Abstract
Background
In adults with chronic kidney disease (CKD), cigarette smoking is associated with an increased risk for CKD progression and transplant failure. In children, secondhand smoke (SHS) exposure has been associated with elevated blood pressure. There are no studies on the prevalence and effect of SHS exposure in CKD.
Methods
Subjects were enrolled in the Chronic Kidney Disease in Children (CKiD) Study, an observational cohort of 366 children aged 1 to 16 years with CKD. Secondhand smoke exposure was obtained via questionnaire. SHS exposure was also determined based on urine cotinine (Ucot) measurements (1 ng/mL≤Ucot<75 ng/mL). The cross-sectional association of SHS exposure with proteinuria was assessed.
Results
Using Ucot, 22 % of subjects were exposed to SHS. SHS exposure was significantly associated with lower maternal education and African American race, and a greater prevalence of nephrotic range proteinuria and left ventricular hypertrophy. In a multivariate model (including sex, age, race, maternal education, income level, private insurance status, abnormal birth history and CKD diagnosis), the prevalence odds of nephrotic range proteinuria was 2.64, (95 % confidence interval 1.08, 6.42) higher in children exposed to SHS compared to those unexposed.
Conclusions
In our cohort of children with CKD, SHS exposure was common (22 %) and independently associated with nephrotic range proteinuria. Exposure to SHS may be an important factor to consider in CKD progression.
Keywords: Proteinuria, Tobacco use, Chronic kidney disease progression, Secondhand smoke exposure, Urine cotinine, Pediatric chronic kidney disease
Introduction
Tobacco use and secondhand smoke (SHS) exposure remain the leading preventable cause of death in the USA [1] and contribute to increased cardiovascular and kidney disease worldwide. Children with chronic kidney disease (CKD) are at an increased risk for cardiovascular morbidity and mortality due to a multitude of cardiovascular disease (CVD) risk factors, making this group highly susceptible to complications from smoking [2]. The use of tobacco products by adults with CKD is a risk factor for transplant nephropathy and progression of CKD to end-stage renal disease [3–6]. There is also evidence linking tobacco exposure to proteinuria in those with and without kidney disease [6, 7].
In children without CKD, SHS exposure has been associated with increased blood pressure (BP) load [8], endothelial dysfunction [9] and dyslipidemia [10, 11]. A recent publication revealed a high prevalence of tobacco use and SHS exposure in adolescents with CKD [12], suggesting that this may be an unrecognized risk factor for CVD and CKD progression.
To evaluate the effect of SHS exposure on health, an objective assessment of the level of SHS is required. Self-reported smoking and measured metabolites of nicotine in body fluids have been shown to be discrepant [13]. Hence, direct measurement of nicotine levels is preferable. Urine cotinine (Ucot) is a stable metabolite of nicotine that is not affected by the presence of other substances in urine, with a half-life of about 20 h and a molecular weight of 176.22 g/mol. Ucot measurement in urine is minimally affected by mild to moderate reductions in glomerular filtration rate (GFR) [14, 15] and any contribution from non-tobacco sources is nominal [16]. In addition, Ucot levels are highly correlated with plasma cotinine concentrations in adults with normal renal function [17]. A major advantage of Ucot over serum cotinine is that the cotinine concentration in urine can be up to fivefold higher than serum levels, thereby providing a more accurate measure in low level exposure conditions such as SHS [17].
Using a large cohort of children with mild to moderate CKD from the Chronic Kidney Disease in Children (CKiD) Study [18], we investigated the associations between Ucot levels and various renal and cardiovascular outcomes.
Materials and methods
Subjects were participants of the CKiD study, an observational cohort of children aged 1 to 16 years with an estimated GFR of between 30 and 90 mL/min per 1.73 m2 based on the Schwartz formula who were enrolled at 48 participating sites. Detailed enrollment characteristics have been published previously [18]. Specific CKD diagnosis was collected at baseline and categorized as glomerular or non-glomerular CKD. The specific CKD diagnoses and classifications have been previously published [19]. Exposure to SHS was obtained by parental questionnaire, while smoking status was collected from a self-administered survey among subjects aged ≥12 years at enrollment. The study was approved by the review board or ethics committee of all institution’s that recruited subjects and permission was obtained from the patients and/or parents who participated.
Urine samples were collected 6 months after enrollment, measurements of cardiovascular risk factors, including echocardiography, ambulatory BP monitoring and lipid panel data, were collected 1 year after enrollment.
Fresh urine was collected and processed as per the CKiD protocol. Frozen urine was batched and shipped on dry ice from the participating sites to the repository and stored at −70 °C. Ucot and urine creatinine (Ucr) concentrations were determined at the Clinical Pharmacology Laboratory, San Francisco General Hospital, University of California, San Francisco, CA. Liquid chromatography–tandem mass spectrometry (LC MS/MS) was used to quantify Ucot with a limit of quantification of 0.05 ng/mL [20].
Since evidence suggests there is no safe level of exposure to tobacco in children [21], this analysis used a limit of ≥1 ng/mL of Ucot to define exposure to SHS. This threshold corresponds to a serum cotinine level of 0.2 ng/mL [17]. Several studies have noted adverse health effects in children at this level of cotinine exposure [22, 23]. Ucot levels of ≥75 ng/mL defined subjects as active smokers and these subjects were excluded from the analysis [24].
