Abstract
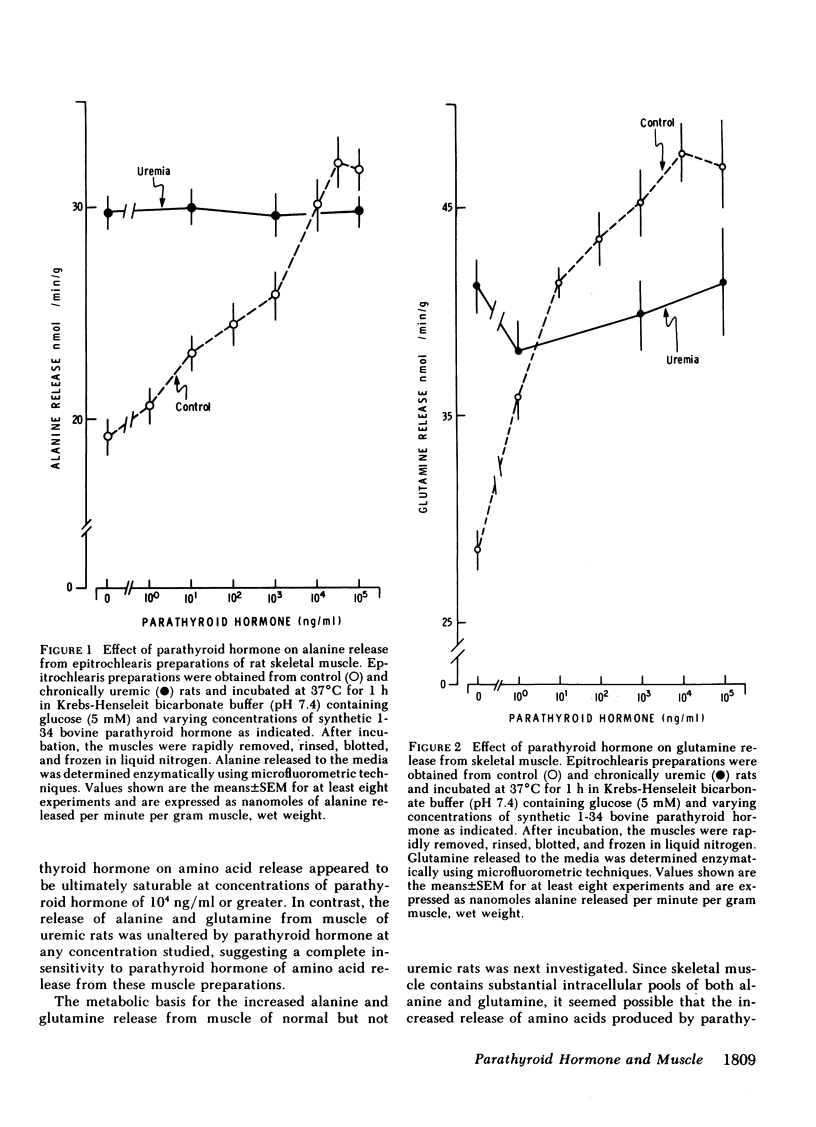
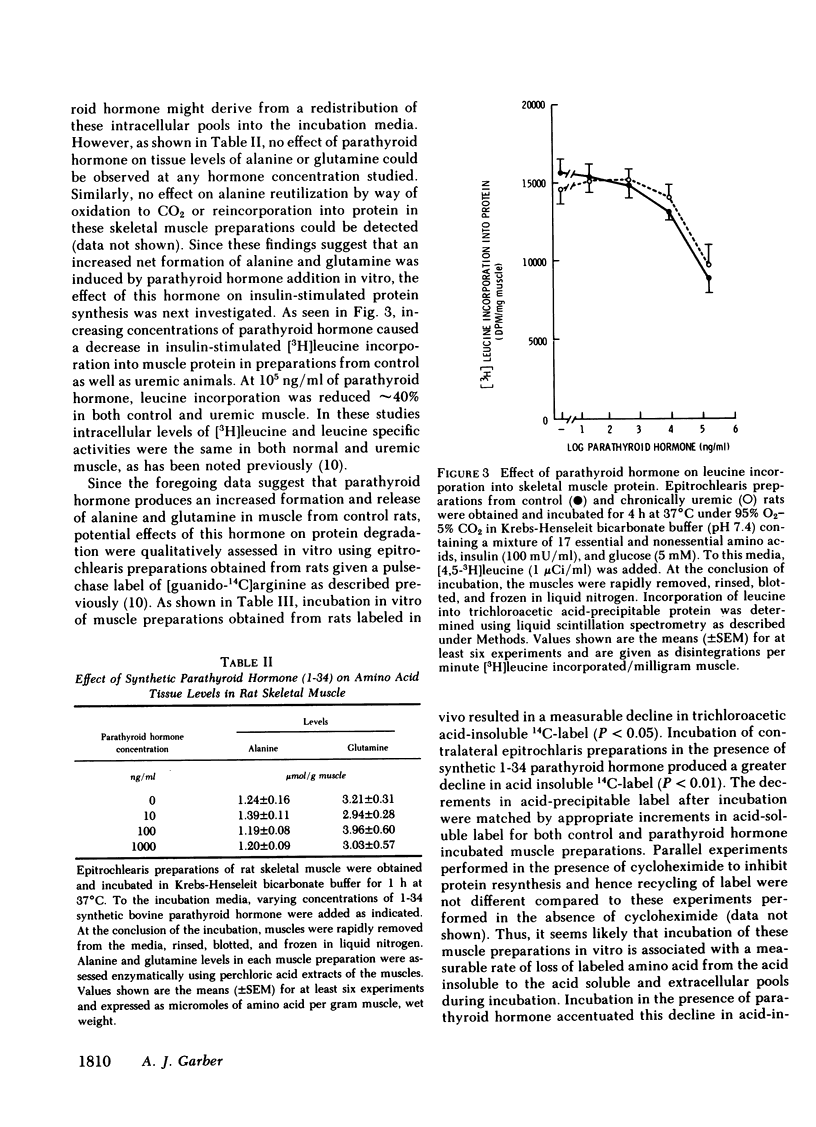
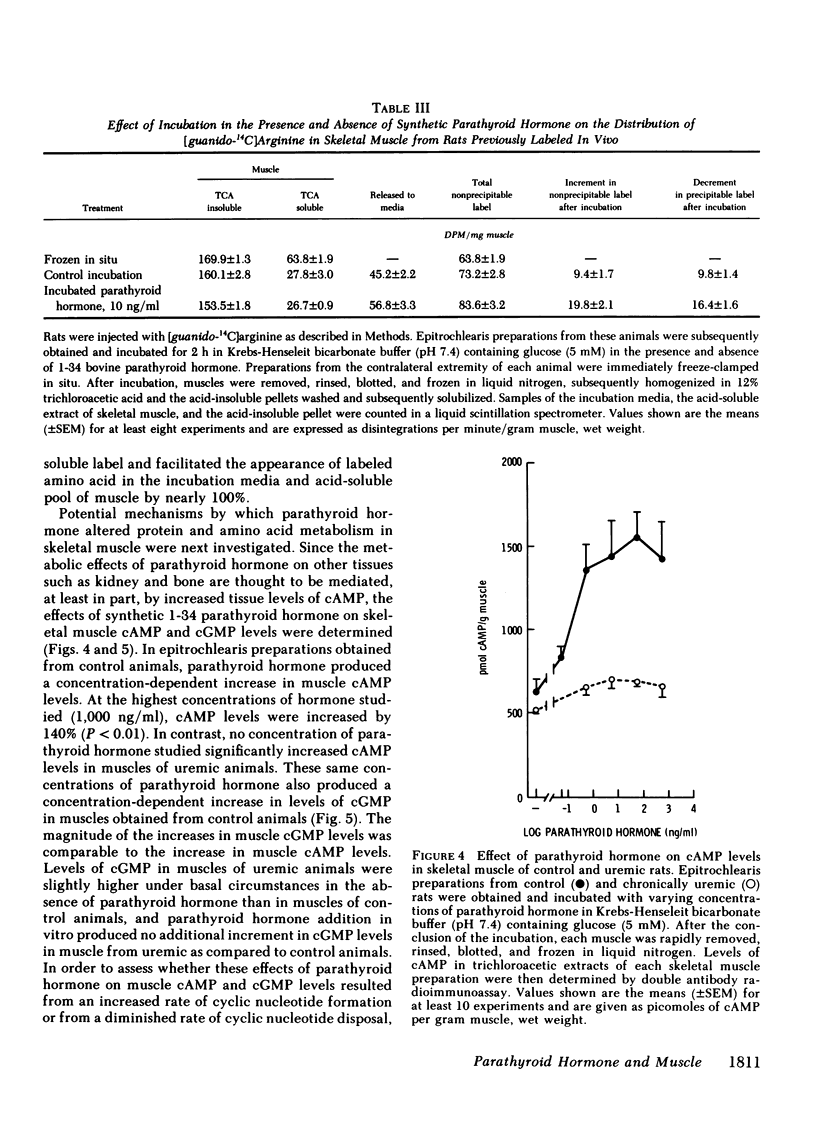
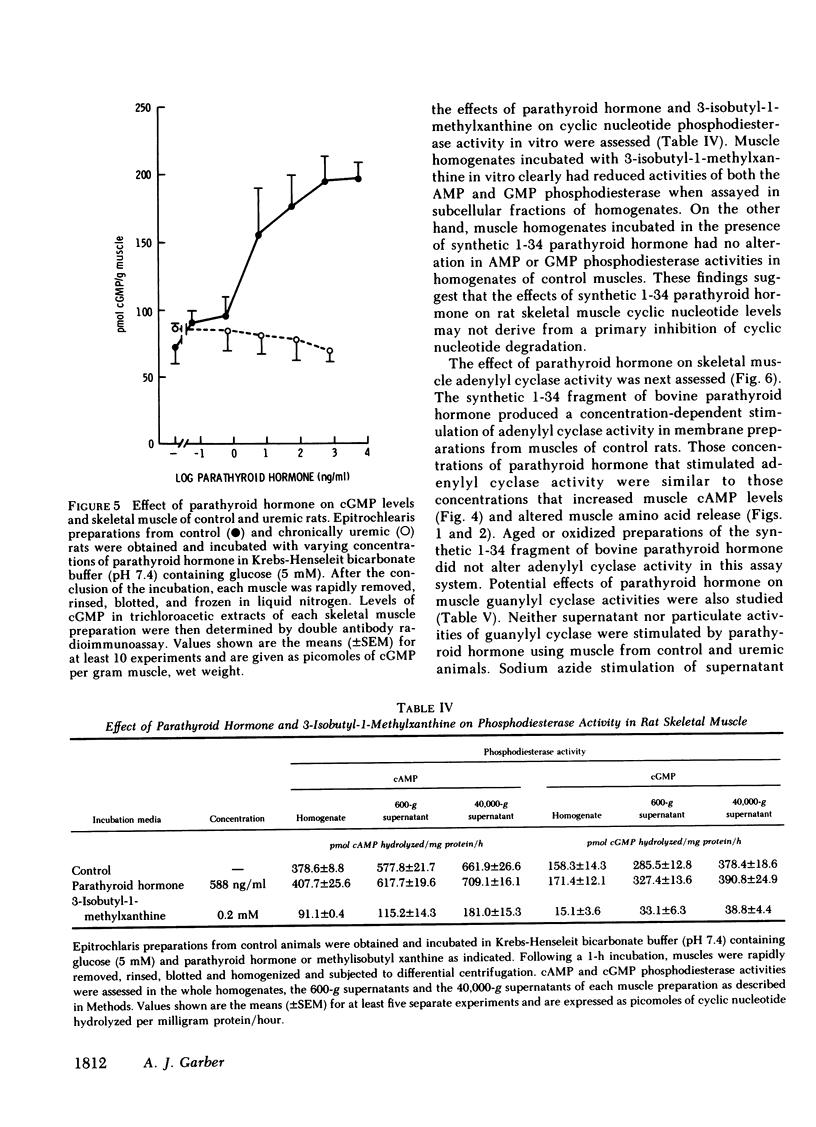
Because prominent skeletal muscle dysfunction and muscle wasting are seen in both chronic uremia and in primary hyperparathyroidism, and because markedly elevated parathyroid hormone levels occur in both disorders, potential effects of parathyroid hormone on skeletal muscle protein, amino acid, and cyclic nucleotide metabolism were studied in vitro using isolated intact rat epitrochlearis skeletal muscle preparations. Intact bovine parathyroid hormone and the synthetic 1-34 fragment of this hormone stimulated the release of alanine and glutamine from muscle of control but not from chronically uremic animals. This stimulation was dependent upon the concentration of parathyroid hormone added: At 105 ng/ml parathyroid hormone increased alanine release 84% and glutamine release 75%. Intracellular levels of alanine and glutamine were not altered by parathyroid hormone. Increasing concentrations of the 1-34 polypeptide decreased [3H]leucine incorporation into protein of muscles from both control and uremic animals. Using muscles from animals given a pulse-chase label of [guanido-14C]arginine in vivo, parathyroid hormone increased the rate of loss of 14C label from acid-precipitable protein during incubation and correspondingly increased the rate of appearance of this label in the incubation media. Parathyroid hormone increased muscle cAMP levels by 140% and cGMP levels by 185%, but had no effect on skeletal muscle cyclic nucleotide phosphodiesterase activities as assayed in vitro. Adenylyl cyclase activity in membrane preparations from control but not uremic rats was stimulated by parathyroid hormone in a concentration-dependent fashion. However, no stimulation of guanylyl cyclase activity was noted by parathyroid hormone, although stimulation by sodium azide was present. Incubation of muscles with added parathyroid hormone produced a diminished responsiveness towards epinephrine or serotonin regulation of amino acid release and cAMP formation in the presence compared to the absence of parathyroid