Abstract
Acute kidney injury (AKI) is associated with a poor prognosis after transcatheter aortic valve replacement (TAVR). A paucity of data exists regarding the incidence and effect of AKI after TAVR using the new recommended Valve Academic Research Consortium criteria. At Columbia University Medical Center, 218 TAVR procedures (64.2% transfemoral, 35.8% transapical) were performed from 2008 to July 2011. The creatinine level was evaluated daily until discharge. Using the Valve Academic Research Consortium definitions, the 30-day and 1-year outcomes were compared between patients with significant AKI (AKI stage 2 or 3) and those without significant AKI (AKI stage 0 or 1). Significant AKI occurred in 18 patients (8.3%). Of these 18 patients, 10 (55.6%) had AKI stage 3 and 9 (50%) required dialysis. AKI was associated with a lower baseline mean transvalvular gradient (37.6 ± 11.4 vs 45.6 ± 14.8 mm Hg for no AKI, p = 0.03). After TAVR, the AKI group had a greater hemoglobin decrease (3.6 ± 2.0 vs 2.4 ± 1.3 g/dl, p = 0.01), greater white blood cell elevation at 72 hours (21.09 ± 12.99 vs 13.18 ± 4.82 × 103/μl, p = 0.001), a more severe platelet decrease (118 ± 40 vs 75 ± 43 × 103/μl, p <0.0001), and longer hospitalization (10.7 ± 6.4 vs 7.7 ± 8.5 days, p <0.001). One stroke (5.6%) occurred in the AKI group compared with 3 (1.5%) in the group without AKI (p = 0.29). The 30-day and 1-year rates of death were significantly greater in the AKI group than in the no-AKI group (44.4% vs 3.0%, hazard ratio 18.1, 95% confidence interval 6.25 to 52.20, p <0.0001; and 55.6% vs 16.0%, hazard ratio 6.32, 95% confidence interval 3.06 to 13.10, p <0.0001, respectively). Periprocedural life-threatening bleeding was the strongest predictor of AKI after TAVR. In conclusion, the occurrence of AKI, as defined by the Valve Academic Research Consortium criteria, is associated with periprocedural complications and a poor prognosis after TAVR.
In an effort to improve the quality of clinical research and enable meaningful comparisons among studies, the Valve Academic Research Consortium (VARC) has proposed standardized consensus definitions for important clinical end points after transcatheter aortic valve replacement (TAVR).1 For acute kidney injury (AKI), The VARC has suggested adopting the serum creatinine criteria from the modified Risk, Injury, Failure, Loss, End-stage kidney disease (RIFLE) classification.2,3 In published studies, a paucity of data exists regarding the occurrence and effect of AKI after TAVR using the new recommended VARC criteria.4–9 Therefore, we sought to determine the incidence, effect, and predictors of AKI after TAVR using the new VARC definitions and to characterize its temporal pattern.
Methods
We prospectively studied consecutive high-risk patients who presented to the Valve Center at Columbia University Medical Center/New York-Presbyterian Hospital with severe aortic stenosis and received TAVR as a part of the Placement of AoRTic traNscathetER valve (PARTNER) trial (Clinicaltrials.gov trial no. NCT00530894) from July 2008 to July 2011. The subjects were ≥60 years old and had severe, calcific aortic stenosis (aortic valve area <0.8 cm2 and mean gradient >40 mm Hg or jet velocity >4.0 m/s). All subjects had cardiac symptoms of advanced aortic valve disease. According to iliac and femoral vessel diameter suitability, TAVR was performed using either the transfemoral (22F or 24F sheath) or transapical (29F dedicated system) approach. The Columbia University Medical Center institutional review board approved the study protocol, and all participants provided written informed consent.
