Abstract
Analogues of benztropines (BZTs) are potent inhibitors of the dopamine transporter (DAT) but are less effective than cocaine as behavioral stimulants. As a result, there have been efforts to evaluate these compounds as leads for potential medication for cocaine addiction. Here we use computational modeling together with site-directed mutagenesis to characterize the binding site for BZTs in DAT. Docking into molecular models based on the structure of the bacterial homologue LeuT supported a BZT binding site that overlaps with the substrate binding pocket. In agreement, mutations of residues within the pocket, including Val1523.46* to Ala or Ile, Ser4228.60 to Ala and Asn1573.51 to Cys or Ala, resulted in decreased affinity for BZT and the analog JHW007, as assessed in [3H]dopamine uptake inhibition assays and/or [3H]CFT competition binding assay. A putative polar interaction of one of the phenyl ring fluorine substituents in JHW007 with Asn1573.51 was used as a criterion for determining likely binding poses and establish a structural context for the mutagenesis findings. The analysis positioned the other fluorine substituted phenyl ring of JHW007 in close proximity to Ala47910.51/Ala48010.52 in transmembrane segment (TM) 10. The lack of such an interaction for BZT led to a more tilted orientation, as compared to JHW007, bringing one of the phenyl rings even closer to Ala47910.51/Ala48010.52. Mutation of Ala47910.51 and Ala48010.52 to valines supported these predictions with a larger decrease in the affinity for BZT than for JHW007. Summarized, our data suggest that BZTs display a classical competitive binding mode with binding sites overlapping those of cocaine and dopamine.
Keywords: Neurotransmitter:sodium symporters, monoamine transporters, dopamine transporter, cocaine, molecular modeling, mutagenesis
1. Introduction
The dopamine transporter (DAT) mediates rapid reuptake of dopamine from the synaptic cleft and is thereby responsible for termination of dopaminergic signalling (Chen et al., 2004; Gether et al., 2006; Torres and Amara, 2007). Alteration in dopamine signalling and DAT function is coupled to neurological and psychiatric diseases including schizophrenia, bipolar disorder, ADHD (Attention Deficit Hyperactivity Disorder), Tourette’s syndrome and Parkinson’s’ disease (Gainetdinov and Caron, 2003; Gether et al., 2006; Torres and Amara, 2007). The transporter belongs to the family of neurotransmitter:sodium symporters (NSS) (also called the SLC6 [solute carrier 6] family or Na+/Cl− coupled transporters) that in addition includes the transporters for other neurotransmitters such as the norepinephrine transporter (NET), the serotonin transporter (SERT), the glycine transporters and the GABA transporters (Chen et al., 2004; Gether et al., 2006; Torres and Amara, 2007). NSS proteins utilize the transmembrane Na+ gradient as a driving force for transport of substrate and are characterized by additional co-transport of Cl− (Chen et al., 2004; Gether et al., 2006; Torres and Amara, 2007; Zomot et al., 2007). Insight into the structural basis of NSS function has emerged from crystallographic analysis of LeuT, a bacterial NSS homolog from Aquifex aeolicus (Krishnamurthy et al., 2009; Singh et al., 2008; Singh et al., 2007; Yamashita et al., 2005).
DAT is the principal target for psychostimulants including cocaine and amphetamines (Chen et al., 2004; Gether et al., 2006; Torres and Amara, 2007). Because of wide abuse of these compounds it has been a long pursued goal to develop medication(s) that can aid in the treatment of this addiction, but success has been elusive. In this pursuit, novel dopamine uptake inhibitors have been targeted as leads to potential medications for cocaine addiction (Dutta et al., 2003). Among these compounds are analogues of benztropine (BZT) or rimcazole that have similar or higher affinity and selectivity for the DAT than cocaine (Newman and Katz, 2009; Newman and Kulkarni, 2002). The BZTs tested so far readily cross the blood-brain barrier (Raje et al., 2003; Syed et al., 2008) and produce increases in extracellular levels of dopamine even for longer durations than cocaine (Raje et al., 2005). Nonetheless, several of these DAT inhibitors are less effective than cocaine as behavioral stimulants (Desai et al., 2005; Katz et al., 2004; Katz et al., 2003; Newman et al., 1994). Furthermore, one BZT analogue, JHW007, was found to potently antagonize the behavioral effects of cocaine (Desai et al., 2005; Hiranita et al., 2009).
We have demonstrated recently a remarkable relationship between cocaine-like subjective effects of uptake inhibitors and the conformational state promoted by these compounds in the DAT protein (Loland et al., 2008). Our data suggested that the stimulatory effect of an inhibitor might be predicted directly from its interaction mode with the transporter (Loland et al., 2008). Whereas cocaine and its analogs induced an outside-open conformation of the transporter, several BZTs with reduced stimulatory effect, including BZT itself and the analog JHW007, promoted a less outward-open configuration of the transport protein (Loland et al., 2008). Subsequent molecular models of the binding sites in DAT, based on the LeuT structure and a previously reported sequence alignment (Beuming et al., 2006), suggested that the data were consistent with binding sites for both cocaine and the BZTs being deeply buried in the transporter structure and overlapping with that of dopamine (Beuming et al., 2008). Moreover, the models suggested that whereas cocaine and the cocaine analog CFT ((−)-2β-carbomethoxy-3β-(4-fluorophenyl)tropane) stabilized a rather open conformation of the binding pocket by breaking a stabilizing intramolecular hydrogen bond between Asp791.45 and Tyr1563.50, BZT and JHW007 bound in the model with a more closed configuration in which the hydrogen bond between the two residues was not broken (Beuming et al., 2008). In agreement with this binding model, mutational disruption of the hydrogen bond by mutating Tyr1563.50 to Phe did not affect binding of cocaine and CFT but decreased the binding affinity of BZT and JHW007, indicating an indirect role of the hydrogen bond in stabilizing the BZT/JHW007 binding mode (Beuming et al., 2008).
The deeply buried binding site for cocaine and analogues was further validated with detailed mutational analysis (Beuming et al., 2008). However, a similar detailed analysis validating the proposed binding site for BZTs was not done, and the characteristics of the binding site were not thoroughly explored. The specific interest in the mode of binding and the position of the binding site within the transporter for BZTs was rekindled by the results of a recent analysis of the binding site for antidepressants and small molecule inhibitors of the closely related serotonin transporter (SERT). Thus, the crystallographic analysis of low affinity antidepressant binding to LeuT, together with mutational analysis in SERT, has been interpreted as evidence for the binding of SERT inhibitors, including tricyclic antidepressants and selected SSRIs (selective serotonin reuptake inhibitors) in a vestibule extracellular to this pocket (Singh et al., 2007; Zhou et al., 2007; Zhou et al., 2009) rather than deep in the transporter structure, in the primary substrate binding pocket. Notably, the vestibule had been proposed to harbor a secondary substrate-binding site (S2) in LeuT with a critical role in the translocation mechanism (Shi et al., 2008; Zhao et al., 2010). We provide here strong evidence that BZT and BZT analogs can bind to the primary substrate binding pocket and thus that the compounds display a classical competitive binding mode with binding sites overlapping that of cocaine and dopamine.
