Introduction
Asthma is a prevalent and heterogeneous disease that not only has a marked effect on the quality of life of affected patients but also imparts a significant economic burden on society. The term “refractory asthma” (RA) is used for patients with persistent asthma symptoms in whom co-morbidities have been treated, triggers addressed, compliance with treatment evaluated, and alternative diagnoses excluded(1). Various phenotypes of chronic asthma with persistent inflammation have been recognized, and a link between bacterial infections and RA has emerged(2–4). Some studies have implicated Mycoplasma pneumoniae (Mp) in the initiation and persistence of asthma, although its precise role and pathogenic mechanisms remain elusive(5,6). Prior studies on the role of Mp in asthma have been limited by the inability to consistently culture this organism, the poor performance of Mp serology in defining active infection, and variable sensitivities of PCR assays in detecting Mp.
Our group has identified a 68-kDa protein unique to Mp called the Community Acquired Respiratory Distress Syndrome Toxin (CARDS Tx) that possesses adenosine diphosphate -ribosyltransferase activity similar to pertussis toxin(7). We have developed assays to detect CARDS Tx by PCR and antigen capture (AC) and to detect IgM and IgG antibodies directed against CARDS Tx(8–10). CARDS Tx gene sequences are more sensitive for the detection of Mp by PCR than other sequences such as P1 adhesin (P1) and ATPase(11,12).
We studied 143 (53 acute asthma, 26 refractory asthma, 64 healthy controls) pediatric subjects to define the prevalence of Mp using CARDS Tx- and P1-specific assays. The purpose of this study was to identify the frequency and persistence of Mp in respiratory secretions by measuring CARDS Tx protein by AC, as well as both CARDS Tx and P1 by PCR. In addition, we evaluated IgG and IgM antibody levels to CARDS Tx and P1, exhaled breath condensate (EBC) pH and asthma control and quality of life.
Methods
Study Subjects
This single-center, prospective cohort study was conducted from December 2009 through June 2011 and was approved by the institutional review board. We obtained written informed consent from the parent or legal guardian of each subject. We enrolled three groups of children 5–17 years of age: 1) children hospitalized for an acute exacerbation of asthma, 2) children with refractory asthma (poorly controlled moderate-severe persistent asthma who met RA criteria described above), and 3) healthy children without asthma (controls). We enrolled subjects from the inpatient setting (acute asthma) or from pulmonary or general pediatric clinics during routine visits (groups 2 and 3, respectively). The diagnosis of asthma was based on physician assessment according to national guidelines(13). Exclusion criteria included: pneumonia, bleeding disorders, pulmonary disease other than asthma, other serious medical conditions (e.g. cerebral palsy, pregnancy, malignancy, cystic fibrosis) and, for healthy control subjects only, any infection within the month prior to enrollment. Subjects with asthma were monitored for 2–5 follow-up visits over a 12 month period of time. We examined data at enrollment by study group and over time.
Specimens were collected from the throat and nasopharynx using nylon-tipped swabs (Copan, Murrieta, CA), and blood to measure CARDS Tx by AC and PCR, P1 by PCR and serum IgG and IgM antibody responses to CARDS Tx and P1. We examined the persistence of Mp and corresponding antibody responses in subjects with at least 3 visits. We collected EBC using the RTube (Respiratory Research, Austin, TX) and measured EBC pH with an Orion Ross® Micro pH electrode (Thermo Scientific, Beverly, MA) after argon degassing as described(14). Throat and nasopharyngeal swabs were placed in transport media immediately following specimen collection. All samples were stored at 4 degrees C, transported within 24 hours for processing, and subsequently stored at −20 degrees C (serum, throat, nasopharyngeal) or −80°C (exhaled breath condensate) until analyzed.
Surveys
For subjects with asthma, we measured asthma symptoms (Asthma Control Test [ACT]) (15,16) and quality of life (Pediatric Asthma Quality of Life Questionnaire [PAQLQ](17). Subjects with asthma returned for 2–5 follow-up visits. The Childhood Asthma Control Test (C-ACT) was administered to children less than 12 years of age; older children completed the ACT. Scores below 20 on either questionnaire indicate poor asthma control over the past 4 weeks. Subjects 7 years of age and older completed the PAQLQ. Scores on this questionnaire range from 1–7. Higher scores indicate better quality of life over the past 7 days; the minimal clinically important difference in scores is 0.5. We examined asthma symptom control (ACT) and quality of life at enrollment by group. In addition, we examined these variables by combining all visits for subjects with asthma, excluding the enrollment visit for the acute asthma group. We recorded exposure to environmental tobacco smoke (parent report) for all subjects and hospital length of stay (medical record review) for the acute asthma group.
Detection of Mp DNA, protein and antibodies
DNA from airway and serum samples was purified using the QIAmp DNA Mini Kit (Qiagen, Valencia, CA). Real-time PCR for CARDS Tx (MPN372) and P1 (MPN141) was performed as described(11,12). CARDS Tx protein was detected and quantified using AC ELISA methods as previously described(10). Because CARDS Tx shares limited amino acid sequence identity with the S1 subunit of Bordetella pertussis toxin (7), we examined the cross-reactivity of CARDS Tx-specific reagents to pertussis toxin, and none was detected (data not shown). The detection of antibodies against recombinant CARDS Tx and recombinant P1 immunodominantcarboxy domain was performed using ELISA as described(7,9,10,18). Mp positive subjects were defined as those with any PCR or AC value in a detectable range, and Mp negative subjects were defined as those in whom all testing was negative.