hormone. In the absence of parathyroid hormone, detectable inhibition of alanine and glutamine release was produced by 10−9 M epinephrine, whereas in the presence of parathyroid hormone (1,000 ng/ml) inhibition of alanine and glutamine release required 10−6 M or greater epinephrine. Resistance to cyclic AMP action as well as inhibition of cyclic AMP formation by parathyroid hormone was found. Preincubation of rat sarcolemma with 1-34 parathyroid hormone produced a decreased activity of the isoproterenol-stimulable adenylyl cyclase activity but there was no apparent change in the concentration of isoproterenol required for one-half maximal and maximal stimulation of the enzyme.
These findings suggest that high levels of parathyroid hormone have direct effects on skeletal muscle protein, amino acid, and cyclic nucleotide metabolism in muscle of normal but not uremic animals. Treatment with these high levels of parathyroid hormone in vitro appears to reproduce in normal muscle, the metabolic deficits and loss of hormone responsiveness observed in muscle of chronically uremic animals. It is therefore possible that direct effects of parathyroid hormone on skeletal muscle may account in part for the muscle dysfunction and wasting of primary hyperparathyroidism and chronic uremia.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. II. Comparison between glucagon- and fluoride-stimulated activities. J Biol Chem. 1971 Mar 25;246(6):1857–1860. [PubMed] [Google Scholar]
- Bockaert J., Hunzicker-Dunn M., Birnbaumer L. Hormone-stimulated desensitization of hormone-dependent adenylyl cyclase. Dual action of luteninizing hormone on pig graafian follicle membranes. J Biol Chem. 1976 May 10;251(9):2653–2663. [PubMed] [Google Scholar]
- Chase L. R., Fedak S. A., Aurbach G. D. Activation of skeletal adenyl cyclase by parathyroid hormone in vitro. Endocrinology. 1969 Apr;84(4):761–768. doi: 10.1210/endo-84-4-761. [DOI] [PubMed] [Google Scholar]
- Chiasson J. L., Dietz M. R., Shikama H., Wootten M., Exton J. H. Insulin regulation of skeletal muscle glycogen metabolism. Am J Physiol. 1980 Jul;239(1):E69–E74. doi: 10.1152/ajpendo.1980.239.1.E69. [DOI] [PubMed] [Google Scholar]