2. Materials and Methods
2.1 Molecular biology and cell culture
Synthetic cDNAs encoding the human DAT (synDAT) were subcloned into pcDNA3 (Invitrogen, Carlsbad, CA) (Loland et al., 2004). All mutations were generated by the QuickChange method (adapted from Stratagene, La Jolla, CA) and confirmed by restriction enzyme mapping and DNA sequencing. COS7 cells were grown and transiently transfected as described with indicated constructs using the calcium phosphate precipitation method (Loland et al., 2004)
2.2. Indexing of residues
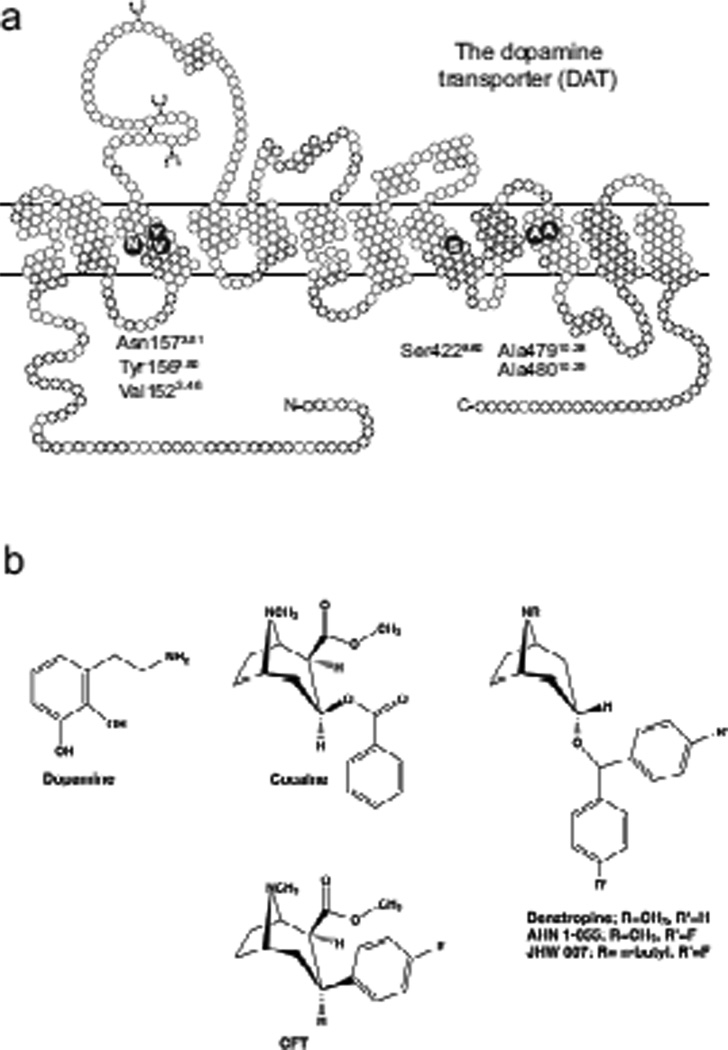
A generic numbering scheme for amino acid residues in NSS proteins has been proposed to facilitate direct comparison of positions between the individual members of the family (Beuming et al., 2006; Goldberg et al., 2003). According to this scheme, the most conserved residue in each transmembrane segment has been given the number 50, and each residue is numbered according to its position relative to this conserved residue. For example, 1.55 indicates a residue in TM1 five residues carboxyl-terminal to the most conserved residue in this TM (Trp1.50). For DAT, the most conserved residues in each transmembrane segment is as follows (generic number being indicated in superscript): TM1, Trp841.50; TM2, Pro1122.50; TM3, Tyr1563.50; TM4, Cys2434.50; TM5, Pro2735.50; TM6, Gln3176.50; TM7, Ser3667.50; TM8, Phe4128.50; TM9, Phe4579.50; TM10, Phe47810.50; TM11, Pro52911.50; TM12, Pro57312.50. Compared to the generic numbering scheme defined in (Beuming et al., 2006), we extend the TM index into the immediate conserved loop regions, specifically TM1 to N-terminus, TM6 to IL3, and TM8 to IL4.
2.3 DAT Inhibitors
The drugs tested are displayed in Fig. 1. Cocaine HCl (Sigma-Aldrich, St. Louis, MO); CFT (Sigma-Aldrich); and BZT (Sigma-Aldrich) were obtained from the designated sources. AHN 1-055 and JHW 007 were synthesized in the Medicinal Chemistry Section (Intramural Research Program, National Institute on Drug Abuse, Baltimore, MD) according to published procedures: AHN 1-055 (Agoston et al., 1997; Newman et al., 1994).
Fig. 1.
DAT diagram and ligands. (A) Two-dimensional schematic representation of the human dopamine transporter (hDAT). Residues relevant to the present study are shown as enlarged dark circles with single letter code in white. (B) Structure of dopamine, cocaine, the cocaine analogue CFT ((−)-2β -carbomethoxy-3β -(4-fluorophenyl)tropane), BZT, AHN 1-055 and JHW007.
2.4. [3H]DA uptake measurement
Uptake assays were performed as described (Loland et al., 2004) using 3,4-[Ring-2,5,6-3H]-dihydroxyphenylethylamine (30–60 Ci/mmol) (Perkin Elmer, NET67300). Briefly, transfected COS7 cells were plated in either 24-well dishes (105 cells/well) or 12-well dishes (3×105 cells/well) coated with poly-ornithine (Sigma) and the uptake assays were carried out 2 days after transfection for 5 min at room temperature in uptake buffer (25 mM HEPES, 130 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 1 mM L-ascorbic acid, 5 mM D-glucose, and 1 µM of the catechol-O-methyltransferase inhibitor Ro 41-0960 (Sigma), pH 7.4). Indicated non-labeled compounds were added to the cells prior to initiation of uptake by addition of 40 nM [3H]dopamine. Nonspecific uptake was determined with 100 µM nomifensine.
2.5. [3H]CFT binding experiments
Binding assays were carried out on intact COS7 cells as described (Loland et al., 1999) using 2–4 nM [3H]CFT (WIN 35,428 from PerkinElmer) (87 Ci/mmol) as radioligand. The assay was performed 2 days after transfection in uptake buffer (see above) for 2 hours at 4°C on cells in either 24-well dishes (105 cells/well) or 12-well dishes (3×105 cells/well). Nonspecific binding was determined with 100 µM nomifensine.
2.6. Data calculations
Uptake data and binding data were analyzed by nonlinear regression analysis using Prism 5.0 from GraphPad Software, San Diego, CA. The Km and Kd values were calculated from the pIC50 values determined by non-linear regression analysis of [3H]dopamine uptake and [3H]CFT binding data as described previously (Loland et al., 2004; Loland et al., 1999). The S.E. intervals were calculated from pK ± S.E. The Ki values were calculated from IC50 values determined by non-linear regression analysis of [3H]dopamine uptake or [3H]CFT binding data using the equation Ki=IC50/(1+(L+K)) where L= concentration of [3H]dopamine or [3H]CFT and K is Km for [3H]dopamine uptake or Kd fo [3H]CFT binding.
2.7. Modeling of DAT/ligand complexes
Ligand-receptor complexes were modeled using a flexible docking approach, using multiple copies of the transporter, together with a well established induced-fit docking (IFD) protocol (Sherman et al., 2006). Four distinct DAT models, equilibrated in various configurations, bound with either substrate or inhibitor, were used as starting points for the IFD procedure. The four models are: 1) A homology model of DAT constructed and equilibrated in explicit water and lipid environment as described before (Guptaroy et al., 2009). This occluded state model of DAT had a dopamine molecule in S1, two Na+, and one Cl−; 2) A dual substrate-occupied DAT model derived from (1), with dopamine in the S1 as well as the S2 site (Shan et al, submitted); 3) Equilibrated DAT models with either CFT or 4) with JHW007 bound in the S1 site. All ligands were constructed and prepared for docking using LigPrep (Schrodinger Inc.). Docking with the IFD method (Sherman et al., 2006) was carried out with default options, using the SP scoring function in the first stage and XP scoring function in the second stage. Because all ligands (CFT, BZT, JHW007, and AHN 1-055) contain a pyramidal nitrogen that may bind preferably with one of the two configurations that exist as an equilibrium in solution, the docking was sampled for both configurations around this nitrogen in each ligand.