Statistical analysis
Data were analyzed using SPSS (ver 19.0). We examined data for normality as well as for other specific test assumptions, such as homogeneity of variance. If variables did not meet the assumption of normality, a non-parametric test was used. Statistical differences between group means were determined using parametric (i.e. two-tailed T test or one-way analysis of variance) or non-parametric analyses (i.e., Mann Whitney U). Differences in categorical data were determined using Pearson’s Chi-Squared tests. Correlation analyses (i.e., Pearson’s r and Kendall’s tau) were performed to test for significant relationships between variables. For categorical variables, the Kendall tau rank correlation coefficient was used. Results for normally distributed variables were expressed as means ± standard deviation. We used non-parametric test for non-normally distributed variables and reported results as median and 25–75 percentile interquartile range (IQR). P values of less than or equal to 0.05 were considered to indicate statistically significant differences and associations.
Results
We enrolled 143 subjects (mean age 10.33 ± 3.13 years, range 5–17; 62% male) with and without asthma from an ethnically diverse population (57% White, 14% Black, 2.8% Asian, 2.1% American Indian, 24.5% Other; 81% Hispanic ethnicity). We enrolled 53 subjects with an acute exacerbation of asthma requiring hospitalization, 26 subjects with refractory asthma (poorly controlled moderate to severe persistent asthma), and 64 healthy control subjects with no history of asthma and no symptoms of a respiratory tract infection within the prior four weeks. Baseline demographic characteristics were compared across groups. Significant differences were found in gender and BMI (Table I).
Table I.
Baseline Characteristics
| Study Group | ||||
|---|---|---|---|---|
| Acute Asthma (n=53) | Refractory Asthma (n= 26) | Healthy Controls (n=64) | P value | |
| Age (years)1 | 9.53 ± 3.02 | 10.58 ± 2.64 | 10.90 ± 3.3 | .057 |
| Race, no. (%) | .15 | |||
| White | 29 (54.7) | 10 (38.5) | 42 (65.6) | |
| Black | 10 (18.9) | 6 (23.1) | 4 (6.3) | |
| American Indian | 1 (1.9) | 0 (0) | 2 (3.1) | |
| Asian | 2 (3.8) | 0 (0) | 2 (3.1) | |
| Other | 11 (20.8) | 10 (38.5) | 14 (21.9) | |
| Hispanic Ethnicity, no. (%) | 40 (75.5) | 19 (73.1) | 57 (89.1) | .089 |
| Male Sex, no. (%) | 38 (71.7) | 20 (76.9) | 31 (48.4) | .008 |
| BMI Percentile | 62.81± 36.8 | 79.14 ± 28.4 | 76.49 ± 26.9 | .035 |
| ETS Exposure, no. (%) | 19 (35.8) | 6 (23.1) | 14 (22.2) | .22 |
| Mycoplasma Positive, no.(%) | 34 (64.2) | 17 (65.4) | 36 (56.3) | .60 |
| ACT Scores < 20, no. (%) | 41 (64.2) | 25(51.0) | N/A | .050 |
| PAQLQ Score | 3.9 ± 1.2 | 4.7 ± 1.5 | N/A | .011 |
Numbers represent means ± standard deviations, unless otherwise noted
Abbreviations: ACT, asthma control test; BMI Percentile (body mass index) percentile (calculated for age and gender); ETS, environmental tobacco smoke; PAQLQ, Pediatric Asthma Quality of Life Questionnaire.
Using CARDS Tx AC and CARDS Tx and P1 PCR, Mp was detected in 64% of subjects with acute asthma, 65% of those with refractory asthma, and 56% of healthy controls (Table I), with no significant differences among the three groups. The proportion of positive samples varied by method (AC vs. PCR) and sampling site (nasopharynx, throat, and serum) (See eTable 1 in the Online Repository). In all subjects, CARDS Tx AC was more sensitive than any PCR (55% vs. 16%, P=.04), and CARDS Tx PCR was more sensitive than P1 PCR (14% vs. 5.1%, P<.001). Regardless of test, nasopharyngeal specimens were more likely to be positive than other sites e.g. comparing nasopharyngeal versus throat samples by AC (51.8% vs. 17%, P<.001). We have previously observed discordance between the PCR and AC assays and between different sites of collection (nasal, throat, sputum)(19). Additionally, in a separate study, we identified a subject with confirmed detection of Mp by immunofluorescence to both CARDS Tx and P1 in a sputum sample, but who was PCR negative (unpublished observations).