- Cholod E. J., Haust M. D., Hudson A. J., Lewis F. N. Myopathy in primary familial hyperparathyroidism. Clinical and morphologic studies. Am J Med. 1970 Jun;48(6):700–707. doi: 10.1016/s0002-9343(70)80004-3. [DOI] [PubMed] [Google Scholar]
- Clark C. M., Jr, Beatty B., Allen D. O. Evidence for delayed development of the glucagon receptor of adenylate cyclase in the fetal and neonatal rat heart. J Clin Invest. 1973 May;52(5):1018–1025. doi: 10.1172/JCI107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deftos L. J., Parthemore J. G. Secretion of parathyroid hormone in patients with medullary thyroid carcinoma. J Clin Invest. 1974 Aug;54(2):416–420. doi: 10.1172/JCI107777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3',5'-monophosphate on gluconeogenesis in the perfused rat liver. J Biol Chem. 1968 Aug 25;243(16):4189–4196. [PubMed] [Google Scholar]
- Frizzell M., Larsen P. R., Field J. B. Spontaneous hypoglycemia associated with chronic renal failure. Diabetes. 1973 Jul;22(7):493–498. doi: 10.2337/diab.22.7.493. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Garber A. J., Bier D. M., Cryer P. E., Pagliara A. S. Hypoglycemia in compensated chronic renal insufficiency. Substrate limitation of gluconeogenesis. Diabetes. 1974 Dec;23(12):982–986. doi: 10.2337/diab.23.12.982. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Entman M. L., Birnbaumer L. Cholinergic stimulation of skeletal muscle alanine and glutamine formation and release. Evidence for mediation by a nicotinic cholinergic receptor and guanosine 3':5'-monophosphate. J Biol Chem. 1978 Nov 10;253(21):7924–7930. [PubMed] [Google Scholar]
- Garber A. J., Harari Y., Entman M. L. Cholinergic stimulation of alanine and glutamine formation and release from skeletal muscle. J Biol Chem. 1978 Nov 10;253(21):7918–7923. [PubMed] [Google Scholar]
- Garber A. J. Inhibition of serotonin of amino acid release and protein degradation in skeletal muscle. Mol Pharmacol. 1977 Jul;13(4):640–651. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. I. Glycolysis and amino acid release. J Biol Chem. 1976 Feb 10;251(3):826–835. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. II. The precursor role of amino acids in alanine and glutamine synthesis. J Biol Chem. 1976 Feb 10;251(3):836–843. [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. IV. beta-Adrenergic inhibition of amino acid release. J Biol Chem. 1976 Feb 10;251(3):851–857. [PubMed] [Google Scholar]