The primary renal outcome of interest was proteinuria. Nephrotic range proteinuria was defined as a urine protein to creatinine ratio of ≥2.0. Cardiovascular outcomes were elevated BP (clinic and ambulatory), abnormal lipid levels, left ventricular hypertrophy (LVH) and inflammation [elevated wide-range C-reactive protein (wrCRP)]. Elevated clinic BP was defined as systolic BP (SBP) or diastolic BP (DBP) of ≥90th percentile for gender, height and age [25]. Ambulatory BP was considered abnormally elevated if the average wake or sleep SBP or DBP was ≥95th percentile [26] or wake or sleep load was ≥25 % [27]. Dyslipidemia was defined as triglycerides of >130 mg/dL, high-density lipoprotein-cholesterol (HDL-C) of <40 mg/dL or non-HDL-C of >160 mg/dL. LVH was defined as a left ventricular mass index of ≥95th percentile [28]. Elevated inflammatory processes were defined as a wrCRP of ≥3.0 mg/L and measured within 6 months of enrollment.
Statistical methods
Descriptive statistics of the demographic and clinical characteristics are presented as percentages (for categorical variables) or medians and interquartile ranges (for continuous variables). Differences between exposed and unexposed SHS groups were determined by Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables.
To quantify the association between exposure to SHS as determined by Ucot and the clinical outcomes of interest, standard logistic regression models were used to calculate prevalence odds ratios (OR). Each model was adjusted for variables identified a priori as important confounders: age, gender, race (African American or non-African American), maternal education as a measure of socioeconomic status (less than high school, high school or some college or college graduate), abnormal birth history (birth weight <2,500 g, small for gestational age [birth weight <10th percentile for gestational age] or premature birth [gestational age <36 weeks]) and CKD diagnosis (underlying glomerular or non-glomerular cause). The prevalence odds ratios (PrOR) correspond to the ratio of the odds of having a particular comorbidity among those exposed to SHS (1 ng/mL≤Ucot<75 ng/mL) to the odds among those not exposed to SHS (Ucot <1 ng/mL). To evaluate the impact of urine concentration on Ucot and since there is no Ucot/Ucr ratio that is accepted as a threshold to identify SHS exposure in those with CKD, we standardized Ucot to the median Ucr of the cohort, which was 0.4 (mg/mL). A threshold of 1 ng/mL Ucot divided by the median urine creatinine (0.4 mg/mL) was used to categorize exposure status to SHS in analyses, e.g., Ucot/Ucr ratio of ≥2.5 ng/mg was classified as exposed and that of <2.5 ng/mg as unexposed. We also analyzed Ucot and Ucot/Ucr data as continuous variables in the log scale. Finally, as a sensitivity analysis we incorporated self-reported exposure data to develop a stricter criteria, whereby exposed subjects were defined as those with both self-reported exposure and detectable cotinine; unexposed subjects were defined as those with self-reported unexposure and undetectable cotinine.
Statistical significance was assessed at the α=0.05 level. All analyses were conducted in SAS ver. 9.2 statistical software (SAS Institute, Cary, NC).
Results
At the time of analysis, 373 urine samples were available for Ucot measurement. Seven of these with levels >75 ng/mL were excluded from the analysis on SHS exposure because they were deemed to have been collected from active smokers. A total of 286 subjects (78 %) had Ucot of <1 ng/mL and were categorized as not exposed to SHS, including 49 subjects with Ucot below the detection limit. The complement comprising 80 (22 %) subjects with Ucot between 1 and 75 ng/mL were categorized as exposed to SHS. The median Ucot/Ucr ratio for the exposed versus unexposed subjects was significantly different (8.0 vs. 0.3, p<0.001).
Of the 366 subjects with Ucot of ≤75 ng/mL, self-reported data on SHS exposure was missing for one subject. Twenty-eight percent (n=102) reported exposure to SHS. Self-reported tobacco use data indicated 21 ever-smokers (4 current and 17 former smokers) among the adolescent subjects (age ≥12 years).
Table 1 presents the agreement between reported exposure status and exposure status based on Ucot. Forty-five percent of those who reported exposure did not have Ucot in the 1–75 ng/mL range, and 9 % of those who did not report SHS exposure had Ucot between 1 and 75 ng/mL. The kappa statistic was equal to 0.49 [95% confidence interval (CI) 0.39, 0.59], indicating moderate agreement between the self-reported data and measured Ucot levels.
Table 1.