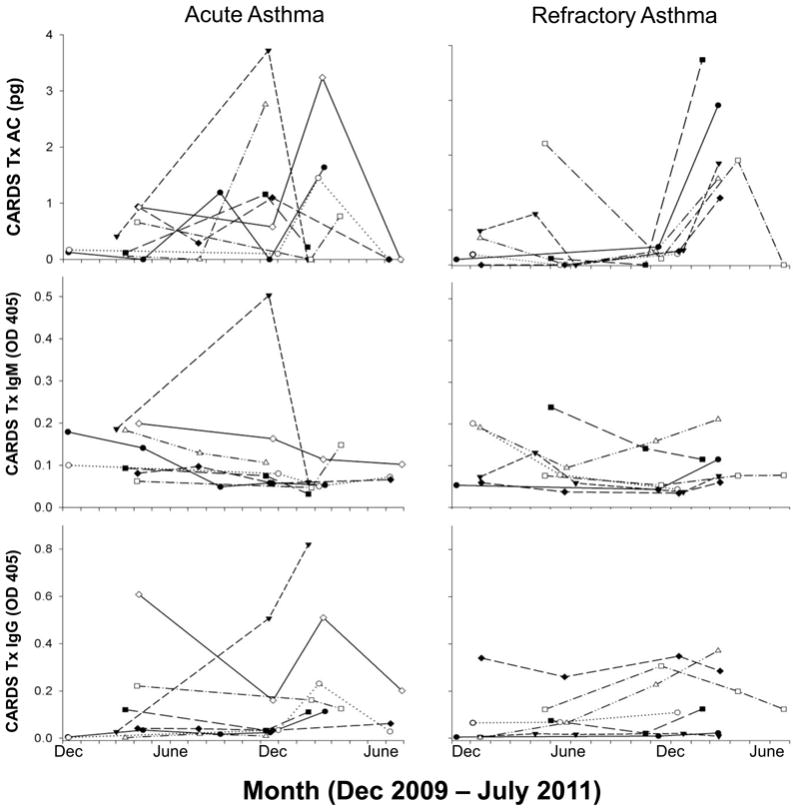
Subjects with asthma were monitored for 2–5 follow-up visits while control subjects had a single visit. While there were no differences in the proportion of subjects testing positive for Mp among the three groups, there was a distinct seasonal variation in the detection of Mp, with the highest rates occurring from December to March and the lowest from June to October (Figure 1). The proportion of subjects with asthma that tested positive was slightly higher during the summer months compared to controls. Many asthma subjects, who were initially positive for CARDS Tx, had positive testing on follow-up visits (Figure 2). Some individuals were persistently positive for CARDS Tx AC throughout the study while some had negative testing during the summer months but were again positive during the following winter/spring season. Despite this persistence of CARDS Tx detection, most subjects with asthma failed to demonstrate significant IgM or IgG antibody levels to CARDS Tx. However, a few subjects clearly demonstrated robust antibody responses that paralleled the CARDS Tx levels measured by AC (Figure 2).
Figure 1.
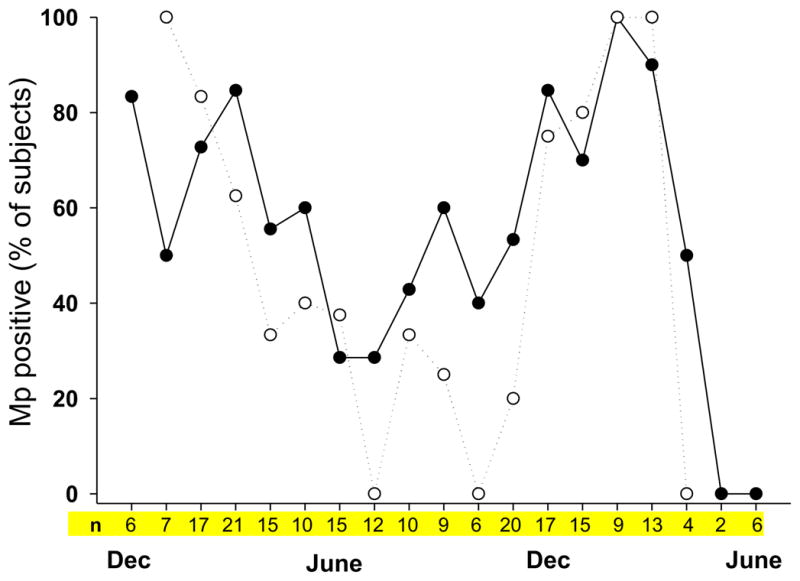
Figure 2.