The baseline demographic, clinical, and echocardiographic information was collected for all patients according to the PARTNER trial protocol recommendations. The echocardiographic measurements were adjudicated by an independant echocardiographic core laboratory. The left ventricular (LV) volumes and LV ejection fraction were measured using the biplane Simpson volumetric method combining apical 4- and 2-chamber views.10 A visual LV ejection fraction assessment was also performed. The stroke volume and cardiac output were calculated using the LV volumes measured using the biplane Simpson method and Doppler methods.11 The atrioventricular peak and mean gradients were obtained using the view showing the maximal velocity. The atrioventricular area or effective orifice area was calculated according to the continuity equation.12 Using these data, the Society of Thoracic Surgeons risk score for an isolated aortic valve replacement was computed for each subject and is reported as the predicted mortality at 30 days. The creatinine level was evaluated the day before TAVR and daily after TAVR until discharge. Significant AKI was defined as stage 2 or 3 kidney injury, and no significant AKI was defined as stage 0 or 1, according to the VARC recommended modified Risk, Injury, Failure, Loss, End-stage kidney disease criteria (Table 1).1,3 The glomerular filtration rate (GFR) was estimated at baseline and then on each day after TAVR using the Cockcroft-Gault equation. The intervals to the peak creatinine level and lowest GFR were recorded.
Table 1.
Acute kidney injury (AKI) according to Valve Academic Research Consortium (VARC) definition
| Change in serum creatinine (≤72 h) compared to baseline |
| Stage 1: increase in serum creatinine to 150–200% (1.5–2.0 times increase compared to baseline) or increase of >0.3 mg/dl (>26.4 mmol/L) |
| Stage 2: increase in serum creatinine to 200–300% (2.0–3.0 times increase compared to baseline) or increase of >0.3 mg/dl (>26.4 mmol/L) but <4.0 mg/dl (<354 mmol/L) |
| Stage 3*: increase in serum creatinine to ≥300% (>3 times increase compared to baseline) or serum creatinine of ≥4.0 mg/dl (≥354 mmol/L), with acute increase of ≥0.5 mg/dl (44 mmol/L) |
Patients receiving renal replacement therapy were considered to meet stage 3 criteria, irrespective of other criteria.
All-cause mortality and procedural outcomes were prospectively assessed and adjudicated. The procedural outcomes included in-hospital bleeding, in-hospital stroke, in-hospital vascular complications, and 30-day mortality. Bleeding, vascular complications, and stroke were assessed according to the VARC criteria.1 One-year mortality was assessed at semiannual follow-up visits or by telephone calls by trained research personnel when follow-up visits were not feasible.
The baseline characteristics between the AKI group (AKI stage 2–3) and no-AKI group (AKI stage 0–1) were compared using the Student t test, chi-square test, or Fisher exact test, as appropriate. The univariate and multivariate predictors of AKI were identified using logistic regression analysis. The association of AKI with clinical outcomes was evaluated using Cox proportional hazards models and tabulated according to the Kaplan-Meier method. All analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina). p Values <0.05 were considered statistically significant.
Results
From July 2008 to July 2011, 218 consecutive suitable patients with severe symptomatic aortic stenosis underwent TAVR using a 23- or 26-mm Edwards SAPIEN transcatheter valve. The transfemoral approach was used in 140 patients (64.2%) and the transapical approach in 78 patients (35.8%). Of the total population, AKI occurred in 18 patients (8.3%) and no AKI (stage 0–1) in 200 (91.7%; Figure 1). Of the 18 AKIs, 12 occurred in the first half (12 of 109; 11.0%) and 6 in the second half (6 of 109; 5.5%) of our experience. In the AKI group, 10 patients (55.6%) had AKI stage 3, 9 of whom (50% of 18) required dialysis. No differences were seen in the baseline characteristics between the 2 groups, except for a lower baseline transvalvular mean gradient (37.6 ± 11.4 vs 45.6 ± 14.8 mm Hg, p = 0.03) in the AKI group (Table 2).
Figure 1.