Urine cotinine levels versus reported exposure to secondhand smoke with exposed and unexposed classificationsa
| Self-report classification | Ucot <1 ng/mL | Ucot 1–75 ng/mL | Total |
|---|---|---|---|
| Self-report unexposed | 239 | 24 | 263 (72 %) |
| Self-report exposed | 46 | 56 | 102 (28 %) |
| Total | 285 (78 %) | 80 (22 %) | 365 |
Ucot, Urine cotinine
Data are presented as the number of subjects with the marginal percentage in parenthesis
Kappa statistic=0.490 [95 % confidence interval (CI) 0.388, 0.592]
Total exposed=126 subjects; unexposed subjects=239
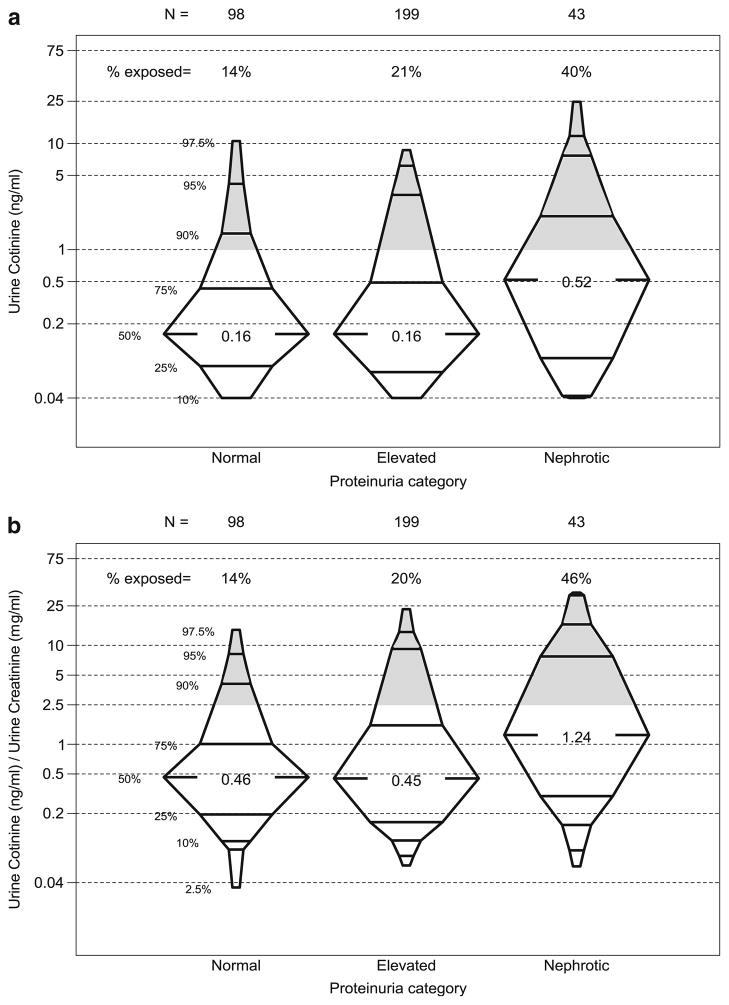
Table 2 provides descriptive statistics for the exposed and unexposed groups, based on Ucot levels, revealing significant clinical and demographic differences between groups. Compared to the unexposed group, the exposed group was older (12 vs. 10 years, p=0.04), was more likely to be female (53 vs. 37 %, p=0.02), have lower maternal education (12 vs. 14 years of education, p<0.01) be African American (34 vs. 15 %, p<0.01) and have a glomerular CKD diagnosis (29 vs. 18 %, p= 0.04). The exposed group had a higher prevalence of comorbidities and indicators of higher disease severity, including a higher prevalence of LVH (26 vs. 13 %, p=0.02), a higher urine protein-to-creatinine ratio (0.6 vs. 0.4, p<0.01), and a higher prevalence of nephrotic proteinuria (23 vs. 10 %, p<0.01). Figure 1a, b highlights this univariate association and demonstrates the differences in the distribution of Ucot and Ucot/Ucr levels by category of urine protein to creatinine ratio. The distribution of Ucot can be seen to be markedly shifted upward for those with nephrotic range proteinuria. The shaded portions of Fig. 1a, b show the proportion of subjects in each category of proteinuria exposed to SHS defined as Ucot >1 ng/mL and Ucot/Ucr >2.5, respectively.
Table 2.
Descriptive statistics between exposed and unexposed subjects
| Characteristica | Cotinine groups
|
p valueb | |
|---|---|---|---|
| Unexposed (Ucot <1 ng/mL) (n=286) | Exposed (Ucot 1–75 ng/mL) (n=80) | ||
| Age, years | 10.1 [6.8, 13.8] | 12.1 [8.1, 15.0] | 0.04* |
| Male | 63 % (179) | 48 % (38) | 0.02* |
| Race | <0.01* | ||
| Caucasian | 72 % (206) | 57 % (46) | |
| African American | 15 % (44) | 34 % (28) | |
| Other | 13 % (36) | 8 % (6) | |
| Maternal education, years | 14 [12, 16] | 12 [11, 14] | <0.01* |
| Glomerular CKD diagnosis | 18 % (51) | 29 % (23) | 0.04* |
| Iohexol-based GFR, mL/min|1.73 m2 | 43.4 [32.8, 56.8] | 44.7 [32.6, 55.7] | 0.90 |
| Serum creatinine, mg/dL | 1.2 [0.9, 1.7] | 1.3 [1.0, 1.8] | 0.21 |
| Urine protein:creatinine (Up/c) ratio | 0.4 [0.2, 1.0] | 0.6 [0.2, 1.8] | <0.01* |
| Proteinuria (Up/c) category | <0.01* | ||
| Elevated, 0.2≤Up/c<2.0 | 59 % (156) | 58 % (43) | |
| Nephrotic, Up/c≥2.0 | 10 % (26) | 23 % (17) | |
| Hemoglobin, g/dL | 12.4 [11.6, 13.5] | 12.5 [11.6, 13.6] | 0.87 |
| Age-sex specific height percentile | 25.3 [8.2, 54.6] | 16.0 [5.2, 43.4] | 0.18 |
| Age-sex specific weight percentile | 45.9 [18.5, 76.0] | 45.4 [11.8, 82.6] | 0.93 |
| Age-sex specific BMI percentile | 62.6 [35.1, 89.4] | 63.3 [37.7, 89.2] | 0.60 |
| BMI>85th percentile | 29 % (83) | 30 % (24) | 0.89 |
| Age-sex-height specific SBP percentile | 66 [36, 88] | 67 [47, 87] | 0.63 |
| SBP >95th percentile | 14 % (40) | 9 % (7) | 0.26 |
| Age-sex-height specific DBP percentile | 68 [44, 88] | 69 [53, 89] | 0.70 |
| DBP percentile >95th percentile | 13 % (37) | 9 % (7) | 0.43 |
| Cholesterol, mg/dLc | 175 [158, 200] | 175 [154, 192] | 0.27 |
| Total triglyceride, mg/dLc | 102 [74, 142] | 118 [75, 153] | 0.29 |
| HDL, mg/dLc | 49 [41, 56] | 45 [41, 55] | 0.15 |
| LDL, mg/dLc | 105 [84, 123] | 103 [81,117] | 0.22 |
| wrCRP groups | 0.06 | ||
| ≤0.3 mg/L | 59 % (161) | 46 % (35) | |
| 0.3–3.0 mg/L | 23 % (63) | 21 % (16) | |
| >3.0 mg/L | 17 % (47) | 33 % (25) | |
| Left ventricular mass, gc | 75 [52, 107] | 84 [62, 122] | 0.08 |
| Left ventricular hypertrophyc | 13 % (31) | 26 % (16) | 0.02* |
| ABPM statusc | 0.34 | ||
| Normotensive | 46 % (82) | 37 % (15) | |
| White coat | 5 % (9) | 5 % (2) | |
| Masked | 34 % (61) | 49 % (20) | |
| Hypertensive | 16 % (28) | 9 % (4) | |
Significant at p<0.05
