). MYO7A appears to be associated with the membrane of the connecting cilium, as well as the periciliary membrane that forms a partial pocket around the connecting cilium [9, 14].
MELANOSOME MOTILITY
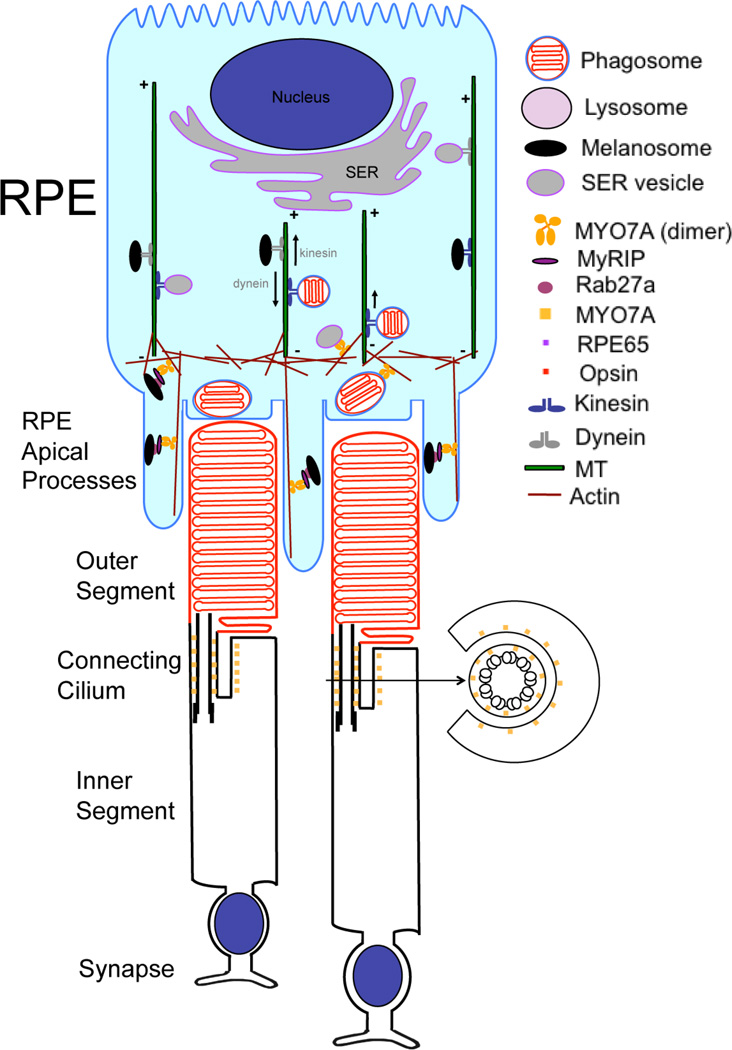
The most obvious defect in the retinas of shaker1 mice is that of melanosome mislocalization. In pigmented mammals, some of the melanosomes of the retinal pigment epithelium (RPE) extend into the apical processes that surround the tips of the photoreceptor outer segments. However, by microscopy, it is evident that this apical region is completely devoid of melanosomes in the RPE of shaker1 retinas, suggesting that a function of MYO7A is to move melanosomes into this region [15] (Fig. 1). In primary cultures of mouse RPE cells, melanosomes can be observed undergoing short movements. When the motility of melanosomes in wild-type cells is compared with that of shaker1 RPE cells, it is clear that the melanosomes in the mutant cells move faster and further [16].
Figure 1.
Illustration of MYO7A functions in an RPE cell and its localization in photoreceptor cells. MYO7A is present in the apical RPE where it is involved in the transport of melanosomes, phagosomes and smooth ER vesicles, in conjunction with microtubule motors. In photoreceptor cells, MYO7A is associated with the ciliary and periciliary plasma membranes.
This behavior resembles that of melanosomes in melanocytes. When MYO5A is lacking in melanocytes, melanosomes similarly move more rapidly [17]. Loss of MYO7A in the RPE or MYO5A in melanocytes allows melanosomes to travel more freely on microtubules, indicating that the role of these myosins in their respective cells is to capture the melanosomes from the microtubule motors and deliver them to the apical processes or dendrites, respectively. The comparison to MYO5A in melanocytes extends to the molecular linkage to the melanosome. Both motors require RAB27A on the melanosomes membrane, plus an exophilin protein that links the motor to RAB27A: melanophilin, in the case of MYO5A [18–21], and MYRIP, in the case of MYO7A [16, 22–25]. Interestingly, MYO5A has been demonstrated to function as a dimeric motor that progresses along actin filaments [26]. Since MYO7A and MYO5 have such comparable in vivo functions, it suggests that MYO7A in RPE cells may function in a mechanistically similar manner.