3. Results
Docking models for the binding of dopamine, cocaine, and selected inhibitors including CFT, BZT and the BZT analog JHW007 had been described previously (Beuming et al., 2008). We based these models on the high-resolution structure of the bacterial NSS member, LeuT (PDB code 2A65)(Yamashita et al., 2005), and a refined structure-based sequence alignment of NSSproteins (Beuming et al., 2006). The initial docked complexes were further refined with molecular dynamics (MD) simulations in explicit water membrane environments at atomic detail (Beuming et al., 2008). The final docking models positioned dopamine, cocaine, CFT, BZT and JHW007 (Fig. 1) in a shared binding site at the center of DAT, comprised of residues in TM1, 3, 6, and 8, and located in a position corresponding to the primary substrate binding cavity in LeuT (S1) (Beuming et al., 2008). The binding models for dopamine, CFT and cocaine in DAT were validated experimentally by verifying their predictions of commonalities and differences in the modes of DAT-ligand interactions (Beuming et al., 2008).
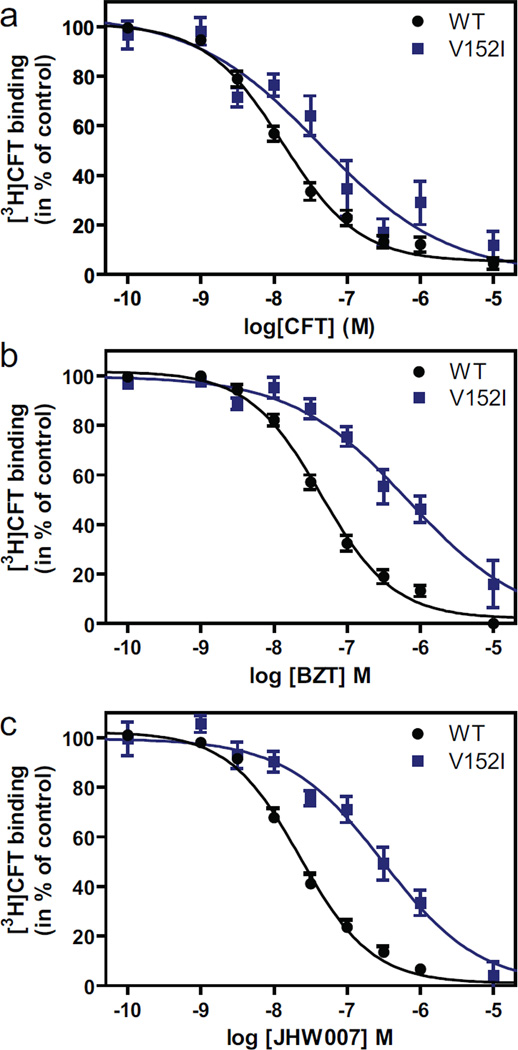
Val1523.46: Val1523.46 in TM3 (Fig. 1) was predicted to be a site of interaction shared by dopamine and cocaine/CFT, as well as BZT and JHW007 (Beuming et al., 2008). Similar to our previous observations (Beuming et al., 2008) and in agreement with these predictions, mutation of Val1523.46 to Ile or Ala increased the Km value for [3H]dopamine uptake (Table 1) and decreased the binding affinity of [3H]CFT (Table 2 and Fig. 2). To assess whether Val1523.46 represented a site of interaction also for BZT and JHW007, we determined their binding affinity for V152A and V152I in [3H]CFT competition binding experiments. For BZT we observed ~15-fold reduction in apparent affinity in both V152A (Table 2) and V152I (Fig. 2 and Table 2). Correspondingly, we observed for JHW007 10–20-fold reduction in apparent affinity in V152A (Table 2) and V152I (Fig. 2 and Table 2). Although indirect effects of the mutations cannot be excluded, these data support a role for Val1523.46 in the interaction with both BZT and JHW007. In parallel, the two compounds exhibited reduced potency in the inhibition of [3H]dopamine uptake, ~2-fold decrease or JHW007 in V152A and ~6-fold in V152I (Table 1), again consistent with an interaction of the compound with Val1523.46. For BZT we observed a ~3-fold decrease at V152A and no change at V152I (Table 1). Curiously, the smaller effect of the two mutations in uptake inhibition assays as compared to the [3H]CFT binding assay was also seen for CFT and cocaine (Table 1 and (Beuming et al., 2008))(Beuming et al., 2008). We have no immediate explanation for the typically smaller effect of the mutations in the uptake assay than in the binding competition assay; however, one possible difference is that the uptake assay is performed under non-equilibrium conditions (5 min incubation) whereas the binding assay is performed under equilibrium conditions (2 hours incubation).
Table 1.
[3H]dopamine uptake properties and inhibition potencies of indicated compounds assessed in COS7 cells transiently expressing wild type DAT or mutants
| DAT mutants |
[3H]Dopamine Km (nM) [S.E. interval] |
Vmax (fmol/min/105 cells) ± S.E. |
CFT Ki (nM) [S.E. interval] |
JHW007 Ki (nM) [S.E. interval] |
BZT Ki (nM) [S.E. interval] |
|---|---|---|---|---|---|
| WT | 986 [861–1130] | 5275 ± 555 | 22 [19–24] | 48 [43–53] | 111 [96–127] |
| V152A | 3090 [2310–4130] | 614 ± 167 | 37 [32–43] | 92 [58–147] | 316 [231–431] |
| V152I | 4370 [3180–6010] | 242 ± 37 | 32 [24–38] | 295 [123–710] | 79 [58–107] |
| N157A | 3240 [2720–3870] | 598 ± 179 | 131 [77–221] | 432 [298–625] | 385 [304–488] |
| N157C | 2710 [2170–3380] | 1768 ± 159 | 163 [108–246] | 358 [278–461] | 284 [170–474] |
| S422A | 3950 [3730–4190] | 320 ± 40 | 614 [486–777] | 1510 [1280–1770] | 1810 [1230–2670] |
| A479V/A480V | 1620 [1350–1940] | 2231 ± 157 | 35 [25–49] | 305 [227–410] | 2000 [1370–2920] |
The KM values were calculated from IC50 values determined by non-linear regression analysis of [3H]dopamine uptake assays. The KM values for [3H]dopamine were used to calculate KI for CFT, JHW007, and BZT. Data were obtained from COS7 cells transiently expressing the DAT WT or mutants. The S.E. interval for each KM value is indicated and was calculated from the pKI ± S.E. Data are means ± S.E. of 3 to 17 experiments performed in triplicate.
Table 2.
[3H]CFT binding properties and calculated affinities of indicated compounds assessed in COS7 cells transiently expressing wild type DAT or mutants
| DAT construct |
CFT Kd (nM) [S.E. interval] |
Bmax (fmol/105 cells) ± S.E. |
JHW007 Ki (nM) [S.E. interval] |
BZT Ki (nM) [S.E. interval] |
|---|---|---|---|---|
| WT | 18 [14–23] | 386 ± 89 | 23 [19–28] | 43 [37–49] |
| V152A | 261 [116–593] | 380 ± 99 | 270 [218–336] | 655 [484–888] |
| V152I | 145 [55–380] | 215 ± 58 | 387 [293–512] | 633 [343–1170] |
| N157A | ND | ND | ND | ND |
| N157C | ND | ND | ND | ND |
| S422A | ND | ND | ND | ND |
| A479V/A480V | 63 [49–82] | 310 ± 44 | 240 [168–341] | 872 [686–1110] |
The Kd values were calculated from IC50 value determined by non-linear regression analysis of [3H]CFT binding assays. The Kd values for [3H]CFT were used to calculate KI for JHW007, and BZT. Data were obtained from COS7 cells transiently expressing the DAT WT or mutants. The S.E. interval for each Kd and Ki value is indicated and was calculated from the pKI ± S.E. Data are means ± S.E. of 3 to 17 experiments performed in triplicate. ND = not detectable.