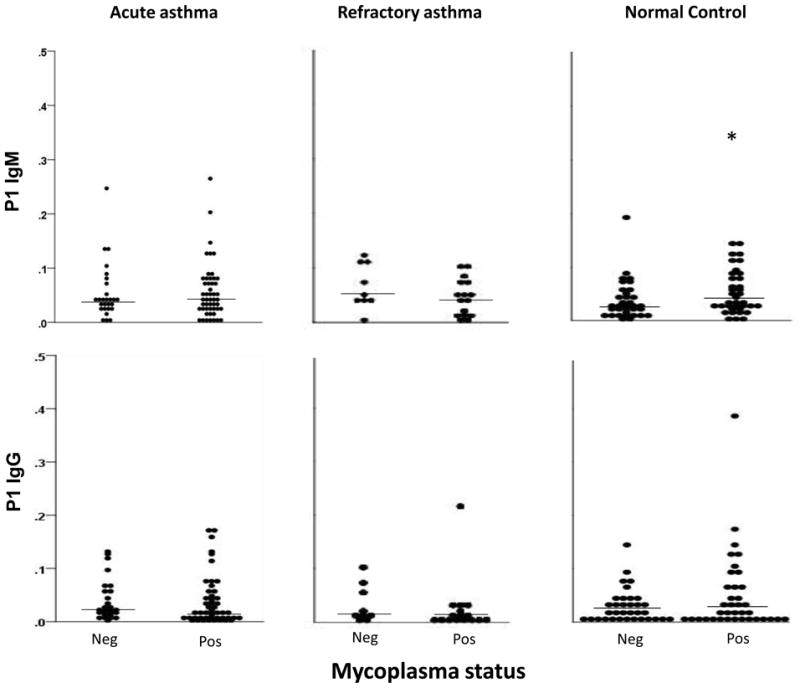
Overall, most subjects with asthma had low serum IgM and IgG levels to Mp (Figures 3 and 4) that were not significantly different between Mp positive and negative subjects for either visit 1 (Figures 3 and 4) or all visits combined. In healthy control subjects, IgM levels directed against P1 were significantly higher in Mp positive versus Mp negative subjects (0.049 [0.027–0.09 IQR] vs. 0.026 [0.014–0.052 IQR], P=.048). IgG levels to CARDS Tx were also slightly higher in Mp positive compared to Mp negative subjects but not significantly (0.080 [0.029–0.282 IQR] vs. 0.059 [0.033–0.104 IQR], P=.099).
Figure 3.
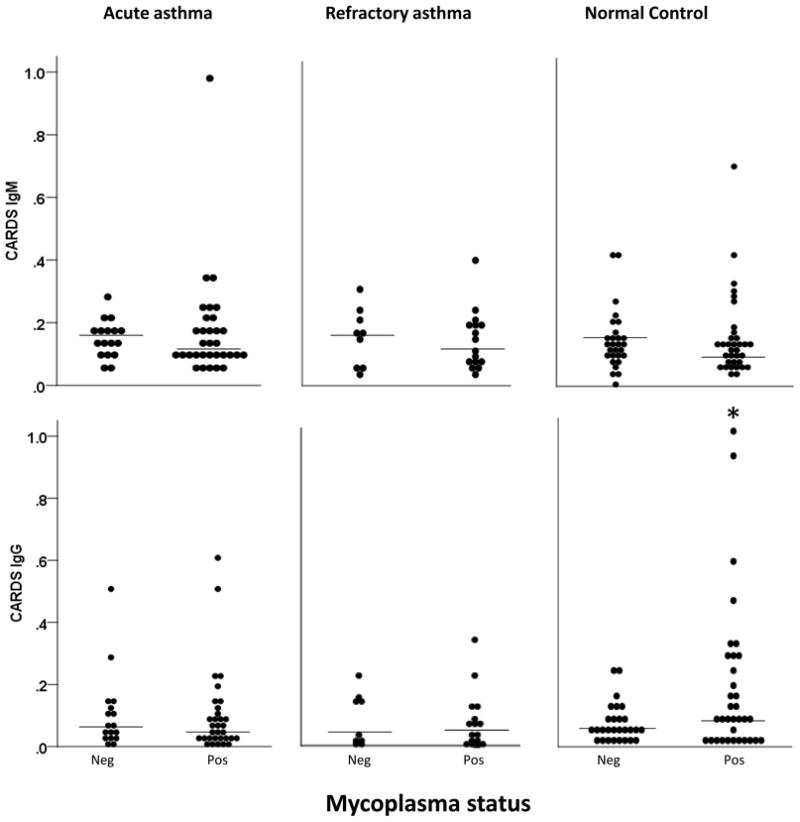
Figure 4.
In subjects with an acute asthma exacerbation, the hospital length of stay was slightly longer in Mp positive compared to Mp negative subjects, but not significantly (3.09 vs 2.21; P=.068). EBC pH was lower in acute asthma compared to healthy controls (7.55 [7.08–8.05 IQR] vs. 8.07 [7.9–8.22 IQR], P<.001). EBC pH was also lower in Mp positive asthma subjects when analyzing all visits (7.88 [7.26–8.17 IQR] vs. 8.12 [7.76–8.25 IQR], P=.017) even when the initial hospitalization visit for the acute asthma group was excluded (P=.034) and was lower in Mp positive subjects with refractory asthma (7.93 [7.68–8.17 IQR] vs. 8.15 [7.99–8.30 IQR], P=.007) (eFigure 1). This relationship remained significant (P=.026) even when pH values < 6 were excluded from the analysis (values < 6 could represent contamination from gastric acid).
The ACT mean score was 16.1 ±4.7 in subject with an acute asthma exacerbation and 16.9 ±5.0 in refractory asthma (scores < 20 indicate poor asthma control over the past 4 weeks). In all visits, except the hospitalization visit for acute asthma, subjects who were Mp positive were more likely to score <20 on the ACT than those who tested negative (70% vs. 30%, P=.001). In addition, poor asthma control (ACT <20) correlated with Mp status (r= −.35, P= .001) and with the CARDS Tx antigen level (r= −.27, P=.01).