CKD, Chronic kidney disease; GFR, glomerular filtration rate; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, LDL, high-, low-density lipoprotein, respectively; wrCRP, wide-range C-reactive protein; ABPM, ambulatory blood pressure monitoring
For continuous variables, data are presented as the median, with the interquartile range [QR] within square parentheses; for categorical variables, as the percentage with the frequency within round parentheses.
Missing data: maternal education, n=6; GFR, n=29; serum creatinine, n=2; Up/c ratio, n=13; hemoglobin, n=5; height percentile, n=7; weight percentile, n=1; BMI percentile, n=17; SBP percentile, n=11; SBP >95th percentile, n=10; DBP percentile, n=11; DBP >95th percentile, n=2; cholesterol, n=30; triglyceride, n=30; HDL, n=30; LDL, n=31; wrCRP group, n=19; left ventricular mass, n=68; left ventricular hypertrophy, n=68; ABPM status, n=145
The Wilcoxon rank sum test was used for continuous variables; the Fisher’s exact test was used for categorical variables
Baseline collection at first follow-up visit
Fig. 1.
a Urine cotinine levels vs. proteinuria category. b Urine cotinine/urine creatinine ratio vs. proteinuria category. Shaded area represents proportion of subjects in each category of proteinuria exposed to secondhand smoke
Table 3 presents the results from the multivariate logistic models describing the association between renal and cardiovascular outcomes and exposure to SHS adjusted for sex, age, race, maternal education, income level and having private insurance (as a proxy for socioeconomic status), abnormal birth history and CKD diagnosis. In general, exposure to SHS was associated with increased odds of having a comorbidity of interest, relative to those who were unexposed. Specifically, the odds of having nephrotic range proteinuria was 2.64-fold higher among those exposed to SHS compared to the unexposed group (PrOR 2.64, 95% CI 1.08, 6.42). The PrORs were consistently above 1.0 for all cardiovascular outcomes comparing those exposed to SHS versus unexposed, although the estimates did not reach statistical significance [PrOR =1.23 for elevated BP; PrOR=1.83 for LVH; PrOR= 2.39 for elevated wrCRP].
Table 3.
Prevalence odds ratios of markers of renal and cardiovascular risk factors
| Outcomes | PrOR of Ucot 1–75 ng/ml vs. Ucot<1 ng/ml | PrOR of Ucot/UCr≥2.5 ng/mg vs. Ucot/Ucr<2.5 ng/mg | PrOR for a 1 log increase in Ucot as a continuous variable | PrOR for a 1 log increase in Ucot/Ucr as a continuous variable |
|---|---|---|---|---|
| Renal outcome | ||||
| Nephrotic proteinuriaa | 2.64 (1.08, 6.42) | 2.66 (1.04, 6.76) | 1.20 (0.99, 1.46) | 1.35 (1.11, 1.65) |
| Cardiovascular outcomes | ||||
| Elevated BP | 1.23 (0.62, 2.41) | 0.91 (0.42, 1.99) | 0.98 (0.84, 1.15) | 1.00 (0.87, 1.17) |
| Abnormal ABPMb | 1.29 (0.57, 2.97) | 1.41 (0.59, 3.38) | 1.04 (0.87, 1.23) | 1.08 (0.90, 1.28) |
| Dyslipidemia | 1.21 (0.62, 2.37) | 1.15 (0.54, 2.43) | 1.05 (0.90, 1.22) | 1.09 (0.94, 1.27) |
| LVH | 1.83 (0.76, 4.43) | 0.95 (0.33, 2.71) | 1.07 (0.88, 1.31) | 1.07 (0.88, 1.31) |
| wrCRP≥3 | 2.39 (0.90, 6.39) | 1.36 (0.44, 4.22) | 1.03 (0.81, 1.31) | 1.06 (0.84, 1.34) |
PrOR, Prevalence odds ratio
Data are presented as the PrOR with the 95 % CI in parenthesis
Adjusted for sex, age, race, maternal education, income level and having private insurance (as proxies for socioeconomic status), abnormal birth history (low birth weight, small for gestational age or prematurity) and CKD diagnosis
This model also included adjustment for angiotensin converting enzyme inhibitor and angiotensin II receptor blocker use
This model was based on 218 subjects (184 with UCot≥1 ng/mL; 44 with UCot<1 ng/mL)
We repeated the analysis on exposure to SHS using the Ucot/Ucr ratio (Table 3). This approach assessed the robustness of inferences given differences in urine concentrations across individuals. Using a Ucot/Ucr cut-off ratio of 2.5 ng/mg, the estimated PrOR for the association between nephrotic range proteinuria and SHS exposure was 2.66 (95 % CI 1.04, 6.76), which was similar to the unstandardized Ucot analysis. The odds of having elevated, but not nephrotic range proteinuria, was 1.94-fold higher (95 % CI: 0.180, 4.71) among those exposed compared to those unexposed. The analyses using Ucot and the Ucot/Ucr ratio as continuous variables (as opposed to dichotomized exposed/unexposed) showed similar associations nephrotic range proteinuria.