PHAGOSOME MOTILITY
Studies with Dictyostelium found that myosin VII was required for the initial stages of particle adhesion during phagocytosis [27]. Given the importance of phagocytosis of photoreceptor outer segment disk membranes by the RPE [28], as part of the turnover of the disk membranes [29], it was thus considered that a phagocytic defect might underlie the retinal pathology associated with Usher syndrome. However, in studies of shaker1 mice, the ingestion of disk membranes by the RPE appeared to be normal. Nevertheless, the disposal of the phagosomes was found to be defective. The phagosomes took longer to be cleared from the apical RPE to the basal RPE, and their digestion is slower [30]. The delivery of phagosomes from the apical to the basal RPE has been shown very vividly in the opossum retina, which has an RPE that is up to 100 µm deep. Colchicine blocks this delivery [31], suggesting the involvement of microtubule motors. A plausible role for MYO7A is that it transports phagosomes through the apical actin meshwork until they can be coupled to microtubule motors, as illustrated in Fig. 1. In this manner, MYO7A would be functioning cooperatively with microtubule motors – in contrast to the competitive nature of its role in capturing melanosomes from microtubule motor activity. Alternatively, in a less direct role, MYO7A might transport lysosomes to the apical RPE, so that lysosomes could fuse with the phagosomes, somehow enabling their release from the apical region. Support for this second hypothesis comes from the finding that some MYO7A is associated with lysosomes [32].
THE VISUAL CYCLE
Shaker1 retinas have been reported to have a mild electrophysiological defect [33], and a greater resistance to acute light damage [34]. The latter has been linked to a defect in the visual retinoid cycle. Following the absorption of a photon of light, the visual pigment becomes bleached. Regeneration of the visual pigment occurs through a series of reactions, known as the visual retinoid cycle [35–38]. A key step in this cycle is the reisomerization of the retinoid chromophore, a reaction that is catalyzed by the enzyme RPE65 [39–41]. RPE65 undergoes a light-dependent translocation within the RPE cells [34]. In shaker1 retinas, RPE65 does not undergo this translocation, and the visual cycle is inhibited, which, in turn, most likely affords the protection against acute light damage [34].
RPE65 associates with smooth ER in the RPE, suggesting that MYO7A might translocate the enzyme by transporting smooth ER tubules and vesicles. Interestingly, MYO5 has been shown to associate with and move ER membrane in a variety of systems, such as squid axon [42], budding yeast [43], and dendrites of Purkinje cells [44–47]. Perhaps MYO7A is comparable to MYO5 in an additional function (other than melanosome transport) – namely, ER membrane transport. ER tubules are also moved along microtubules by microtubule motors [48], so that if MYO7A proves to be an actin-based motor for ER in the RPE, its function is likely to be linked to that of microtubule motors, as appears to be the case in melanosomes and phagosome motility (Fig. 1).
OPSIN DELIVERY
The localization of MYO7A in the connecting cilium of photoreceptor cells suggests that it might participate in moving proteins along this connection between the inner and outer segments. Consistent with this suggestion, immunogold labeling of opsin of shaker1 retinas shows that, in the absence of functional MYO7A, abnormally high levels of opsin accumulate in the connecting cilium [10]. However, this phenotype could arise from a less direct link to opsin transport. Transport along cilia appears to be a highly regulated process, and proteins involved in this regulation, such as in the maintenance of a diffusion barrier [49], would be required for the normal distribution of proteins within the structure. Kinesin-2 motors seem to be the primary motors required for the transport of opsin along the connecting cilium – their absence has a much more severe effect on opsin delivery to the outer segment [50]. MYO7A’s role is more likely to be an auxiliary one.
CONCLUSION
Analyses of MYO7A-deficient mice indicate that MYO7A has a variety of roles in the RPE and photoreceptor cells of the mammalian retina. In the RPE at least, the roles appear to involve organelle transport. Each of the mutant phenotypes resulting from lack of MYO7A is likely to compromise the health of the retina, suggesting that retinal degeneration in Usher 1B patients results from a combination of different cellular defects.
Acknowledgments
Funding:
Studies on the retinal function of MYO7A in the Williams lab have been supported by a grant from the National Institutes of Health [EY07042].