- Garber A. J., Schwartz R. J., Seidel C. L., Silvers A., Entman M. L. Skeletal muscle protein and amino acid metabolism in hereditary mouse muscular dystrophy. Accelerated protein turnover and increased alanine and glutamine formation and release. J Biol Chem. 1980 Sep 10;255(17):8315–8324. [PubMed] [Google Scholar]
- Garber A. J. Skeletal muscle protein and amino acid metabolism in experimental chronic uremia in the rat: accelerated alanine and glutamine formation and release. J Clin Invest. 1978 Sep;62(3):623–632. doi: 10.1172/JCI109169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J. The impact of streptozotocin-induced diabetes mellitus on cyclic nucleotide regulation of skeletal muscle amino acid metabolism in the rat. J Clin Invest. 1980 Feb;65(2):478–487. doi: 10.1172/JCI109691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J. The regulation of skeletal muscle alanine and glutamine formation and release in experimental chronic uremia in the rat: subsensitivity of adenylate cyclase and amino acid release to epinephrine and serotonin. J Clin Invest. 1978 Sep;62(3):633–641. doi: 10.1172/JCI109170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Graff G., Haddox M. K., Stephenson J. H., Glass D. B., Moser M. E. Oxidative activation of guinea pig splenic cell guanylate cyclase activity by dehydroascorbate, ascorbate, fatty acid hydroperoxides and prostaglandin endoperoxides. Adv Enzyme Regul. 1977 Oct 3;16:165–191. doi: 10.1016/0065-2571(78)90072-9. [DOI] [PubMed] [Google Scholar]
- Goltzman D., Goltzmann D., Peytremann A., Callahan E., Tregear G. W., Potts J. T., Jr Analysis of the requirements for parathyroid hormone action in renal membranes with the use of inhibiting analogues. J Biol Chem. 1975 Apr 25;250(8):3199–3203. [PubMed] [Google Scholar]