A sensitivity analysis strict criteria of classifying exposed (Ucot 1–75 ng/mL + self-reported exposure, n=56) and unexposed (Ucot<1 ng/mL + no self-reported exposure, n=239) subjects revealed a PrOR of 2.21 (95% CI 0.79, 6. 17) for nephrotic range proteinuria.
Discussion
A major finding of this analysis is that exposure to SHS (as determined by Ucot) in children with mild to moderate CKD is independently associated with nephrotic range proteinuria. This is the first study linking SHS to increased proteinuria in children with CKD and adds to the list of important adverse health effects of tobacco in children. Reports that side stream smoke (smoke released directly from the tip of a burning cigarette into the air, the major component of SHS) is potentially more dangerous than main stream smoke (smoke inhaled by active smokers) [29, 30] underscore the health risks of SHS.
Tobacco smoke is known to cause significant mesangial proliferation, glomerulosclerosis and tubulointestitial fibrosis [31, 32] in animals. In humans, nicotine has been shown to promote mesangial cell and extracellular matrix production via recently discovered nicotinic receptors in mesangial tissue [33]. Apart from the direct effect of nicotine and its metabolites on renal tissue, tobacco smoke is known to contain over 4,000 chemicals including known toxic compounds [34]. Serum levels of carbon monoxide, arsenic, vinyl chloride, cadmium, lead and acrolein have been directly related to SHS exposure [35]. Exposure to low levels of environmental lead has been independently associated with CKD progression [36], masked hypertension and increased reactive oxygen species (ROS) accompanied by a reduction in urinary nitric oxide excretion [37]. Acrolein, an aldehyde present in large amounts in tobacco smoke, has been shown to induce apoptosis in renal tissue [38] and to generate ROS [39]. Other mechanisms by which SHS may contribute to proteinuria and CKD progression include induction of hypoxia, stimulation of pro-inflammatory cytokines, endothelial dysfunction and intrarenal vasoconstriction [3, 40]. Although we are unsure of the exact mechanism by which SHS exposure is associated with proteinuria in our cohort, we speculate that chronic exposure may have similar renal effects to those seen among active smokers.
Other studies that have evaluated tobacco exposure by Ucot in children have investigated cohorts with normal renal function and used different assays than ours for cotinine measurement, thus making comparisons difficult [41, 42]. Since this study investigated Ucot in children with CKD, it remains unclear if Ucot is a reliable measure of exposure in light of compromised renal function. Based on the absence of published data on the Ucot/Ucr level consistent with SHS exposure in those with CKD, we chose to analyze Ucot/Ucr both continuously and dichotomously, using a data-driven threshold for classification of exposure. Similar inferences were made regardless of the definition of exposure. It should be noted that dilute urine is common in children with non-glomerular CKD and this may explain the higher proportion of non-glomerular CKD among those unexposed (82 %) compared to those exposed (71 %; Table 2).
Research regarding metabolism and pharmacokinetics of nicotine in individuals with CKD is limited, and the degree to which cotinine clearance decreases with decreasing GFR is somewhat conflicting [14, 15]. However, results from the analysis using Ucot/Ucr were consistent with those from the main analysis of Ucot, suggesting that neither variability in renal function nor urine concentration had an impact on the results. It is unlikely that the noted association between nephrotic range proteinuria and SHS smoke exposure could be the result of protein binding of cotinine. Benowitz et al. [43] demonstrated minimal (only 2.5 %) binding of cotinine to serum proteins. Additionally, renal clearance of cotinine is increased in acidified urine [43]; however, we were unable to determine if this affected our Ucot results. More research in the pharmacokinetics and the metabolism of nicotine in those with CKD is clearly warranted in order to understand the nature of the cross sectional association found here.
As is clear from Table 1, self or parental report of exposure to SHS does not correlate well with measured cotinine levels. Although this discrepancy has been noted in the literature [13], our study is the first time it has been observed in a large pediatric CKD population and suggests that future studies evaluating the role of SHS in the pediatric CKD population should not be based solely on self or parental report. The discrepancy could also be the result of limitations in exposure assessment using Ucot. The limited half-life of Ucot may result in misclassification of sporadically exposed children or those who were not recently exposed. Despite the short half-life, cotinine is still the most widely used tobacco biomarker, and its efficacy has been validated in several peer-reviewed cross-sectional and longitudinal studies [13, 17, 20, 44–49], including CKD [14].