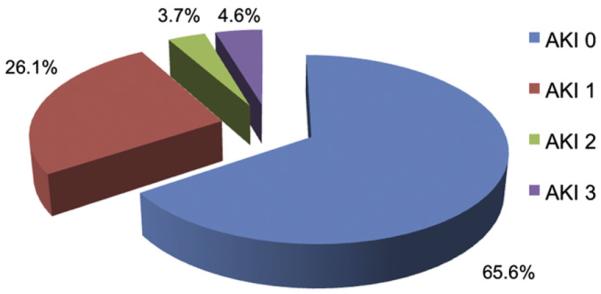
Rates of AKI According to VARC definitions. Significant AKI (stage 2–3) occurred in 18 patients (8.3%) after TAVR.
Table 2.
Baseline characteristics according to occurrence of acute kidney injury (AKI)
| Variable | AKI 0–1 (n = 200) | AKI 2–3 (n = 18) | Combined (n = 218) | p Value |
|---|---|---|---|---|
| Age (yrs) | 85.5 ± 7.7 | 84.0 ± 7.4 | 85.4 ± 7.7 | 0.39 |
| Women | 49% (97) | 50% (9) | 48% (106) | 0.90 |
| Society of Thoracic Surgeons score | 11.65 ± 4.74 | 12.84 ± 4.32 | 11.75 ± 4.71 | 0.23 |
| Weight (kg) | 67.5 ± 16.6 | 67.9 ± 17.5 | 67.5 ± 16.6 | 0.91 |
| Height (cm) | 163.6 ± 12.0 | 163.3 ± 9.8 | 163.6 ± 11.8 | 0.96 |
| Body surface area (m2) | 1.72 ± 0.27 | 1.72 ± 0.24 | 1.72 ± 0.27 | 0.84 |
| Body mass index (kg/m2) | 25.15 ± 5.40 | 25.35 ± 5.94 | 25.16 ± 5.44 | 0.89 |
| Diabetes | 28% (55) | 38% (7) | 29% (62) | 0.31 |
| Hypertension | 83% (165) | 83% (15) | 83% (180) | 1.00 |
| Dyslipidemia | 63% (125) | 67% (12) | 63% (137) | 0.73 |
| Atrial fibrillation/flutter | 41% (82) | 44% (8) | 41% (90) | 0.78 |
| Previous percutaneous coronary intervention | 40% (80) | 44% (8) | 40% (88) | 0.71 |
| Previous coronary artery bypass grafting | 40% (80) | 50% (9) | 40% (89) | 0.41 |
| Previous pacemaker placement | 30% (59) | 44% (8) | 31% (67) | 0.19 |
| Previous stroke | 10% (19) | 11% (2) | 10% (21) | 0.69 |
| Peripheral vascular disease | 24% (47) | 33% (6) | 24% (53) | 0.39 |
| Tobacco use | 9% (18) | 17% (3) | 10% (21) | 0.39 |
| Severe chronic obstructive pulmonary disease | 29% (57) | 39% (7) | 29% (64) | 0.35 |
| Ejection fraction (%) | 48.2 ± 15.8 | 49.5 ± 16.4 | 48.3 ± 15.8 | 0.63 |
| Mean gradient (mm Hg) | 45.6 ± 14.8 | 37.6 ± 11.4 | 44.9 ± 14.7 | 0.03 |
| Aortic valve area (cm2) | 0.58 ± 0.17 | 0.55 ± 0.18 | 0.58 ± 0.17 | 0.72 |
| Annulus size (mm) | 22.8 ± 1.9 | 23.0 ± 2.1 | 22.8 ± 1.9 | 0.80 |
| Approach | ||||
| Transfemoral | 65% (131) | 50% (9) | 64% (140) | 0.19 |
| Transapical | 35% (69) | 50% (9) | 36% (78) | 0.19 |
| Valve size (mm) | 0.41 | |||
| 23 | 38 (75) | 44% (8) | 38% (83) | |
| 26 | 63% (125) | 56% (10) | 62% (135) |
Data are presented as mean ± SD or % (n).