Abbreviations
- RPE
retinal pigmented epithelium
- ER
endoplasmic reticulum
References
- 1.Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, Kelley PM, Kimberling WJ, Wagenaar M, Levi-Acobas F, Larget-Piet D, Munnich A, Steel KP, Brown SDM, Petit C. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 2.Smith RJ, Berlin CI, Hejtmancik JF, Keats BJ, Kimberling WJ, Lewis RA, Moller CG, Pelias MZ, Tranebjaerg L. Clinical diagnosis of the Usher syndromes. Usher Syndrome Consortium. Am J Med Genet. 1994;50:32–38. doi: 10.1002/ajmg.1320500107. [DOI] [PubMed] [Google Scholar]
- 3.Udovichenko IP, Gibbs D, Williams DS. Actin-based motor properties of native myosin VIIa. J Cell Sci. 2002;115:445–450. doi: 10.1242/jcs.115.2.445. [DOI] [PubMed] [Google Scholar]
- 4.Umeki N, Jung HS, Watanabe S, Sakai T, Li XD, Ikebe R, Craig R, Ikebe M. The tail binds to the head-neck domain, inhibiting ATPase activity of myosin VIIA. Proc Natl Acad Sci U S A. 2009;106:8483–8488. doi: 10.1073/pnas.0812930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Baboolal TG, Siththanandan V, Chen M, Walker ML, Knight PJ, Peckham M, Sellers JR. A FERM domain autoregulates Drosophila myosin 7a activity. Proc Natl Acad Sci U S A. 2009;106:4189–4194. doi: 10.1073/pnas.0808682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakai T, Umeki N, Ikebe R, Ikebe M. Cargo binding activates myosin VIIA motor function in cells. Proc Natl Acad Sci U S A. 2011;108:7028–7033. doi: 10.1073/pnas.1009188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M, Beisel KW, Steel KP, Brown SDM. A type VII myosin encoded by mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 8.Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci U S A. 1995;92:9815–9819. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Vansant G, Udovichenko IP, Wolfrum U, Williams DS. Myosin VIIa, the product of the Usher 1B syndrome gene, is concentrated in the connecting cilia of photoreceptor cells. Cell Motil. Cytoskel. 1997;37:240–252. doi: 10.1002/(SICI)1097-0169(1997)37:3<240::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Udovichenko IP, Brown SDM, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J. Neurosci. 1999;19:6267–6274. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Besharse JC, Horst CJ. The photoreceptor connecting cilium: a model for the transition zone. In: Bloodgood RA, editor. Ciliary and flagellar membranes. New York: Plenum Press; 1990. pp. 409–431. [Google Scholar]
- 12.Horst CJ, Johnson LV, Besharse JC. Transmembrane assemblage of the photoreceptor connecting cilium and motile cilium transition zone contain a common immunologic epitope. Cell Motil Cytoskeleton. 1990;17:329–344. doi: 10.1002/cm.970170408. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs D, Williams DS. Usher 1 protein complexes in the retina. Invest Ophthalmol Vis Sci. 2004 May 26;45 e-letter. [Google Scholar]
- 14.Williams DS. Usher syndrome: Animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res. 2008;48:433–441. doi: 10.1016/j.visres.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Ondek B, Williams DS. Mutant myosin VIIa causes defective melanosome distribution in the RPE of shaker-1 mice. Nat. Genet. 1998;19:117–118. doi: 10.1038/470. [DOI] [PubMed] [Google Scholar]
- 16.Gibbs D, Azarian SM, Lillo C, Kitamoto J, Klomp AE, Steel KP, Libby RT, Williams DS. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J Cell Sci. 2004;117:6473–6483. doi: 10.1242/jcs.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Bowers B, Rao K, Wei Q, Hammer JA., 3rd Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem. 2002;277:12432–12436. doi: 10.1074/jbc.C200005200. [DOI] [PubMed] [Google Scholar]
- 19.Nagashima K, Torii S, Yi Z, Igarashi M, Okamoto K, Takeuchi T, Izumi T. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett. 2002;517:233–238. doi: 10.1016/s0014-5793(02)02634-0. [DOI] [PubMed] [Google Scholar]