Similarly, active smokers may have been included in the analysis if their Ucot levels had declined to <75 ng/mL prior to testing. Relying on both self-report and high cotinine levels to define exposure (i.e. performing the analysis using only participants concordant on those assessments) did not impact the magnitude and direction of the association with proteinuria, although the reduction in sample size did render the results non-significant in the sensitivity analysis.
The modest association between cardiovascular outcomes and exposure was somewhat surprising, considering results from other studies. While the associations pointed towards a higher risk of cardiovascular outcomes, there were no significant effects. This should be interpreted cautiously as our data may not have been powered enough to detect differences. In addition, subjects could be on medications, such as antihypertensives, that alter BP and cardiac structure. The time difference of about 6 months between Ucot levels and the collection of cardiovascular parameters may have been a factor as well, although other studies have utilized cotinine levels to evaluate health effects for a longer period after cotinine was measured [9].
In the CKiD cohort, the prevalence of adolescent self-reported smoking was lower than that in the general population and also lower than that reported in a recent survey of teens with CKD [12]. The prevalence of SHS exposure was also low compared to reports of children with other chronic medical conditions [50]. Even with the use of Ucot, the prevalence of current smokers was low. The difference in prevalence may be the result of self-selection for participation in the CKiD study, whereby those that enroll represent a subset with healthier behaviors than those of the general population. Other potential causes include inaccurate answers to current or former smoking status as a result of the perceived stigma of smoking, abstinence from tobacco products within the 24 h prior to a study visit or misclassification of smokers as exposed to SHS due to limitations of Ucot evaluation in those with CKD. Nonetheless, the implications of tobacco use among adolescents with CKD should not be underestimated considering the association of high risk behavior and medication non-compliance with tobacco use in teenagers with a chronic illness [51].
There are several limitations to this cross-sectional analysis that should be noted. Detailed data on exposure histories that would have allowed us to quantify exposure frequency and exposure dose were unavailable. We also did not have serum cotinine levels, which would have provided a confirmatory exposure level given the limitations of Ucot. However, interpreting serum cotinine levels can be complicated based on its dependency on the volume of distribution at steady state [15]. Although we adjusted for potential cofounders, it is also feasible that residual confounding factors, such as GFR differences or medications other than angiotensin converting enzyme inhibitor, could have accounted for our findings. However, the CKiD study collects rich data on factors that describe socioeconomic status—a potentially strong confounder in the current analysis—and the models were adjusted for race, income, insurance status and maternal education. Despite the above limitations, this study highlights the largely unaddressed impact SHS may have in pediatric CKD.
In summary, SHS exposure via self report is common in the CKiD cohort. Using Ucot as a reflection of exposure, SHS may be associated with nephrotic range proteinuria independent of important confounders. A longitudinal investigation of this cohort to better understand SHS exposure as a potentially important determinant of renal and cardiovascular outcomes in the pediatric CKD population is warranted.
Acknowledgments
We would like to acknowledge the assistance of Adrienne Stolfi in the conceptualization of the project. CKiD is funded by the NIDDK, with additional funding from NINDS, NICHD and NHLBI (U01-DK-66143, U01-DK-66174, and U01-DK-66116). The Clinical Pharmacology Laboratory at the University of California San Francisco receives support from the NIH (S10 RR026437 and P30 DA012393). Funding for urine cotinine and creatinine evaluation was provided by the Research Foundation of Dayton Children’s Medical Center, Dayton, Ohio, USA.
Footnotes
The study was presented in part as a platform presentation at the American Society of Pediatric Nephrology Annual Meeting 2011 held at Denver, Colorado.
Contributor Information
Abiodun Omoloja, Email: omolojaa@childrensdayton.org, Department of Pediatrics, Wright State University, One Children’s Plaza, Dayton, OH 45404, USA.