The quality of life mean scores at enrollment were lower in subjects during an acute exacerbation of asthma compared with refractory asthma (3.9 ±1.2 vs 4.7 ±1.5, P=.01; lower scores indicate poorer quality of life over the past 7 days). To examine the effect of Mp in chronic asthma, we examined all visits except acute asthma exacerbations. PAQLQ scores trended lower for Mp positive vs. Mp negative subjects (4.9 ±1.4 vs. 5.5 ±1.2, P=.06). In refractory asthma, Mp positive subjects scored significantly lower on the PAQLQ than Mp negative subjects (4.9 ±1.4 vs. 5.7 ±1.1, P=.029) (eFigure 2).
Discussion
Mp infects the upper and lower respiratory tract(20) causing pneumonia, bronchitis, and possibly asthma(21). Prior evidence has linked Mp to new-onset asthma(22), exacerbations of asthma(23), chronic worsening of asthma and long-term decrements in pulmonary function(24–26). Biscardi et al. reported that Mp is associated with the initiation and recurrence of asthma exacerbations in children(27). However, defining a clear cause-and-effect relationship between infection and asthma has been elusive, and the role of colonization by Mp is unclear.
In the past, the detection of Mp has been difficult because Mp is a fastidious organism that is capable of extracellular and intracellular growth and persistence in vivo; even in laboratory conditions, growth is very difficult(28). Mp may be more easily cultured in cases with severe pneumonia(29)and more difficult to culture in states of colonization, where organism burdens are presumably lower. In an outbreak of Mp community-acquired pneumonia, the Centers for Disease Control and Prevention demonstrated that a PCR assay to CARDS Tx was the most sensitive assay tested(11). Our results in the current study confirm that the CARDS Tx PCR assay is superior to detection by P1 PCR, but that the CARDS Tx AC assay was more sensitive than either PCR assay. We further observed that CARDS Tx antigen detection in samples derived from the nasopharynx was higher than in samples from the throat or serum.
In our study, we have found very high rates of Mp detection in the winter/spring seasons. Other recent reports have shown similar seasonal patterns and a dramatically increased incidence of Mp in 2010 and 2011(30,31), suggesting that our study may have coincided with a worldwide epidemic of Mp. Additionally, a high rate of Mp was detected in healthy children (56%), despite no reported respiratory symptoms within 4 weeks of study enrollment. Although some investigators have found low rates of Mp detection in healthy children (4–8%) (32,33), others have identified higher rates. One large study identified that 30% of household contacts were Mp positive, half of whom were asymptomatic(34). Another study found 21% of asymptomatic children were Mp positive(35). Based on our findings, as well as those of other investigators, it would appear that Mp may persist for long periods of time in asymptomatic children. This finding prompts the question as to whether healthy children possess protective factors that allow them to recover from or resist Mp infections without sequelae. Indeed, higher levels of Mp antibodies in our healthy control group compared to the asthma groups may provide insight into a protective immune response.
In the current study, we have shown a higher rate of Mp detection in acute asthma (64%) than previous studies. In hospitalized children with asthma older than 2 years, Freymuth et al. identified only 3% Mp positive subjects(36); Maffey found 6.6% positive in children 4–16 years old(37); and Biscardi found 20% positive in children 2–15 years old(27). Some studies have shown higher rates of 29% in children with acute wheezing(38) and 48% in acutely ill children with recurrent wheezing or asthma(39). In most of these studies, Mp was detected using P1 PCR or by acute and convalescent antibody titers, which are not as sensitive as our assays. In our study, the rate of Mp detection was higher in subjects with asthma than in healthy controls, although this difference was not significant. The higher rate of Mp detection in children with asthma compared to healthy controls may be due to the fact that we selected children with ongoing asthma symptoms (acute asthma or refractory asthma) and Mp may have contributed to these symptoms.
We have shown previously that detection of Mp in adults with refractory asthma is more frequent (52%) and persists for longer than previously recognized(19). In the current study, we show that children with refractory asthma also have high rates of Mp detection (65%) and tend to be persistently positive on multiple follow-up visits. Whether this represents true persistence, reinfection, reactivation, or colonization is unclear, since many subjects also had at least one visit at which they were Mp negative.
We found lower EBC pH values in subjects with acute and chronic asthma. Although it was not surprising that subjects with acute asthma had lower EBC pH, as this has been reported previously(14), lower EBC pH correlated with Mp detection in refractory asthma subjects, who were not experiencing an acute exacerbation. Lower EBC pH was recently reported to correlate with higher scores on the Asthma Predictive Index in children, and thus may represent a biological marker of chronic asthma(40). In addition, subjects with refractory asthma who were positive for Mp had lower symptoms scores (ACT) and lower quality of life scores (PAQLQ). Taken together, these data indicated that Mp detection is associated with worsening chronic asthma.
The direct effect of Mp infection is unknown, but we speculate that immune modulation mediated by CARDS Tx may occur. Animal studies show that low-dose M. pneumoniae infection enhances lung eosinophilia and IL-4(41). We recently reported that CARDS Tx alone recapitulates an “asthma-like” phenotype in mice with promotion of TH2 cytokines(42), and induces pulmonary inflammation, mucous hypersecretion, and hyperreactivity in mice and baboons(43). Differences between Mp strains may also contribute to the severity of the resultant pathological phenotypes (12).