- 20.Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem. 2002;277:25423–25430. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- 21.Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA., 3rd Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4:271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 22.El-Amraoui A, Schonn JS, Kussel-Andermann P, Blanchard S, Desnos C, Henry JP, Wolfrum U, Darchen F, Petit C. MyRIP, a novel Rab effector, enables myosin VIIa recruitment to retinal melanosomes. EMBO Rep. 2002;3:463–470. doi: 10.1093/embo-reports/kvf090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Futter CE, Ramalho JS, Jaissle GB, Seeliger MW, Seabra MC. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol Biol Cell. 2004;15:2264–2275. doi: 10.1091/mbc.E03-10-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klomp AE, Teofilo K, Legacki E, Williams DS. Analysis of the linkage of MYRIP and MYO7A to melanosomes by RAB27A in retinal pigment epithelial cells. Cell Motil Cytoskeleton. 2007;64:474–487. doi: 10.1002/cm.20198. [DOI] [PubMed] [Google Scholar]
- 25.Lopes VS, Ramalho JS, Owen DM, Karl MO, Strauss O, Futter CE, Seabra MC. The ternary Rab27a-Myrip-Myosin VIIa complex regulates melanosome motility in the retinal pigment epithelium. Traffic. 2007;8:486–499. doi: 10.1111/j.1600-0854.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vale RD. Myosin V motor proteins: marching stepwise towards a mechanism. J Cell Biol. 2003;163:445–450. doi: 10.1083/jcb.200308093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuxworth RI, Weber I, Wessels D, Addicks GC, Soll DR, Gerisch G, Titus MA. A role for myosin VII in dynamic cell adhesion. Curr Biol. 2001;11:318–329. doi: 10.1016/s0960-9822(01)00097-5. [DOI] [PubMed] [Google Scholar]
- 28.Bok D, Hall MO. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci U S A. 2003;100:6481–6486. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herman KG, Steinberg RH. Phagosome movement and the diurnal pattern of phagocytosis in the tapetal retinal pigment epithelium of the opossum. Invest Ophthalmol Vis Sci. 1982;23:277–290. [PubMed] [Google Scholar]
- 32.Soni LE, Warren CM, Bucci C, Orten DJ, Hasson T. The unconventional myosin-VIIa associates with lysosomes. Cell Motil Cytoskeleton. 2005;62:13–26. doi: 10.1002/cm.20080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Libby RT, Steel KP. Electroretinographic anomalies in mice with mutations in Myo7a, the gene involved in human Usher syndrome type 1B. Invest Ophthalmol Vis Sci. 2001;42:770–778. [PubMed] [Google Scholar]
- 34.Lopes VS, Gibbs D, Libby RT, Aleman TS, Welch DL, Lillo C, Jacobson SG, Radu RA, Steel KP, Williams DS. The Usher 1B protein, MYO7A, is required for normal localization and function of the visual retinoid cycle enzyme, RPE65. Hum. Mol. Genet. 2011 doi: 10.1093/hmg/ddr155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wald G, Brown PK. Synthesis and bleaching of rhodopsin. Nature. 1956;177:174–176. doi: 10.1038/177174a0. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein PS, Law WC, Rando RR. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc Natl Acad Sci U S A. 1987;84:1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 38.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tabb JS, Molyneaux BJ, Cohen DL, Kuznetsov SA, Langford GM. Transport of ER vesicles on actin filaments in neurons by myosin V. J Cell Sci. 1998;111(Pt 21):3221–3234. doi: 10.1242/jcs.111.21.3221. [DOI] [PubMed] [Google Scholar]
- 43.Estrada P, Kim J, Coleman J, Walker L, Dunn B, Takizawa P, Novick P, Ferro-Novick S. Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J Cell Biol. 2003;163:1255–1266. doi: 10.1083/jcb.200304030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dekker-Ohno K, Hayasaka S, Takagishi Y, Oda S, Wakasugi N, Mikoshiba K, Inouye M, Yamamura H. Endoplasmic reticulum is missing in dendritic spines of Purkinje cells of the ataxic mutant rat. Brain Res. 1996;714:226–230. doi: 10.1016/0006-8993(95)01560-4. [DOI] [PubMed] [Google Scholar]
- 45.Takagishi Y, Oda S, Hayasaka S, Dekker-Ohno K, Shikata T, Inouye M, Yamamura H. The dilute-lethal (dl) gene attacks a Ca2+ store in the dendritic spine of Purkinje cells in mice. Neurosci Lett. 1996;215:169–172. doi: 10.1016/0304-3940(96)12967-0. [DOI] [PubMed] [Google Scholar]
- 46.Wagner W, Hammer JA., 3rd Myosin V and the endoplasmic reticulum: the connection grows. J Cell Biol. 2003;163:1193–1196. doi: 10.1083/jcb.200311077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner W, Brenowitz SD, Hammer JA., 3rd Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat Cell Biol. 2010;13:40–48. doi: 10.1038/ncb2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wozniak MJ, Bola B, Brownhill K, Yang YC, Levakova V, Allan VJ. Role of kinesin-1 and cytoplasmic dynein in endoplasmic reticulum movement in VERO cells. J Cell Sci. 2009;122:1979–1989. doi: 10.1242/jcs.041962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]