Judith Jerry-Fluker, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Derek K. Ng, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Alison G. Abraham, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
Susan Furth, Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Bradley A. Warady, Children’s Mercy Hospital, Kansas City, MO, USA
Mark Mitsnefes, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
References
- 1.Marshall L, Schooley M, Ryan H, Cox P, Easton A, Healton C, Jackson K, Davis KC, Homsi G Centers for Disease Control and Prevention . Youth tobacco surveillance-united states, 2001–2002. MMWR Surveill Summ. 2006;55:1–56. [PubMed] [Google Scholar]
- 2.Mitsnefes MM. Cardiovascular complications of pediatric chronic kidney disease. Pediatr Nephrol. 2008;23:27–39. doi: 10.1007/s00467-006-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol. 2008;3:226–236. doi: 10.2215/CJN.03740907. [DOI] [PubMed] [Google Scholar]
- 4.Zitt N, Kollerits B, Neyer U, Mark W, Heininger D, Mayer G, Kronenberg F, Lhotta K. Cigarette smoking and chronic allograft nephropathy. Nephrol Dial Transplant. 2007;22:3034–3039. doi: 10.1093/ndt/gfm275. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto R, Nagasawa Y, Shoji T, Iwatani H, Hamano T, Kawada N, Inoue K, Uehata T, Kaneko T, Okada N, Moriyama T, Horio M, Yamauchi A, Tsubakihara Y, Imai E, Rakugi H, Isaka Y. Cigarette smoking and progression of IgA nephropathy. Am J Kidney Dis. 2010;56:313–324. doi: 10.1053/j.ajkd.2010.02.351. [DOI] [PubMed] [Google Scholar]
- 6.Jones-Burton C, Seliger SL, Scherer RW, Mishra SI, Vessal G, Brown J, Weir MR, Fink JC. Cigarette smoking and incident chronic kidney disease: a systematic review. Am J Nephrol. 2007;27:342–351. doi: 10.1159/000103382. [DOI] [PubMed] [Google Scholar]
- 7.Hogan SL, Vupputuri S, Guo X, Cai J, Colindres RE, Heiss G, Coresh J. Association of cigarette smoking with albuminuria in the united states: the third national health and nutrition examination survey. Ren Fail. 2007;29:133–142. doi: 10.1080/08860220601098888. [DOI] [PubMed] [Google Scholar]
- 8.Pijanowska M, Zajaczkowska M. Passive smoking and patterns of 24-hour ambulatory blood pressure in healthy children. Pol Merkur Lekarski. 2004;16:320–322. [PubMed] [Google Scholar]
- 9.Kallio K, Jokinen E, Raitakari OT, Hamalainen M, Siltala M, Volanen I, Kaitosaari T, Viikari J, Ronnemaa T, Simell O. Tobacco smoke exposure is associated with attenuated endothelial function in 11-year-old healthy children. Circulation. 2007;115:3205–3212. doi: 10.1161/CIRCULATIONAHA.106.674804. [DOI] [PubMed] [Google Scholar]
- 10.Neufeld EJ, Mietus-Snyder M, Beiser AS, Baker AL, Newburger JW. Passive cigarette smoking and reduced HDL cholesterol levels in children with high-risk lipid profiles. Circulation. 1997;96:1403–1407. doi: 10.1161/01.cir.96.5.1403. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz WB, Schwartz PF, Schieken RM. Childhood passive smoking, race, and coronary artery disease risk: the MCV twin study. Medical college of Virginia. Arch Pediatr Adolesc Med. 1999;153:446–453. doi: 10.1001/archpedi.153.5.446. [DOI] [PubMed] [Google Scholar]
- 12.Omoloja A, Chand D, Greenbaum L, Wilson A, Bastian V, Ferris M, Bernert J, Stolfi A, Patel H. Cigarette smoking and second-hand smoking exposure in adolescents with chronic kidney disease: a study from the Midwest pediatric nephrology consortium. Nephrol Dial Transplant. 2010;26:908–913. doi: 10.1093/ndt/gfq475. [DOI] [PubMed] [Google Scholar]
- 13.Caraballo RS, Giovino GA, Pechacek TF. Self-reported cigarette smoking vs. serum cotinine among U.S. adolescents. Nicotine Tob Res. 2004;6:19–25. doi: 10.1080/14622200310001656821. [DOI] [PubMed] [Google Scholar]
- 14.Jones-Burton C, Vessal G, Brown J, Dowling TC, Fink JC. Urinary cotinine as an objective measure of cigarette smoking in chronic kidney disease. Nephrol Dial Transplant. 2007;22:1950–1954. doi: 10.1093/ndt/gfm075. [DOI] [PubMed] [Google Scholar]
- 15.Molander L, Hansson A, Lunell E, Alainentalo L, Hoffmann M, Larsson R. Pharmacokinetics of nicotine in kidney failure. Clin Pharmacol Ther. 2000;68:250–260. doi: 10.1067/mcp.2000.109006. [DOI] [PubMed] [Google Scholar]
- 16.Domino EF. Nontobacco sources of cotinine in the urine of nonsmokers. Clin Pharmacol Ther. 1995;57:479. doi: 10.1016/0009-9236(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 17.Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P., 3rd Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res. 2009;11:954–960. doi: 10.1093/ntr/ntp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Muñoz A, Warady BA. Design and methods of the chronic kidney disease in children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA Chronic Kidney Disease in Children Study Group . Blood pressure in children with chronic kidney disease: a report from the chronic kidney disease in children study. Hypertension. 2008;52:631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernert JT, Jr, Turner WE, Pirkle JL, Sosnoff CS, Akins JR, Waldrep MK, Ann Q, Covey TR, Whitfield WE, Gunter EW, Miller BB, Patterson DG, Jr, Needham LL, Hannon WH, Sampson EJ. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43:2281–2291. [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 22.Kallio K, Jokinen E, Hamalainen M, Saarinen M, Volanen I, Kaitosaari T, Viikari J, Ronnemaa T, Simell O, Raitakari OT. Decreased aortic elasticity in healthy 11-year-old children exposed to tobacco smoke. Pediatrics. 2009;123:e267–e273. doi: 10.1542/peds.2008-2659. [DOI] [PubMed] [Google Scholar]
- 23.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect. 2005;113:98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the third national health and nutrition examination survey, 1988 to 1991. JAMA. 1996;275:1233–1240. [PubMed] [Google Scholar]