Serological responses involving IgM and IgA can be an early indicator of Mp infection. The IgG antibody response in humans and primates is reported to peak 4–6 weeks after the onset of infection(44). The importance of an intact adaptive humoral immune response for protection against Mp infection is evidenced by the increased rate of severe infections and systemic complications in patients with humoral immune deficiency(45,46). One possible clue to the pathophysiology of Mp infection in children with asthma may be the IgM and IgG responses. While healthy control children in our study who were Mp positive appeared to mount somewhat higher antibody responses, subjects with acute or refractory asthma had low IgM and IgG levels to Mp. Other investigators have reported reduced or absent IgG levels to Mp during acute infections and in chronic infection/colonization states(3,47–49). Thus, the inability to mount an appropriate immune response to Mp may be germane to understanding the impact of Mp on asthma.
The salient findings of the current study are that the detection of Mp in children with asthma 1) had a seasonal pattern with the highest prevalence in the winter/spring; 2) tended to be recurrent or persistent, 3) was associated with lower QOL and ACT scores, 4) was associated with lower EBC pH, and 5) was associated with poor antibody responses. However, this study had several limitations. We did not test for viral respiratory pathogens or other bacterial pathogens, and thus are unable to comment on the role of other organisms, either alone or in combination with Mp, on asthma morbidity. Most subjects had a limited number of follow-up visits, and thus future studies should examine the role of Mp on asthma control and morbidity over time by monitoring subjects longitudinally.
Supplementary Material
Acknowledgments
Sources of financial support:
This work is supported by the National Institute of Allergy and Infectious Disease (NIAID; 3U19AI070412-04S1) and by a Clinical Science and Translation Award, UL1RR025767.
The authors would like to thank Dana Word, RN for study subject recruitment and management, Brandon Guin and Caleb Herrera for technical assistance in reagent preparation and specimen analysis, and Mercedes Vaughn for assistance with analyses.
Footnotes
Author contributions:
PR Wood: 1,2,3,4
VL Hill: 1,2,3
ML Burks: 2,3,4
JI Peters: 1,3,4
H Singh: 2,3,4
TR Kannan: 2,3,4
S Vale: 2,3,4
MP Cagle: 2,3,4
MFR Principe: 2,3,4
JB Baseman: 1,3,4
EG Brooks: 1,2,3,4
- conception and design
- data generation
- analysis and interpretation of data
- preparation or critical revision of manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pamela R Wood, Department of Pediatrics, University of Texas Health Science Center at San Antonio (UTHSCSA).
Vanessa L Hill, Department of Pediatrics, UTHSCSA.
Margaret L Burks, Department of Pediatrics, UTHSCSA.
Jay I Peters, Department of Medicine, UTHSCSA.
Harjinder Singh, Department of Medicine, UTHSCSA.
Thirumalai R Kannan, Department of Microbiology and Immunology, UTHSCSA.
Shruthi Vale, Department of Pediatrics, UTHSCSA.
Marianna P Cagle, Department of Microbiology and Immunology, UTHSCSA.
Molly F R Principe, Department of Pediatrics, UTHSCSA.
Joel B Baseman, Department of Microbiology and Immunology, UTHSCSA.
Edward G Brooks, Department of Pediatrics, UTHSCSA.
References
- 1.Bel EH, Sousa A, Fleming L, Bush A, Chung KF, Versnel J, et al. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI) Thorax. 2010;76:910–7. doi: 10.1136/thx.2010.153643. [DOI] [PubMed] [Google Scholar]
- 2.Hahn DL, McDonald R. Can acute Chlamydia pneumoniae respiratory tract infection initiate chronic asthma? Annals of Allergy, Asthma & Immunology. 1998;81:339–44. doi: 10.1016/S1081-1206(10)63126-2. [DOI] [PubMed] [Google Scholar]
- 3.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, Marmion BP, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 4.Good JT, Jr, Kolakowski CA, Groshong SD, Murphy JR, Martin RJ. Refractory asthma: importance of bronchoscopy to identify phenotypes and direct therapy. Chest. 2012;141:599–606. doi: 10.1378/chest.11-0741. [DOI] [PubMed] [Google Scholar]
- 5.Leaver R, Weinberg EG. Is Mycoplasma pneumoniae a precipitating factor in acute severe asthma in children? S Afr Med J. 1985;68:78–9. [PubMed] [Google Scholar]
- 6.Sutherland ER, King TS, Icitovic N, Ameredes BT, Bleecker E, Boushey HA, et al. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 2010;126:747–53. doi: 10.1016/j.jaci.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannan TR, Baseman JB. ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. PNAS. 2006;103:6724–9. doi: 10.1073/pnas.0510644103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannan TR, Musatovova O, Balasubramanian S, Cagle M, Jordan JL, Krunkosky TM, et al. Mycoplasma pneumoniae Community Acquired Respiratory Distress Syndrome toxin expression reveals growth phase and infection-dependent regulation. Mol Microbiol. 2010;76:1127–41. doi: 10.1111/j.1365-2958.2010.07092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg CP, Kannan TR, Klein R, Gregor M, Baseman JB, Wesselborg S, et al. Mycoplasma antigens as a possible trigger for the induction of antimitochondrial antibodies in primary biliary cirrhosis. Liver Int. 2009;29:797–809. doi: 10.1111/j.1478-3231.2008.01942.x. [DOI] [PubMed] [Google Scholar]