The absolute and relative changes in renal function after TAVR compared to baseline are listed in Table 3. No differences were seen in the baseline creatinine or amount of contrast used between the 2 groups. Of the total population, 96 patients (44.0%) had improved renal function (lower creatinine and greater GFR) from baseline to discharge. The laboratory findings before and after TAVR for both groups are listed in Table 4. The hemoglobin decrease, platelet decrease, and white blood cell elevation at 72 hours after procedure were more pronounced in the AKI group.
Table 3.
Renal function at baseline and after transcatheter aortic valve replacement (TAVR)
| Variable | AKI 0–1 (n = 200) | AKI 2–3 (n = 18) | Combined (n = 218) | p Value |
|---|---|---|---|---|
| Baseline creatinine (mg/dl) | 0.81 | |||
| Mean ± standard deviation | 1.3 ± 0.5 | 1.4 ± 0.6 | 1.3 ± 0.5 | |
| Range | 0.5–2.8 | 0.7–2.8 | 0.5–2.8 | |
| Baseline glomerular filtration rate (ml/min/1.73 m2) | 0.59 | |||
| Mean ± standard deviation | 56.02 ± 21.77 | 52.97 ± 19.21 | 55.77 ± 21.55 | |
| Range | 17.76–175.23 | 22.69–97.89 | 17.76–175.23 | |
| Postoperative | ||||
| Peak creatinine (mg/dl) | 1.42 ± 0.57 | 2.97 ± 1.31 | 1.55 ± 0.78 | <0.0001 |
| Final glomerular filtration rate (ml/min/1.73 m2) | 52.27 ± 22.98 | 22.24 ± 8.95 | 49.79 ± 23.65 | <0.0001 |
| Delta creatinine (peak – baseline) (mg/dl) | <0.0001 | |||
| Mean ± standard deviation | 0.12 ± 0.31 | 1.61 ± 1.21 | 0.25 ± 0.61 | |
| Range | −1.00–1.10 | 0.30–5.00 | −1.00–5.00 | |
| Change in creatinine (%) | <0.0001 | |||
| Mean ± standard deviation | 9.83 ± 22.89 | 131.77 ± 98.49 | 19.90 ± 48.70 | |
| Range | −40.00–88.89 | 10.71–454.55 | −40.00–454.55 | |
| Change in glomerular filtration rate (%) | <0.0001 | |||
| Mean ± standard deviation | −6.08 ± 21.77 | −55.40 ± 18.71 | −10.16 ± 25.44 | |
| Range | −54.69–80.33 | −86.15–11.11 | −86.15–80.33 | |
| Interval to peak creatinine postoperatively | <0.0001 | |||
| Median | 2.0 | 3.0 | 2.0 | |
| Interquartile range | 1.0–3.0 | 2.0–7.0 | 1.0–3.0 | |
| Mean ± standard deviation | 2.1 ± 1.5 | 4.0 ± 2.4 | 2.2 ± 1.7 | |
| Range | 1.0–7.0 | 1.0–8.0 | 1.0–8.0 | |
| Contrast volume (ml) | 103.84 ± 58.53 | 122.61 ± 96.79 | 105.39 ± 62.47 | 0.78 |
| Dialysis required (%) | 0.0% (0) | 50.0% (9) | 4.1% (9) | <0.0001 |
Data in parentheses are numbers of patients.
Table 4.