- 25.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 26.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 27.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee . Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American heart association atherosclerosis, hypertension, and obesity in youth committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433–451. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 28.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709–714. doi: 10.1016/j.echo.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Rubenstein D, Jesty J, Bluestein D. Differences between mainstream and sidestream cigarette smoke extracts and nicotine in the activation of platelets under static and flow conditions. Circulation. 2004;109:78–83. doi: 10.1161/01.CIR.0000108395.12766.25. [DOI] [PubMed] [Google Scholar]
- 30.Hoegg UR. Cigarette smoke in closed spaces. Environ Health Perspect. 1972;2:117–128. doi: 10.1289/ehp.7202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obert DM, Hua P, Pilkerton ME, Feng W, Jaimes EA. Environmental tobacco smoke furthers progression of diabetic nephropathy. Am J Med Sci. 2011;341:126–130. doi: 10.1097/MAJ.0b013e3181f6e3bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boor P, Casper S, Celec P, Hurbankova M, Beno M, Heidland A, Amann K, Sebekova K. Renal, vascular and cardiac fibrosis in rats exposed to passive smoking and industrial dust fibre amo-site. J Cell Mol Med. 2009;13:4484–4491. doi: 10.1111/j.1582-4934.2008.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaimes EA, Tian RX, Raij L. Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol. 2007;292:H76–H82. doi: 10.1152/ajpheart.00693.2006. [DOI] [PubMed] [Google Scholar]
- 34.Taylor AE, Johnson DC, Kazemi H. Environmental tobacco smoke and cardiovascular disease. A position paper from the council on cardiopulmonary and critical care, American Heart Association. Circulation. 1992;86:699–702. doi: 10.1161/01.cir.86.2.699. [DOI] [PubMed] [Google Scholar]
- 35.Mannino DM, Homa DM, Matte T, Hernandez-Avila M. Active and passive smoking and blood lead levels in U.S. adults: data from the third national health and nutrition examination survey. Nicotine Tob Res. 2005;7:557–564. doi: 10.1080/14622200500185264. [DOI] [PubMed] [Google Scholar]
- 36.Yu CC, Lin JL, Lin-Tan DT. Environmental exposure to lead and progression of chronic renal diseases: a four-year prospective longitudinal study. J Am Soc Nephrol. 2004;15:1016–1022. doi: 10.1097/01.asn.0000118529.01681.4f. [DOI] [PubMed] [Google Scholar]
- 37.Vaziri ND, Ding Y, Ni Z, Gonick HC. Altered nitric oxide metabolism and increased oxygen free radical activity in lead-induced hypertension: effect of lazaroid therapy. Kidney Int. 1997;52:1042–1046. doi: 10.1038/ki.1997.426. [DOI] [PubMed] [Google Scholar]
- 38.Schwerdt G, Gordjani N, Benesic A, Freudinger R, Wollny B, Kirchhoff A, Gekle M. Chloroacetaldehyde- and acrolein-induced death of human proximal tubule cells. Pediatr Nephrol. 2006;21:60–67. doi: 10.1007/s00467-005-2006-6. [DOI] [PubMed] [Google Scholar]
- 39.Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–1036. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- 40.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 41.Wakefield M, Banham D, Martin J, Ruffin R, McCaul K, Badcock N. Restrictions on smoking at home and urinary cotinine levels among children with asthma. Am J Prev Med. 2000;19:188–192. doi: 10.1016/s0749-3797(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 42.Oddoze C, Dubus JC, Badier M, Thirion X, Pauli AM, Pastor J, Bruguerolle B. Urinary cotinine and exposure to parental smoking in a population of children with asthma. Clin Chem. 1999;45:505–509. [PubMed] [Google Scholar]
- 43.Benowitz NL, Kuyt F, Jacob P, 3rd, Jones RT, Osman AL. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34:604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Stanton B, Hopper J, Khankari N. Sources, locations, and predictors of environmental tobacco smoke exposure among young children from inner-city families. J Pediatr Health Care. 2011;25:365–372. doi: 10.1016/j.pedhc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 45.Avila-Tang E, Al-Delaimy WK, Ashley DL, Benowitz N, Bernert JT, Kim S, Samet JM, Hecht SS. Assessing secondhand smoke using biological markers. Tob Control. 2012;1:1–2. doi: 10.1136/tobaccocontrol-2011-050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto A, Matsumoto A, Ichiba M, Payton NM, Oishi H, Hara M. Simultaneous measurement of urinary total nicotine and cotinine as biomarkers of active and passive smoking among Japanese individuals. Environ Health Prev Med. 2012;1:1–2. doi: 10.1007/s12199-012-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain R, Balhara YP, Jhanjee S, Sethi H. Concordance between urinary cotinine levels and self-reported tobacco use among drug-dependent persons: a pilot study. Subst Abus. 2012;33:99–102. doi: 10.1080/08897077.2011.630947. [DOI] [PubMed] [Google Scholar]
- 48.Kramer U, Lemmen CH, Behrendt H, Link E, Schafer T, Gostomzyk J, Scherer G, Ring J. The effect of environmental tobacco smoke on eczema and allergic sensitization in children. Br J Dermatol. 2004;150:111–118. doi: 10.1111/j.1365-2133.2004.05710.x. [DOI] [PubMed] [Google Scholar]
- 49.Dolcini MM, Adler NE, Lee P, Bauman KE. An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine Tob Res. 2003;5:473–483. [PubMed] [Google Scholar]
- 50.Tyc VL, Hovell MF, Winickoff J. Reducing secondhand smoke exposure among children and adolescents: emerging issues for intervening with medically at-risk youth. J Pediatr Psychol. 2008;33:145–155. doi: 10.1093/jpepsy/jsm135. [DOI] [PubMed] [Google Scholar]
- 51.Zbikowski SM, Klesges RC, Robinson LA, Alfano CM. Risk factors for smoking among adolescents with asthma. J Adolesc Health. 2002;30:279–287. doi: 10.1016/s1054-139x(01)00394-9. [DOI] [PubMed] [Google Scholar]