Fig. 2.
Evidence for involvement of Val1523.46 in binding of BZT and JHW007. (A) [3H]CFT/CFT competition binding on wild type (WT) DAT (black circles) or V152I (blue square). (B, C) Inhibition of [3H]CFT binding by BZT (B) or JHW007 (C) to wild type DAT (black circles) or I152I (blue square). The binding experiments were performed using indicated concentrations of non-labeled inhibitor on intact COS7 cells transiently expressing WT DAT or V152I. Data are means ± S.E. of 3 to 17 experiments performed in triplicate.
Ser4228.60: This TM8 residue was also predicted to be part of the binding site for CFT/cocaine and dopamine as well as for BZT/JHW007, while also being involved in coordination of the second Na+ (Beuming et al., 2008; Beuming et al., 2006; Yamashita et al., 2005). In agreement with our previous observations, mutation of Ser4228.60 to Ala increased the Km value for [3H]dopamine uptake ~4-fold and decreased, according to uptake competition assays, the apparent affinity for CFT ~30-fold (Table 1 and (Beuming et al., 2008)). Consistent with a role of Ser4228.60 also in BZT/JHW007 binding, the apparent affinity for BZT decreased ~16-fold and for JHW007 ~30-fold (Table 1). It was not possible to perform [3H]CFT binding experiments on S422A, which was not surprising given the dramatic decrease in apparent affinity as assessed from the uptake competition experiments (Table 1).
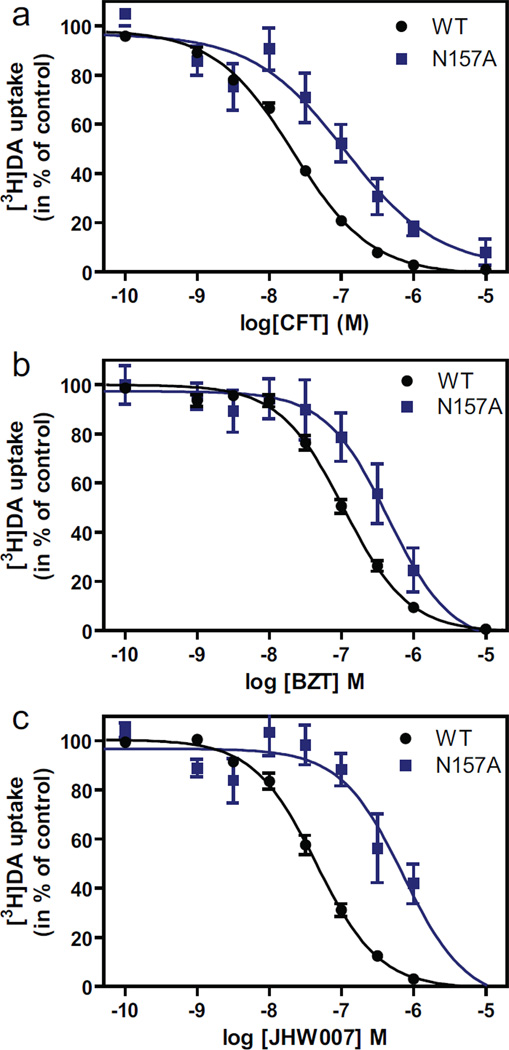
Asn1573.51: This TM 3 residue is in the S1 binding cavity and in our docking models forms a polar interaction with the fluorine substituent of CFT but has no interaction with dopamine when it is bound in S1 (Beuming et al., 2008). Corresponding to our earlier observations, mutation of Asn1573.51 to Cys decreased the apparent affinity for CFT 7–8-fold as determined in [3H]dopamine uptake experiments (Table 1); a similar effect was observed for the Asn1573.51 to Ala mutant (Fig. 3 and Table 1). In agreement with the marked decrease in affinity we were unable to perform reliable [3H]CFT binding experiments for these two mutants. Of note, we did previously report [3H]CFT binding experiments with the N157C mutant (Beuming et al., 2008), but the actual counts per minute determining the specific binding were very low. Possibly due to variation in transfection efficiency and expression, we were not able to obtain reliable data for [3H]CFT binding during the course of the present study. However, we were able to assess the inhibition potency of JHW007 and BZT in [3H]dopamine uptake experiments for both N157C (Table 1) and N157A (Fig. 3 and Table 1). We observed a marked reduction in apparent affinity of JHW007 with ~9-fold increase in the calculated Ki value of N157A and ~7-fold in that of N157C (Fig. 3 and Table 1), suggesting that one of the two fluorine substituents may be engaged in polar interaction with Asn1573.51. Perhaps because it does not contain a fluorine, BZT exhibited only a small reduction, ~3-fold, in apparent affinity for these two mutants, which suggests a weaker interaction of this compound with Asn1573.51 (Figs 3, 4 and Table 1).
Fig. 3.
Evidence for involvement of Asn1573.51 in binding of BZT and JHW007. (A, B, C) Inhibition of [3H]dopamine uptake by CFT (A), BZT (B) or JHW007 (C). The uptake experiments were performed using indicated concentrations of inhibitor on COS7 cells transiently expressing wild type (WT) DAT (black circles) or N157A (blue squares). Data are means ± S.E. of 3 to 17 experiments performed in triplicate.
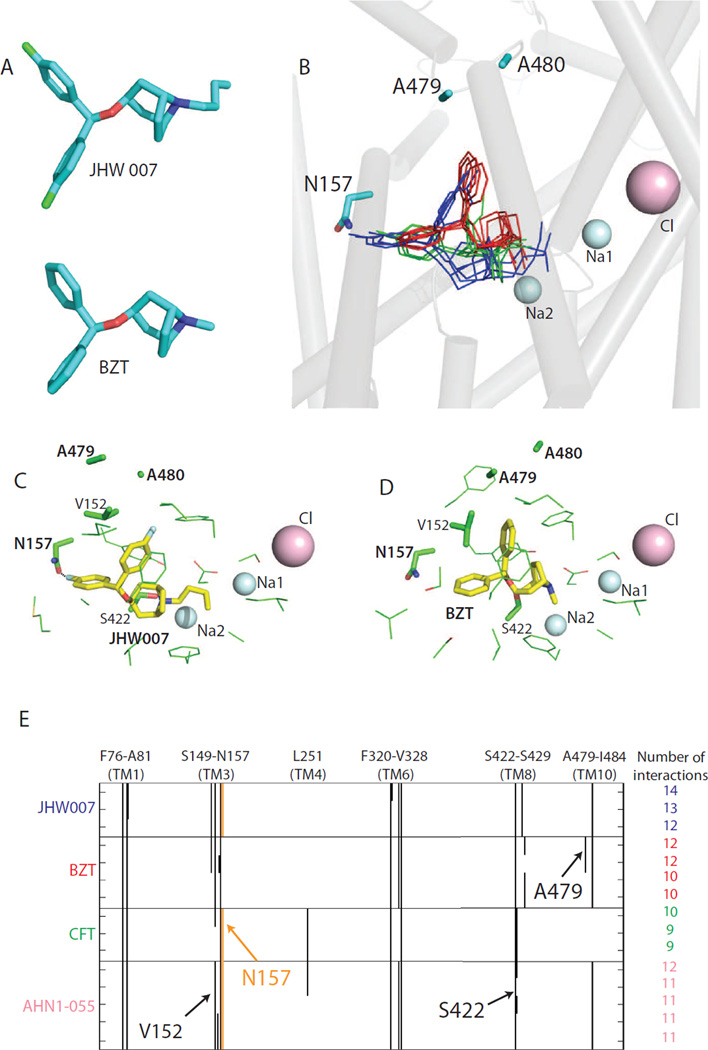
Fig. 4.