Laboratory findings according to occurrence of acute kidney injury (AKI)
| Variable | AKI 0–1 (n = 200) | AKI 2–3 (n = 18) | Combined (n = 218) | p Value |
|---|---|---|---|---|
| Hemoglobin (g/dl) | ||||
| Baseline | 11.2 ± 1.5 | 11.3 ± 1.5 | 11.2 ± 1.5 | 0.71 |
| Postoperative nadir | 8.8 ± 1.4 | 7.8 ± 1.6 | 8.7 ± 1.5 | 0.01 |
| Decrease | 2.4 ± 1.3 | 3.6 ± 2.0 | 2.5 ± 1.4 | 0.01 |
| Transfusion received (n) | 0.60 ± 1.4 | 1.1 ± 1.4 | 0.64 ± 1.2 | 0.17 |
| Transfusion received (≥1) (%) | 26.5% (53) | 44.4% (8) | 28.0% (61) | 0.09 |
| White blood cell count (× 103/μL) | ||||
| Baseline | 7.37 ± 2.65 | 8.45 ± 3.04 | 7.46 ± 2.70 | 0.10 |
| Peak at 72 h | 13.18 ± 4.82 | 21.09 ± 12.99 | 13.83 ± 6.27 | 0.001 |
| Platelet count (× 103/μL) | ||||
| Baseline | 200 ± 65 | 226 ± 80 | 202 ± 66 | 0.22 |
| Postoperative nadir | 125 ± 50 | 107 ± 89 | 124 ± 54 | 0.11 |
| Decrease | 75 ± 43 | 118 ± 40 | 78 ± 44 | <0.0001 |
| Troponin (ng/ml) | ||||
| Baseline | 0.13 ± 0.23 | 0.04 ± 0.02 | 0.12 ± 0.22 | 0.65 |
| Peak | 3.64 ± 13.24 | 6.29 ± 11.25 | 3.90 ± 13.04 | 0.08 |
| Creatine kinase-MB (IU/L) | ||||
| Baseline | 2.99 ± 1.69 | 2.33 ± 1.30 | 2.94 ± 1.67 | 0.20 |
| Peak | 9.68 ± 12.97 | 3.80 ± 2.56 | 9.33 ± 12.67 | 0.10 |
| Brain natriuretic peptide (pg/ml) | ||||
| Baseline | 1,473.97 ± 1,634.93 | 1,250.54 ± 1,159.96 | 1,455.64 ± 1,600.11 | 0.67 |
| At discharge | 1,146.0 ± 1,429.9 | 1,386.4 ± 1,157.3 | 1,162.7 ± 1,410.6 | 0.27 |
Data in parentheses are numbers of patients.
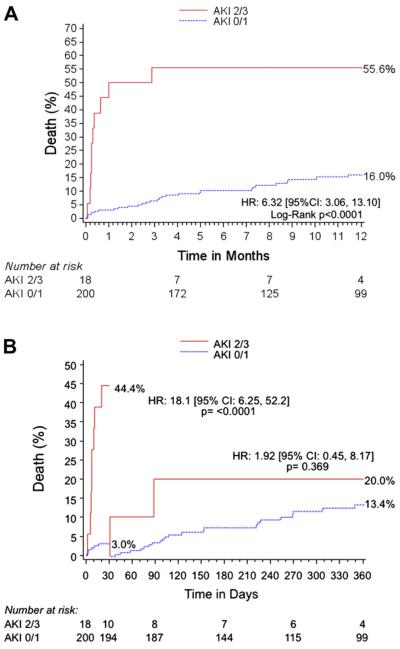
The 30-day clinical outcomes are listed in Table 5. The occurrence of AKI after TAVR was associated with significantly greater rates of VARC life-threatening bleeding, VARC major vascular complications, and longer hospitalization. The 30-day and 1-year mortality were significantly greater in the AKI group (Table 5 and Figure 2).
Table 5.