- 10.Kannan TR, Coalson JJ, Cagle M, Musatovova O, Hardy RD, Baseman J. Synthesis and distribution of CARDS toxin during Mycoplasma pneumoniae infection in a murine model. J Infect Dis. 2011;204:1596–604. doi: 10.1093/infdis/jir557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winchell JM, Thurman KA, Mitchell SL, Thacker WL, Fields BS. Evaluation of Three Real-Time PCR Assays for Detection of Mycoplasma pneumoniae in an Outbreak Investigation. J Clin Microbiol. 2008;46:3116–8. doi: 10.1128/JCM.00440-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Techasaensiri C, Tagliabue C, Cagle M, Iranpour P, Katz K, Kannan TR, et al. Variation in Colonization, ADP-Ribosylating and Vacuolating Cytotoxin, and Pulmonary Disease Severity among Mycoplasmapneumoniae Strains. Am J Respir Crit Care Med. 2010;182:797–804. doi: 10.1164/rccm.201001-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Asthma Education and Prevention Program. NIH Publication no 07–4051. Bethesda, MD: National Heart, Lung, and Blood Institute (US); 2007. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. [Google Scholar]
- 14.Hunt JF, Fang KH, Malik RE, Snyder AL, Malhotra N, Platts-Mills TAE, et al. Endogenous Airway Acidification. Implications for Asthma Pathophysiology. Am J Respir Crit Care Med. 2000;161:694–9. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 15.Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, et al. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119:817–25. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 16.Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–56. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in children with asthma. Qual Life Res. 1996;5:35–46. doi: 10.1007/BF00435967. [DOI] [PubMed] [Google Scholar]
- 18.Dallo SF, Su CJ, Horton JR, Baseman JB. Identification of P1 gene domain containing epitope(s) mediating Mycoplasma pneumoniae cytoadherence. J Exp Med. 1988;167:718–23. doi: 10.1084/jem.167.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters J, Singh H, Brooks EG, Diaz J, Kannan TR, Coalson JJ, et al. Persistence of community-acquired respiratory distress syndrome toxin-producing Mycoplasma pneumoniae in refractory asthma. Chest. 2011;140:401–7. doi: 10.1378/chest.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waites KB, Atkinson TP. The role of Mycoplasma in upper respiratory infections. Current Infectious Disease Reports. 2009;11:198–206. doi: 10.1007/s11908-009-0030-6. [DOI] [PubMed] [Google Scholar]
- 21.Berkovich S, Millian SJ, Snyder RD. The association of viral and mycoplasma infections with recurrence of wheezing in the asthmatic child. Ann Allergy. 1970;28:43–9. [PubMed] [Google Scholar]
- 22.Yano T, Ichikawa Y, Komatu S, Arai S, Oizumi K. Association of Mycoplasma pneumoniae antigen with initial onset of bronchial asthma. Am J Respir Crit Care Med. 1994;149:1348–53. doi: 10.1164/ajrccm.149.5.8173777. [DOI] [PubMed] [Google Scholar]
- 23.Ou CY, Tseng YF, Chiou YH, Nong BR, Huang YF, Hsieh KS. The role of Mycoplasma pneumoniae in acute exacerbation of asthma in children. Acta Paediatr Taiwan. 2008;49:14–8. [PubMed] [Google Scholar]
- 24.Sabato AR, Martin AJ, Marmion BP, Kok TW, Cooper DM. Mycoplasma pneumoniae: acute illness, antibiotics, and subsequent pulmonary function. Arch Dis Child. 1984;59:1034–7. doi: 10.1136/adc.59.11.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mok JY, Waugh PR, Simpson H. Mycoplasma pneuminia infection. A follow-up study of 50 children with respiratory illness. Arch Dis Child. 1979;54:506–11. doi: 10.1136/adc.54.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim CK, Chung CY, Kim JS, Kim WS, Park Y, Koh YY. Late abnormal findings on high-resolution computed tomography after Mycoplasma pneumonia. Pediatrics. 2000;105:372–8. doi: 10.1542/peds.105.2.372. [DOI] [PubMed] [Google Scholar]
- 27.Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, Heilbronner C, et al. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–6. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 28.Dallo SF, Baseman JB. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb Pathog. 2000;29:301–9. doi: 10.1006/mpat.2000.0395. [DOI] [PubMed] [Google Scholar]
- 29.Kannan TR, Hardy RD, Coalson JJ, Cavuoti DC, Siegel JD, Cagle M, et al. Fatal outcomes in family transmission of Mycoplasma pneumoniae. Clin Infect Dis. 2012;54:225–31. doi: 10.1093/cid/cir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polkowska A, Harjunpaa A, Toikkanen S, Lappalainen M, Vuento R, Vuorinen T, et al. Increased incidence of Mycoplasma pneumoniae infection in Finland, 2010–2011. Euro Surveill. 2012;17:20072. doi: 10.2807/ese.17.05.20072-en. [DOI] [PubMed] [Google Scholar]