Molecular models of DAT/ligand complexes. The aligned structures of JHW007 and BZT in A illustrate their large common moiety. The docking poses of CFT (green), BZT (red) and JHW007 (blue) in DAT are superimposed in B based on the S1 binding site residues. Sodium and chloride ions around the S1 site and residues Asn1573.51, Ala47910.51 and Ala48010.52 are indicated. The representative poses for JHW007 and BZT are depicted individually in C and D. In JHW007 (C) docking model, the polar interaction between Asn1573.51 and the fluoride substituent is indicated by a dotted line. The absence of this interaction in the BZT docking model (D) allows the ligand to get closer to Ala47910.51. Panel E shows the side chain interaction fingerprints (Deng et al., 2004) of the filtered docking pose clusters of CFT, BZT, JHW007, and AHN1-055. The interactions between the fluoride substituent and Asn1573.51 are highlighted in orange.
This putative interaction of JHW007 with Asn1573.51 served in the subsequent exploration and further refinement of the ligand-transporter interaction models (Fig. 4). Using several DAT models and an induced fit protocol that allows backbone flexibility (IFD - see Methods), we filtered the JHW007 poses that had any of the fluorine substituents within 4 Å of the Asn1573.51 side chain, and examined the rest of the interactions of the compound in the binding site, subject to this restraint. It is not entirely surprising that the filtered JHW007 poses overlapped with the cocaine and dopamine binding sites (Beuming et al., 2008) so that the tropane ring and the N-butyl substituents contact residues from TMs 1, 3, 6, 8, 10 with the N-butyl substituent inserted between TMs 1 and 6, and the aromatic rings interacting with residues from TMs 3, 6, and 8 (Fig. 4). The side chain interaction fingerprints (Deng et al., 2004) of the filtered docking pose clusters are shown in Fig. 4E, specifically illustrating the differential interaction of CFT and the BZTs with Asn1573.51.
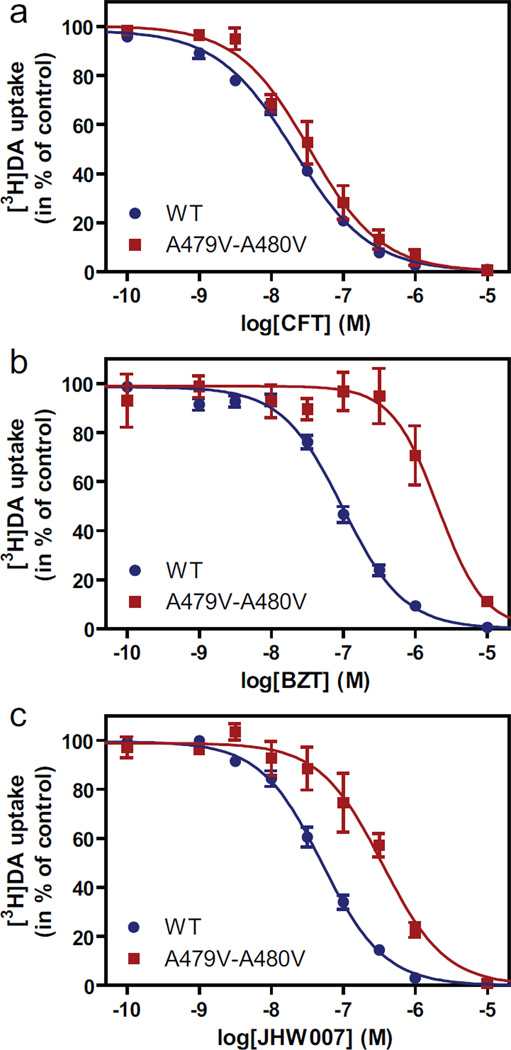
Interestingly, all the filtered poses position the other fluorine substituted aromatic ring in close proximity to Ala47910.51 and Ala48010.52 in TM10 (Fig. 4B, C). Using the same docking protocol and filtering criteria, CFT, which is smaller and has only one fluorine-substituted aromatic ring, is predicted to not interact with these two residues. We tested this predicted difference by mutating both residues to valines (A479V-A480V) assuming that the larger side chain of the valines would sterically impair JHW007 binding, but not that of CFT. In agreement with this prediction, [3H]CFT binding affinity was only slightly affected (Kd ~63 nM in A479V-A480V versus ~18 nM for wild type, Table 2) and the ability of CFT to inhibit [3H]dopamine uptake was almost unaffected (Fig. 5 and Table 1). In contrast, JHW007 displayed ~10-fold decrease in affinity calculated from [3H]CFT competition binding experiments (Table 2) and ~6-fold decrease in affinity calculated from [3H]dopamine uptake inhibition experiments (Fig. 5 and Table 1).
Fig. 5.
Evidence for involvement of Ala47910.51 and Ala48010.52 in binding of BZT and JHW007. (A, B, C) Inhibition of [3H]dopamine uptake by CFT (A), BZT (B) or JHW007 (C). The uptake experiments were performed using indicated concentrations of inhibitor on COS7 cells transiently expressing wild type (WT) DAT (blue circles) or A479V-A480V (red squares). Data are means ± S.E. of 3 to 17 experiments performed in triplicate.
Based on the findings for JHW007 we selected BZT docking poses by taking advantage of their common moiety (assumed to be a key component of the pharmacophore) (Fig. 4A). Thus, BZT poses were filtered with the condition that the common pharmacophore element be in the same orientation as for JHW007 (Fig. 4B), i. e., the RMSD of common pharmacophore is within 3 Å. In these filtered BZT poses, we found that the lack of direct interaction with Asn1573.51 resulted in a tilted orientation of the aromatic rings so as to position one of them even closer to Ala47910.51 and Ala48010.52 than seen for JHW007 (Fig. 4B–D). Consistent with this predicted difference from JHW007, BZT showed at A479V-A480V ~20-fold decrease in affinity calculated from either [3H]CFT competition binding experiments (Table 2) or [3H]dopamine uptake inhibition experiments (Fig. 5 and Table 1).