Clinical outcomes at 30 days
| Variable | AKI 0–1 (n = 200) | AKI 2–3 (n = 18) | Combined (n = 218) | HR (95% CI) | p Value |
|---|---|---|---|---|---|
| Death | |||||
| All cause | 3.0% (6) | 44.4% (8) | 6.4% (14) | 18.10 (6.25–52.20) | <0.0001 |
| Cardiac | 2.0% (4) | 27.8% (5) | 4.1% (9) | 13.70 (3.40–54.90) | <0.0001 |
| Major stroke at 30 days | 1.5% (3) | 5.6% (1) | 1.8% (4) | 3.70 (0.41–33.33) | 0.29 |
| Life-threatening bleeding | 5.5% (11) | 27.8% (5) | 7.3% (16) | 5.00 (1.96–12.51) | 0.005 |
| Major bleeding | 17.5% (35) | 27.8% (5) | 18.3% (40) | 1.58 (0.71–3.57) | 0.34 |
| Transfusion ≥1 | 26.5% (53) | 44.4% (8) | 28.0% (61) | 2.20 (0.82–5.91) | 0.09 |
| Major vascular complications | 7.0% (14) | 27.8% (5) | 8.7% (19) | 4.00 (1.61–10.00) | 0.01 |
| Periprocedural myocardial infarction | 1.0% (2) | 0.0% (0) | 0.9% (2) | 0.47 (0.02–9.49) | 1.00 |
| New pacemaker implantation | 3.0% (6) | 0.0% (0) | 2.8% (6) | 1.23 (0.07–20.98) | 1.00 |
| Postoperative hospitalization (days) | |||||
| Median | 5.0 | 8.0 | 6.0 | — | 0.01 |
| Interquartile range | 4.0–8.0 | 7.0–11.0 | 4.0–8.0 | ||
| Mean ± standard deviation | 7.7 ± 8.5 | 10.7 ± 6.4 | 7.8 ± 8.5 | — | 0.01 |
| Gradient (mm Hg) | 9.0 ± 6.1 (158) | 9.4 ± 3.8 (8) | 9.1 ± 6.0 (166) | — | 0.59 |
| Aortic valve area (cm2) | 1.7 ± 0.4 (87) | 1.7 ± 0.7 (5) | 1.7 ± 0.4 (92) | — | 0.88 |
| Ejection fraction (%) | 50.5 ± 14.0 (162) | 51.9 ± 12.4 (8) | 50.5 ± 13.9 (170) | — | 0.85 |
Data in parentheses are numbers of patients, unless noted otherwise.
CI = confidence interval; HR = hazard ratio.
Figure 2.
Kaplan-Meier curves showing cumulative death over time. (A) Comparison of 1-year cumulative rate of death over time between AKI stage 0–1 and AKI stage 2–3. (B) Thirty-day landmark analysis. Most deaths in AKI stage 2–3 group occurred early after TAVR, suggesting the occurrence of a lethal periprocedural complication. CI = confidence interval; HR = hazard ratio.
The variables associated with the occurrence of AKI are listed in Table 6. After the multivariate analyses, peri-procedural VARC life-threatening bleeding remained the strongest predictor.
Table 6.
Predictors of acute kidney injury (AKI) after transcatheter aortic valve replacement (TAVR)
| Candidate Variables | Unadjusted HR (95% CI) | p Value | Adjusted* HR (95% CI) | p Value |
|---|---|---|---|---|
| Age (10-year increase) | 0.79 (0.44–1.41) | 0.42 | 0.74 (0.37–1.47) | 0.38 |
| Female gender | 1.06 (0.41–2.79) | 0.90 | 0.89 (0.29–2.72) | 0.84 |
| Baseline creatinine | 0.96 (0.64–1.46) | 0.86 | 0.93 (0.57–1.49) | 0.75 |
| Contrast volume used | 0.87 (0.57–1.34) | 0.52 | 0.77 (0.43–1.41) | 0.40 |
| Transapical approach (vs transfemoral) | 1.89 (0.72–5.00) | 0.19 | 2.56 (0.61–10.69) | 0.19 |
| Major vascular complication | 4.00 (1.61–10.00) | 0.01 | 3.24 (1.23–13.52) | 0.08 |
| Life-threatening bleeding | 5.00 (1.96–12.51) | 0.005 | 5.87 (1.84–14.76) | 0.037 |
Adjusted for all included variables.
Discussion
The present report, drawn from a cohort of 218 inoperable or high-risk patients with symptomatic severe aortic stenosis who underwent TAVR, assessed the incidence, predictors, and effect of postprocedural AKI as defined by the new VARC criteria. The major results of the present study were as follows: (1) significant AKI (stage 2–3) was not uncommon after TAVR; (2) significant AKI was associated with the occurrence of periprocedural bleeding and vascular complications; and (3) significant AKI was associated with increased early mortality after TAVR.