- Harter H. R., Karl I. E., Klahr S., Kipnis D. M. Effects of reduced renal mass and dietary protein intake on amino acid release and glucose uptake by rat muscle in vitro. J Clin Invest. 1979 Aug;64(2):513–523. doi: 10.1172/JCI109489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg M., Weinstein I., Kohout M. The effects of glucagon, dibutyryl cyclic adenosine 3',5'-monophosphate, and concentration of free fatty acid on hepatic lipid metabolism. J Biol Chem. 1969 Oct 10;244(19):5131–5139. [PubMed] [Google Scholar]
- Horton E. S., Johnson C., Lebovitz H. E. Carbohydrate metabolism in uremia. Ann Intern Med. 1968 Jan;68(1):63–74. doi: 10.7326/0003-4819-68-1-63. [DOI] [PubMed] [Google Scholar]
- Jacob A. I., Lanier D., Jr, Canterbury J., Bourgoignie J. J. Reduction by cimetidine of serum parathyroid hormone levels in uremic patients. N Engl J Med. 1980 Mar 20;302(12):671–674. doi: 10.1056/NEJM198003203021207. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Rannels D. E., Munger B. L., Morgan H. E. Insulin in the regulation of protein turnover in heart and skeletal muscle. Fed Proc. 1974 Apr;33(4):1098–1104. [PubMed] [Google Scholar]
- Johnson G. L., Wolfe B. B., Harden T. K., Molinoff P. B., Perkins J. P. Role of beta-adrenergic receptors in catecholamine-induced desensitization of adenylate cyclase in human astrocytoma cells. J Biol Chem. 1978 Mar 10;253(5):1472–1480. [PubMed] [Google Scholar]
- Kaplan M. A., Canterbury J. M., Gavellas G., Jaffe D., Bourgoignie J. J., Reiss E., Bricker N. S. The calcemic and phosphaturic effects of parathyroid hormone in the normal and uremic dog. Metabolism. 1978 Dec;27(12):1785–1792. doi: 10.1016/0026-0495(78)90264-0. [DOI] [PubMed] [Google Scholar]
- Karl I. E., Pagliara A. S., Kipnis D. M. A microfluorometric enzymatic assay for the determination of alanine and pyruvate in plasma and tissues. J Lab Clin Med. 1972 Sep;80(3):434–441. [PubMed] [Google Scholar]
- Kimura H., Murad F. Two forms of guanylate cyclase in mammalian tissues and possible mechanisms for their regulation. Metabolism. 1975 Mar;24(3):439–445. doi: 10.1016/0026-0495(75)90123-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee S. H., Davis E. J. Carboxylation and decarboxylation reactions. Anaplerotic flux and removal of citrate cycle intermediates in skeletal muscle. J Biol Chem. 1979 Jan 25;254(2):420–430. [PubMed] [Google Scholar]