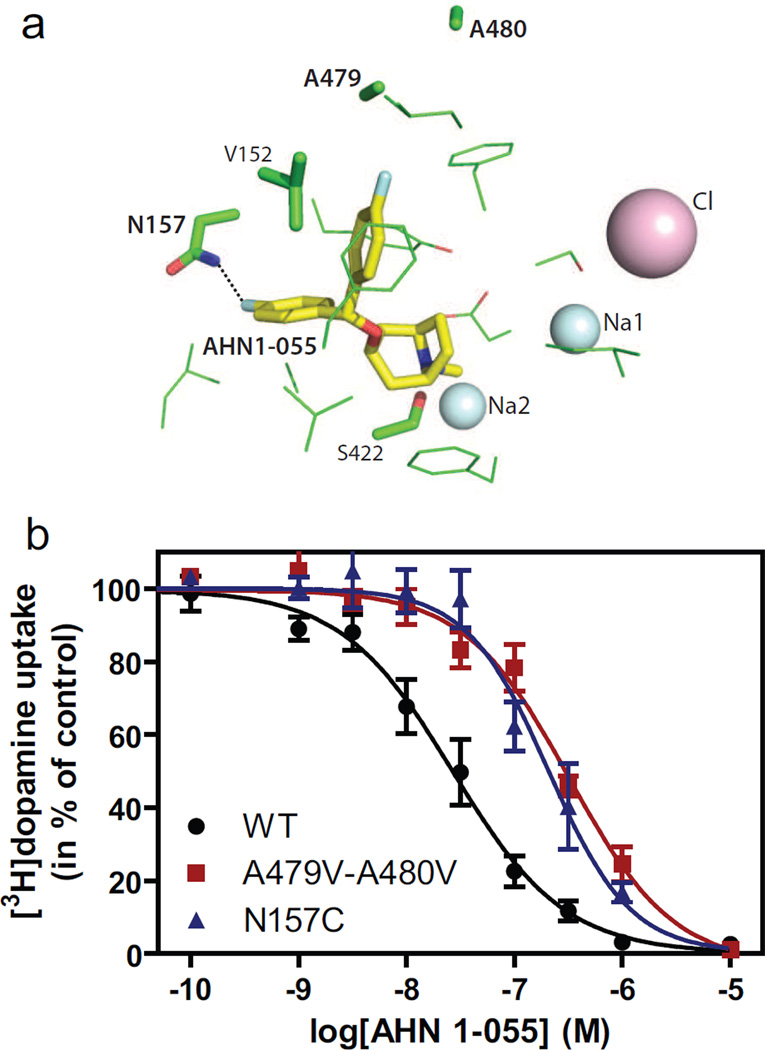
The resulting binding models deduced with the help of structural relations between BZT and JHW007 were probed with another BZT analog, AHN 1-055, which differs from JHW007 by having an N-methyl rather than N-butyl substitution and thereby differing from BZT only by having the para-fluoro-substitutions on the phenyl rings that also are present in JHW007 (Fig. 1). The protocol described above was used to filter the AHN1-055 docking poses and, as expected, the selected poses of AHN1-055 had the same binding mode as JHW007 except for the loss of interactions due the N-methyl replacement of the N-butyl in JHW007. However, like in JHW007, when one of the fluorine substituents interacts with Asn1573.51, the second fluorine–substituted ring is in close proximity to Ala47910.51 and Ala48010.52 in TM 10 (Fig. 6). AHN 1-055 bound DAT with an affinity close to that of JHW007 and had a Ki value for inhibition of [3H]dopamine uptake of 26 [18–37] nM, and for inhibition of [3H]CFT binding of 9 [8–10] nM (means [S.E. interval], n= 3). In full agreement with the proposed interaction with Asn1573.51, the Ki value for inhibition of [3H]dopamine uptake increased 10–15-fold in both N157A and N157C (N157A, Ki = 171 [118–246] nM; N157C, Ki = 206 [151–280] nM, means [S.E. interval], n= 3) (Fig. 6). This binding model was also supported by the observed increase in the Ki value for the A479V-A480V mutant (Fig. 6) (Ki = 274 [253–296] nM, mean [S.E. interval], n= 3), by ~10-fold. [3H]CFT binding experiments confirmed the loss of affinity at A479V-A480V with ~15-fold loss in calculated affinity for AHN 1-055 (Ki = 156 [144–169] nM, mean [S.E. interval]).
Fig. 6.
Evidence for involvement of Asn1573.51, Ala47910.51 and Ala48010.52 in binding of AHN 1-055. (A) Molecular model of DAT/AHN1-055 complex. The filtered binding pose of AHN1-055 is similar to that of JHW007 (see Fig. 4). (B) Inhibition of [3H]dopamine uptake by AHN 1-055 at WT DAT (black circles), N157C (blue triangles) and A479V-A480V (red squares). The uptake experiments were performed using indicated concentrations of inhibitor on COS7 cells transiently expressing indicated constructs. Data are means ± S.E. of 3 experiments performed in triplicate.
4. Discussion
In a recent study we provided evidence that the binding site in DAT for cocaine and cocaine analogs overlap with the binding site of the substrate dopamine (Beuming et al., 2008). Thus, we obtained data arguing strongly against an allosteric mechanism of dopamine binding inhibition for cocaine (Beuming et al., 2008). Our previous dockings also proposed a binding site for BZT and BZT analogs in the primary substrate binding cavity (S1) of DAT and, hence, overlapping binding sites with dopamine for these compounds as well (Beuming et al., 2008). However, the only experimental support for this binding mode for the BZTs was a decrease in affinity upon mutation of Ty1563.50 to Phe, which disrupts a stabilizing hydrogen bond between Asp791.45 and Ty1563.50 (Beuming et al., 2008). In the present study we sought to validate the conclusion that BZTs bind in S1 and, moreover, to create detailed models for the binding of BZTs in the S1 pocket.
The binding mode of inhibitors of NSS proteins has been the subject of intense discussion. Recently, the discussion has been reinforced by crystallization of the bacterial leucine transporter LeuT in complex with various ligands, including both tricyclic antidepressants and the SSRIs sertraline and fluoxetine (Singh et al., 2007; Zhou et al., 2007; Zhou et al., 2009). This crystallization was possible despite a very low affinity of these compounds for LeuT, as compared to the high affinity they display for SERT and/or NET, which both are closely related to DAT (Iversen, 2006). The LeuT structures revealed a binding site in an extracellular-facing vestibule, which is situated right above the occluded substrate binding site (Singh et al., 2007; Zhou et al., 2007; Zhou et al., 2009) and was suggested to represent a secondary substrate binding site (S2) (Shi et al., 2008). To investigate whether the structures described a binding mode for antidepressants that could be generalized to the mammalian transporters, a series of mutations around the tentative binding site in the extracellular-facing vestibule were analyzed in SERT (Zhou et al., 2009). Of these, mutation of e.g. Ile1793.53 caused a dramatic decrease in the uptake inhibition potency of sertraline and to a lesser extent fluoxetine, consistent with a binding site for these compounds in S2 of SERT (Zhou et al., 2009). It is nevertheless unlikely that all the inhibitors bind within the S2 pocket. For example, (S)-Citalopram can be docked successfully in the S1 site and this binding mode has been supported by detailed mutagenesis within the S1 binding pocket (Andersen et al., 2009a, 2010; Henry et al., 2006; Koldso et al., 2010). Moreover, a series of mutations targeting the S2 pocket hardly affected high affinity binding of (S)-citalopram, adding further support that the primary high affinity (S)-citalopram binding site is situated in S1 (Andersen et al., 2009b). It is also important to note that a recent study presented structural models supported by mutagenesis data suggesting that tricyclic antidepressants such as imipramine bind with high affinity in S1 rather than in S2 as indicated by the LeuT structures (Sinning et al., 2010).
In DAT, mutations at several loci had been shown to generate differential effects on cocaine/cocaine analogs and dopamine (Chen et al., 2006; Lin and Uhl, 2002; Loland et al., 2004; Uhl and Lin, 2003; Volz and Schenk, 2005). This was interpreted as support for an allosteric mechanism of action (see also (Huang et al., 2009)) and led to the suggestion that it might be possible to develop a cocaine antagonist that blocks cocaine binding without affecting dopamine transport, for treatment of cocaine addiction (Lin and Uhl, 2002). However, our computational and experimental data are difficult to reconcile with this kind of allosteric mechanism, as they offer rather strong support for a competitive binding mode of cocaine in S1, (Beuming et al., 2008). It should also be noted that several of the mutants previously found to cause differential effects on cocaine/cocaine analogs and dopamine binding, are likely causing indirect conformational effects. One example is the highly conserved Tyr335 in the third intracellular loop that plays a key role in the network of interactions responsible for maintaining the intracellular gate closed (Kniazeff et al., 2008; Loland et al., 2004; Loland et al., 2002).