The present study was the first to evaluate the incidence and effect of the occurrence of AKI after TAVR as appropriately assessed using the VARC-recommended criteria. Nuis et al5 previously appraised the effect of VARC-defined AKI on 118 consecutive patients who had undergone TAVR. AKI occurred in 19% of their cohort and was the only independent predictor of late mortality. However, Nuis et al5 pooled AKI stage 1, 2, and 3 in their analysis; however, the VARC recommends reporting only AKI stage 2 and 3 as significant AKI. This might explain their greater rate of AKI compared to our results (8.3%). The present report has clearly shown that patients with AKI 0–1 have a completely different prognosis, with significantly lower early and late mortality. Including AKI stage 1 as a part of the group with significant AKI would have resulted in an AKI rate of 34.4% in our study, diluting the strength of the association between bleeding events, vascular complications, and, ultimately, death. The present report, by assessing the outcomes after TAVR in conformity with the VARC recommendations, has validated the use of these criteria in this population.
The VARC report recommended evaluating and classifying AKI 72 hours after TAVR.1 In our cohort, we observed that 50% of the patients with AKI stage 2–3 reached their peak creatinine value after 72 hours (Table 3). However, these patients had already met the severity criteria (within 72 hours) before reaching their own maximum value. Of the 18 patients with AKI 2–3, 9 (50%) underwent renal replacement therapy and were classified as having AKI 3 on that basis. However, 75% of patients with AKI stage 0–1 reached their peak creatinine value within 72 hours, suggesting that 25% could have been underclassified, having been categorized “too early” (at day 3), before having reached their maximum creatinine value. This finding has important implications regarding the appropriate length of follow-up of renal function after TAVR, particularly when the creatinine level could potentially continue to increase beyond the early postprocedural phase, and supports the extension to 7 days of follow-up proposed by the VARC-II.13
The mean contrast volume was not significantly greater in patients with AKI stage 2–3. This finding is consistent with those from previous published reports.4,5,7,8 Although these findings might seem counterintuitive, several reasons could explain these results. First, a greater amount of contrast might have been used in patients treated in our very early experience without regard to procedural complications. Second, the use of intraprocedural transesophageal echocardiography for procedural guidance reduces the amount of contrast required for TAVR, to less than that required for conventional cardiovascular invasive procedures. Third, the operators tend to be more conscious of, and careful about, the amount of contrast used with elderly high-risk patients, such as the currently treated TAVR population. Finally, the management of procedural complications by an endovascular procedure might be associated with greater contrast use in some cases. However, surgery might be the intervention of choice for catastrophic and life-threatening complications and does not involve contrast, given the direct visualization.
Of the 218 patients, 96 (44.0% of the entire cohort) actually had their renal function improve after TAVR, showing a decrease in creatinine and an increase in the GFR at discharge compared to baseline. This finding parallels the results of 2 previous studies, in which ≤62% of patients had improved creatinine levels compared to baseline at 48 hours after TAVR4 and ≤55.6% of patients had similar improvement ≤7 days.7 This finding underscores the incremental benefit of relieving the obstruction created by severe aortic stenosis, thereby improving forward flow and kidney perfusion. These findings also reveal how safe TAVR can be, bringing to light the full benefits of the procedure, when it is uneventful.
As previously observed, AKI in our population was associated with a greater leukocyte count 72 hours after the procedure, suggesting a more prominent inflammatory state and/or reflecting a biological response to an important stress (complications).7,8 We also observed a greater decrease in hemoglobin and the platelet count and a trend toward a greater need for transfusion in patients with significant AKI. These findings most likely reflect a more complicated procedure. Significant AKI was strongly associated with VARC life-threatening bleeding, major bleeding, and major vascular complications. This observation suggests a potential causal effect of bleeding and vascular complications on the development of AKI. This relation was reinforced by the similar baseline creatinine levels and GFR between the groups and similar contrast amounts used during the procedure. It is very likely that periprocedural complications without significant renal function impairment were either less “serious” or better managed. Hence, the occurrence of significant AKI, per se, might represent the ultimate marker of the severity of a given complication occurring after TAVR.