- 31.Uldum SA, Bangsborg JM, Gahrn-Hansen B, Ljung R, Molvadgaard M, Fons PR, et al. Epidemic of Mycoplasma pneumoniae infection in Denmark, 2010 and 2011. Euro Surveill. 2012;17:20073. doi: 10.2807/ese.17.05.20073-en. [DOI] [PubMed] [Google Scholar]
- 32.Gil JC, Cedillo RL, Mayagoitia BG, Paz MD. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann Allergy. 1993;70:23–5. [PubMed] [Google Scholar]
- 33.Esposito S, Blasi F, Arosio C, Fioravanti L, Fagetti L, Droghetti R, et al. Importance of acute Mycoplasma pneumoniae and Chlamydia pneumoniae infections in children with wheezing. Eur Respir J. 2000;16:1142–6. doi: 10.1034/j.1399-3003.2000.16f21.x. [DOI] [PubMed] [Google Scholar]
- 34.Dorigo-Zetsma JW, Wilbrink B, van der Nat H, Bartelds AI, Heijnen ML, Dankert J. Results of molecular detection of Mycoplasma pneumoniae among patients with acute respiratory infection and in their household contacts reveals children as human reservoirs. J Infect Dis. 2001;183:675–8. doi: 10.1086/318529. [DOI] [PubMed] [Google Scholar]
- 35.Spuesens E, Fraaij P, Visser E, Hoogenboezem T, Hop W, Schutten M, et al. European Society for Paediatric Infectious Disease. 2012. Asymptomatic carriage of Mycoplasma pneumonia in the upper respiratory tract of children. abstract. [Google Scholar]
- 36.Freymuth F, Vabret A, Brouard J, Toutain F, Verdon R, Petitjean J, et al. Detection of viral, Chlamydia pneumoniae and Mycoplasma pneumoniae infections in exacerbations of asthma in children. J Clin Virol. 1999;13:131–9. doi: 10.1016/S1386-6532(99)00030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maffey AF, Barrero PR, Venialgo C, Fernandez F, Fuse VA, Saia M, et al. Viruses and atypical bacteria associated with asthma exacerbations in hospitalized children. Pediatr Pulmonol. 2010;45:619–25. doi: 10.1002/ppul.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Principi N, Esposito S, Blasi F, Allegra L. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis. 2001;32:1281–9. doi: 10.1086/319981. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca-Aten M, Okada PJ, Bowlware KL, Chavez-Bueno S, Mejias A, Rios AM, et al. Effect of clarithromycin on cytokines and chemokines in children with an acute exacerbation of recurrent wheezing: a double-blind, randomized, placebo-controlled trial. Ann Allergy Asthma Immunol. 2006;97:457–63. doi: 10.1016/S1081-1206(10)60935-0. [DOI] [PubMed] [Google Scholar]
- 40.von Jagwitz M, Pessler F, Akmatov M, Li J, Range U, Vogelberg C. Reduced breath condensate pH in asymptomatic children with prior wheezing as a risk factor for asthma. J Allergy Clin Immunol. 2011;128:50–5. doi: 10.1016/j.jaci.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Wu Q, Martin RJ, LaFasto S, Chu HW. A low dose of Mycoplasma pneumoniae infection enhances an established allergic inflammation in mice: the role of the prostaglandin E2 pathway. Clin Experiment Allergy. 2009;39:1754–63. doi: 10.1111/j.1365-2222.2009.03309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medina JL, Coalson JJ, Brooks EG, Winter VT, Chaparro A, Principe MF, et al. Mycoplasma Pneumoniae CARDS Toxin Induces Pulmonary Eosinophilic and Lymphocytic Inflammation. Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2011-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardy RD, Coalson JJ, Peters J, Chaparro A, Techasaensiri C, Cantwell AM, et al. Analysis of pulmonary inflammation and function in the mouse and baboon after exposure to Mycoplasma pneumoniae CARDS toxin. PLoS One. 2009;4:e7562. doi: 10.1371/journal.pone.0007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barile MF, Grabowski MW, Kapatais-Zoumbos K, Brown B, Hu PC, Chandler DKF. Experimentally induced Mycoplasma pneumoniae pneumonia in chimpanzees. Microbial Pathogenesis. 1993;15:243–53. doi: 10.1006/mpat.1993.1075. [DOI] [PubMed] [Google Scholar]
- 45.Gelfand EW. Unique susceptibility of patients with antibody deficiency to mycoplasma infection. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1993;17 (Suppl 1):S250–S253. [PubMed] [Google Scholar]
- 46.Foy HM, Ochs H, Davis SD, Kenny GE, Luce RR. Mycoplasma pneumoniae infections in patients with immunodeficiency syndromes: report of four cases. J Infect Dis. 1973;127:388–93. doi: 10.1093/infdis/127.4.388. [DOI] [PubMed] [Google Scholar]
- 47.Atkinson TP, Duffy LB, Pendley D, Dai Y, Cassell GH. Deficient immune response to Mycoplasma pneumoniae in childhood asthma. Allergy Asthma Proc. 2009;30:158–65. doi: 10.2500/aap.2009.30.3207. [DOI] [PubMed] [Google Scholar]
- 48.Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs. erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471–7. doi: 10.1097/00006454-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson AC, Bjorkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 2008;8:93. doi: 10.1186/1471-2180-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