In the present study, we compared the details of binding modes for cocaine and BZT analogs in the S1 site of DAT by refining the detailed binding models in conjunction with mutations of selected residues in S1. Thus, Val1523.46 that is predicted to form hydrophobic interactions with dopamine and cocaine/CFT as well as BZT/JHW007 was found here to affect binding of both BZT and JHW007, just as it was shown earlier to be involved in dopamine and cocaine/CFT binding (Beuming et al., 2008). Notably, the position corresponding to Val1523.46 in SERT (Ile1723.46) has also been implicated in inhibitor binding, i.e. mutation of this residue markedly decreased the affinity for both cocaine and (S)-citalopram (Chen et al., 1997; Henry et al., 2006).
We also analyzed mutation of Ser422 to alanine. According to our structural models of the binding sites this residue is located in close proximity to all tested ligands in S1, but is also predicted to be involved in binding the second sodium (Na2) (Beuming et al., 2008; Beuming et al., 2006; Yamashita et al., 2005). In S422A, we observed a marked decrease in the Vmax for [3H]dopamine uptake, which might reflect an impairment of Na+ binding that results in lower transport capacity. For the inhibitors, we observed a marked parallel decrease in apparent affinities. Although these data should be interpreted with care due to possible interference with Na+ binding, the observations are in support of a common binding mode for all three inhibitors tested.
According to our previous data, Asn1573.51 was suggested to form a polar interaction with the para-fluoro-substituted 3β-phenyl ring of CFT and, in agreement, we observed impaired CFT binding upon mutation of Asn1573.51 to Cys (N157C) (Beuming et al., 2008). In addition, in this study we analyzed the mutation of Asn1573.51 to alanine (N157A) that caused a decrease in the apparent affinity of CFT, similar to that seen for N157C. Interestingly, we also observed a marked decrease in the apparent affinity of JHW007 with a smaller effect on BZT itself. This would be consistent with an interaction of one of the para-fluoro-substituted phenyl rings in JHW007 that contrast the unsubstituted phenyl rings in BZT (Fig. 1). To explore this possibility further, we used computational analysis that selected from the docking data those binding poses for JHW007 in which the interaction with Asn1573.51 was present, and then identified all BZT binding poses that positioned common moieties of BZT and JHW007 in the same position within the binding site. According to this analysis, the interaction of one of these phenyl ring fluorine substituents of JHW007 with Asn1573.51, positions the other fluorine-substituted ring close to Ala47910.51 and Ala48010.52 in TM10. In contrast, the BZT oriented according to the rest of JHW007 moieties is in a tilted orientation, as compared to JHW007, and has one of the aromatic rings even closer to Ala47910.51 and Ala48010.52 (including a direct interaction with Ala47910.51). The experimental data obtained for the A479V-A480V mutation were fully consistent with this prediction as they demonstrated the largest effect to be for BZT, a lesser effect on JHW007, and only a small effect on CFT. The generalization of these inferences to an additional BZT analog, AHN 1-055, which shares the fluorine substitutions of JHW007, but lacks the large N-alkyl substituent, provided further support for the proposed orientation of the BZTs in the S1 pocket.
Summarized, our present data suggest that BZT and its analogs bind with high affinity in the primary substrate binding pocket with their diphenyl rings positioned between TM3 and TM10, and thus that they share overlapping but non-identical binding sites with dopamine as well as with cocaine/cocaine analogs. Nevertheless, the different orientations of the compounds are most likely to stabilize distinct conformations of DAT and in turn affect the pharmacological responses of the compounds. Indeed, based on accessibility of a cysteine inserted in position 159 in TM3 we proposed that cocaine and cocaine analogues stabilize more outward open configurations than those induced by BZT and BZT analogs (Loland et al., 2008). We also observed that cocaine and cocaine analogues were more affected by a mutation shifting the transporter to an inward facing conformation (Y335A) as compared to most BZT analogs. We even observed a relationship between the decrease in potencies of inhibitors at this mutant, and cocaine-like responding in rats trained to discriminate cocaine from saline injections, indicating a possible correlation between binding mode and the in vivo behavioral effects (Loland et al., 2008). The present data represent an important framework for further exploring these complicated actions of DAT inhibitors and might in this way form an important basis for future discovery of new leads for treatment of psychostimulant addiction.
Acknowledgments
We thank Pia Elsman for technical assistance and Jianjing Cao for synthesizing the BZT analogues used in this study. The work was supported in part by the National Institute of Health Grants P01 DA12408 (HW and UG), R00 DA023694 (LS), the NIDA-Intramural Research Program (A.H.N), the Danish Health Science Research Council (CJL and UG), the Lundbeck Foundation (CJL and UG), the Novo Nordisk Foundation (CJL and UG), and the Maersk Foundation (CJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
number in superscript describes residue number according to generic numbering scheme (see Materials and Methods)
References
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. Novel N-substituted 3 alpha-[bis(4'-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem. 1997;40:4329–4339. doi: 10.1021/jm970525a. [DOI] [PubMed] [Google Scholar]
- Andersen J, Olsen L, Hansen KB, Taboureau O, Jorgensen FS, Jorgensen AM, Bang-Andersen B, Egebjerg J, Stromgaard K, Kristensen AS. Mutational mapping and modeling of the binding site for (S)-citalopram in the human serotonin transporter. J Biol Chem. 2009a doi: 10.1074/jbc.M109.072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J, Olsen L, Hansen KB, Taboureau O, Jorgensen FS, Jorgensen AM, Bang-Andersen B, Egebjerg J, Stromgaard K, Kristensen AS. Mutational mapping and modeling of the binding site for (S)-citalopram in the human serotonin transporter. J Biol Chem. 2010;285:2051–2063. doi: 10.1074/jbc.M109.072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J, Taboureau O, Hansen KB, Olsen L, Egebjerg J, Stromgaard K, Kristensen AS. Location of the antidepressant binding site in the serotonin transporter: importance of Ser-438 in recognition of citalopram and tricyclic antidepressants. J Biol Chem. 2009b;284:10276–10284. doi: 10.1074/jbc.M806907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, Loland CJ. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci. 2008;11:780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Shi L, Javitch JA, Weinstein H. A comprehensive structure-based alignment of prokaryotic and eukaryotic neurotransmitter/Na+ symporters (NSS) aids in the use of the LeuT structure to probe NSS structure and function. Mol Pharmacol. 2006;70:1630–1642. doi: 10.1124/mol.106.026120. [DOI] [PubMed] [Google Scholar]