Although the low number of patients and events (AKI stage 2–3) precluded a meaningful multivariate analysis, periprocedural VARC life-threatening bleeding was the strongest independent predictor of significant AKI after TAVR. This implication is in line with results from 3 other centers, in which bleeding and/or the number of transfusions appeared consistently among the independent predictors of AKI after TAVR.4,5,7 Furthermore, that the occurrence of AKI was associated mainly with increased early mortality (Figure 2) supports the hypothesis that the main predictor of AKI is an acute periprocedural life-threatening complication.
AKI stage 2–3 was associated with a 10-mm Hg lower gradient at baseline than AKI stage 0–1. This finding might have a pathophysiologic role, with a lower gradient underlying a more severe/advanced disease (low flow, low gradient), putting the patient more at risk of significant AKI after TAVR. However, given the similar ejection fraction at baseline between the 2 groups and the low number of patients in the present study, it was difficult to precisely determine the exact nature of this finding (chance, association, or causality).
Also, the AKI rate seemed to decrease with increased operator/heart team experience. We observed a 50% decrease in the rate of significant AKI between the first and second halves of our experience. This finding suggests that optimization of patient selection and improved implantation and adjunctive techniques, paired with emerging device technologies, can significantly reduce bleeding complications, vascular complications, and, ultimately, AKI.
The present analysis had several important limitations. The present study represents the experience of a single academic center in its early experience using a first-generation device and treating a high-risk population. Increased operator experience, paired with the use of an improved device in a lower-risk population, could result in a lower rate of AKI. In addition, the small number of patients limited the possibility of meaningful and extensive multivariate regression analysis. However, the association between periprocedural bleeding (and vascular complications) and AKI remained strong in the present model. Distinct causes (contrast-induced nephropathy, nephrotoxic drugs, hypoperfusion) of AKI might be associated with different prognoses. Also, unsuspected confounders might have affected our results. The findings from our report should therefore be considered hypothesis generating.
References
- 1.Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, Krucoff MW, Mack M, Mehran R, Miller C, Morel MA, Petersen J, Popma JJ, Takkenberg JJ, Vahanian A, van Es GA, Vranckx P, Webb JG, Windecker S, Serruys PW. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–269. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 4.Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochelliere R, Doyle D, Masson JB, Gutierrez MJ, Clavel MA, Bertrand OF, Pibarot P, Rodes-Cabau J. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865–874. doi: 10.1093/eurheartj/ehp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuis RJ, Van Mieghem NM, Tzikas A, Piazza N, Otten AM, Cheng J, van Domburg RT, Betjes M, Serruys PW, de Jaegere PP. Frequency, determinants, and prognostic effects of acute kidney injury and red blood cell transfusion in patients undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2011;77:881–889. doi: 10.1002/ccd.22874. [DOI] [PubMed] [Google Scholar]
- 6.Genereux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, Smith C, Serruys PW, Kappetein AP, Leon MB. Clinical outcomes after transcatheter aortic valve replacement using Valve Academic Research Consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59:2317–2326. doi: 10.1016/j.jacc.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Aregger F, Wenaweser P, Hellige GJ, Kadner A, Carrel T, Windecker S, Frey FJ. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrol Dial Transplant. 2009;24:2175–2179. doi: 10.1093/ndt/gfp036. [DOI] [PubMed] [Google Scholar]
- 8.Sinning JM, Ghanem A, Steinhauser H, Adenauer V, Hammerstingl C, Nickenig G, Werner N. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:1141–1149. doi: 10.1016/j.jcin.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Barbash IM, Ben-Dor I, Dvir D, Maluenda G, Xue Z, Torguson R, Satler LF, Pichard AD, Waksman R. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J. 2012;163:1031–1036. doi: 10.1016/j.ahj.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee. European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101–102. [DOI] [PubMed] [Google Scholar]
- 13.Kappetein AP, Head SJ, Généreux P, Piazza N, Van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, Van Es GE, et al. Updated standardized endpoint definitions for transcatheter aortic valve replacement: the VARC-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]