- Chen JG, Sachpatzidis A, Rudnick G. The third transmembrane domain of the serotonin transporter contains residues associated with substrate and cocaine binding. J Biol Chem. 1997;272:28321–28327. doi: 10.1074/jbc.272.45.28321. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004;447:519–531. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Chuaqui C, Singh J. Structural interaction fingerprint (SIFt): a novel method for analyzing three-dimensional protein-ligand binding interactions. J Med Chem. 2004;47:337–344. doi: 10.1021/jm030331x. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J. Neurosci. 2005;25:1889–1893. doi: 10.1523/JNEUROSCI.4778-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta AK, Zhang S, Kolhatkar R, Reith ME. Dopamine transporter as target for drug development of cocaine dependence medications. Eur J Pharmacol. 2003;479:93–106. doi: 10.1016/j.ejphar.2003.08.060. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter Transporters: molecular function of important drug targets. Trends Pharmacol Sci. 2006;27:375–383. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Goldberg NR, Beuming T, Soyer OS, Goldstein RA, Weinstein H, Javitch JA. Probing conformational changes in neurotransmitter transporters: a structural context. Eur J Pharmacol. 2003;479:3–12. doi: 10.1016/j.ejphar.2003.08.052. [DOI] [PubMed] [Google Scholar]
- Guptaroy B, Zhang M, Bowton E, Binda F, Shi L, Weinstein H, Galli A, Javitch JA, Neubig RR, Gnegy ME. A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward-facing conformation. Mol Pharmacol. 2009;75:514–524. doi: 10.1124/mol.108.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LK, Field JR, Adkins EM, Parnas ML, Vaughan RA, Zou MF, Newman AH, Blakely RD. Tyr-95 and ile-172 in transmembrane segments 1 and 3 of human serotonin transporters interact to establish high affinity recognition of antidepressants. J Biol Chem. 2006;281:2012–2023. doi: 10.1074/jbc.M505055200. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther. 2009;329:677–686. doi: 10.1124/jpet.108.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Gu HH, Zhan CG. Mechanism for cocaine blocking the transport of dopamine: insights from molecular modeling and dynamics simulations. J Phys Chem B. 2009;113:15057–15066. doi: 10.1021/jp900963n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol. 2006;147(Suppl 1):S82–S88. doi: 10.1038/sj.bjp.0706428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, Newman AH. Effects of N-Substituted Analogues of Benztropine: Diminished Cocaine-Like Effects in Dopamine Transporter Ligands. J Pharmacol Exp Ther. 2004;309:650–660. doi: 10.1124/jpet.103.060525. [DOI] [PubMed] [Google Scholar]
- Katz JL, Libby TA, Kopajtic T, Husbands SM, Newman AH. Behavioral effects of rimcazole analogues alone and in combination with cocaine. Eur J Pharmacol. 2003;468:109–119. doi: 10.1016/s0014-2999(03)01638-8. [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Shi L, Loland CJ, Javitch JA, Weinstein H, Gether U. A conserved intracellular ionic network regulates conformational transitions in neurotransmitter:sodium symporters. 2008 doi: 10.1074/jbc.M800475200. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldso H, Severinsen K, Tran TT, Celik L, Jensen HH, Wiborg O, Schiott B, Sinning S. The two enantiomers of citalopram bind to the human serotonin transporter in reversed orientations. J Am Chem Soc. 2010;132:1311–1322. doi: 10.1021/ja906923j. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy H, Piscitelli CL, Gouaux E. Unlocking the molecular secrets of sodium-coupled transporters. Nature. 2009;459:347–355. doi: 10.1038/nature08143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Uhl GR. Dopamine transporter mutants with cocaine resistance and normal dopamine uptake provide targets for cocaine antagonism. Mol Pharmacol. 2002;61:885–891. doi: 10.1124/mol.61.4.885. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol. 2008;73:813–823. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Granas C, Javitch JA, Gether U. Identification of intracellular residues in the dopamine transporter critical for regulation of transporter conformation and cocaine binding. J Biol Chem. 2004;279:3228–3238. doi: 10.1074/jbc.M304755200. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Gether U. Defining proximity relationships in the tertiary structure of the dopamine transporter. Identification of a conserved glutamic acid as a third coordinate in the endogenous Zn(2+)-binding site. J Biol Chem. 1999;274:36928–36934. doi: 10.1074/jbc.274.52.36928. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Litman T, Gether U. Generation of an activating Zn(2+) switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc Natl Acad Sci U S A. 2002;99:1683–1688. doi: 10.1073/pnas.032386299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Allen AC, Izenwasser S, Katz JL. Novel 3 alpha-(diphenylmethoxy)tropane analogs: potent dopamine uptake inhibitors without cocaine-like behavioral profiles. J Med Chem. 1994;37:2258–2261. doi: 10.1021/jm00041a002. [DOI] [PubMed] [Google Scholar]
- Newman AH, Katz JL. Atypical Dopamine Uptake Inhibitors that Provide Clues About Cocaine’s Mechanism at the Dopamine Transporter. In: Napier S, Bingham M, editors. Transporters as Targets for Drugs. 2009. [Google Scholar]
- Newman AH, Kulkarni S. Probes for the dopamine transporter: new leads toward a cocaine-abuse therapeutic--A focus on analogues of benztropine and rimcazole. Med Res Rev. 2002;22:429–464. doi: 10.1002/med.10014. [DOI] [PubMed] [Google Scholar]
- Raje S, Cao J, Newman AH, Gao H, Eddington ND. Evaluation of the blood-brain barrier transport, population pharmacokinetics, and brain distribution of benztropine analogs and cocaine using in vitro and in vivo techniques. J Pharmacol Exp Ther. 2003;307:801–808. doi: 10.1124/jpet.103.053504. [DOI] [PubMed] [Google Scholar]
- Raje S, Cornish J, Newman AH, Cao J, Katz JL, Eddington ND. Pharmacodynamic assessment of the benztropine analogues AHN-1055 and AHN-2005 using intracerebral microdialysis to evaluate brain dopamine levels and pharmacokinetic/pharmacodynamic modeling. Pharm Res. 2005;22:603–612. doi: 10.1007/s11095-005-2488-8. [DOI] [PubMed] [Google Scholar]
- Sherman W, Day T, Jacobson MP, Friesner RA, Farid R. Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem. 2006;49:534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- Shi K, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter--inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2008;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- Sinning S, Musgaard M, Jensen M, Severinsen K, Celik L, Koldso H, Meyer T, Bols M, Jensen HH, Schiott B, Wiborg O. Binding and orientation of tricyclic antidepressants within the central substrate site of the human serotonin transporter. J Biol Chem. 2010;285:8363–8374. doi: 10.1074/jbc.M109.045401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SA, Newman AH, Othman AA, Eddington ND. Population pharmacokinetics, brain distribution, and pharmacodynamics of 2nd generation dopamine transporter selective benztropine analogs developed as potential substitute therapeutics for treatment of cocaine abuse. J Pharm Sci. 2008;97:1993–2007. doi: 10.1002/jps.21123. [DOI] [PubMed] [Google Scholar]
- Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Lin Z. The top 20 dopamine transporter mutants: structure-function relationships and cocaine actions. Eur J Pharmacol. 2003;479:71–82. doi: 10.1016/j.ejphar.2003.08.058. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Schenk JO. A comprehensive atlas of the topography of functional groups of the dopamine transporter. Synapse. 2005;58:72–94. doi: 10.1002/syn.20183. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Quick M, Shi L, Mehler EL, Weinstein H, Javitch JA. Substrate-dependent proton antiport in neurotransmitter:sodium symporters. Nat Chem Biol. 2010;6:109–116. doi: 10.1038/nchembio.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Goetz RM, Law CJ, Reith ME, Wang DN. LeuT-Desipramine Structure Reveals How Antidepressants Block Neurotransmitter Reuptake. Science. 2007;317:1390–1393. doi: 10.1126/science.1147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith ME, Wang DN. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16:652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomot E, Bendahan A, Quick M, Zhao Y, Javitch JA, Kanner BI. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature. 2007;449:726–730. doi: 10.1038/nature06133. [DOI] [PubMed] [Google Scholar]