- Lee T. P., Kuo J. F., Greengard P. Regulation of myocardial cyclic AMP by isoproterenol, glucagon and acetylcholine. Biochem Biophys Res Commun. 1971 Nov;45(4):991–997. doi: 10.1016/0006-291x(71)90435-9. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Wood C. L., Gore T. B., Mukherjee C. Regulation of prostaglandin receptors by prostaglandins and guanine nucleotides in frog erythrocytes. J Biol Chem. 1977 Aug 10;252(15):5295–5303. [PubMed] [Google Scholar]
- Lowrie E. G., Soeldner J. S., Hampers C. L., Merrill J. P. Glucose metabolism and insulin secretion in uremic, prediabetic, and normal subjects. J Lab Clin Med. 1970 Oct;76(4):603–615. [PubMed] [Google Scholar]
- Mahgoub A., Stern P. H. Carbon dioxide and the effect of parathyroid hormone on bone in vitro. Am J Physiol. 1974 Jun;226(6):1272–1275. doi: 10.1152/ajplegacy.1974.226.6.1272. [DOI] [PubMed] [Google Scholar]
- Marcus R., Aurbach G. D. Bioassay of parathyroid hormone in vitro with a stable preparation of adenyl cyclase from rat kidney. Endocrinology. 1969 Nov;85(5):801–810. doi: 10.1210/endo-85-5-801. [DOI] [PubMed] [Google Scholar]
- PERKOFF G. T., THOMAS C. L., NEWTON J. D., SELLMAN J. C., TYLER F. H. Mechanism of impaired glucose tolerance in uremia and experimental hyperazotemia. Diabetes. 1958 Sep-Oct;7(5):375–383. doi: 10.2337/diab.7.5.375. [DOI] [PubMed] [Google Scholar]
- Patten B. M., Bilezikian J. P., Mallette L. E., Prince A., Engel W. K., Aurbach G. D. Neuromuscular disease in primary hyperparathyroidism. Ann Intern Med. 1974 Feb;80(2):182–193. doi: 10.7326/0003-4819-80-2-182. [DOI] [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschett J. B., Zurbach P., Sylk D. Acute effects of parathyroid hormone on proximal bicarbonate transport in the dog. Kidney Int. 1976 Jun;9(6):501–510. doi: 10.1038/ki.1976.64. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Berger M. The formation of glutamine and alanine in skeletal muscle. J Biol Chem. 1974 Sep 10;249(17):5500–5506. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Segre G. V., Habener J. F., Powell D., Tregear G. W., Potts J. T. Parathyroid Hormone in Human Plasma: IMMUNOCHEMICAL CHARACTERIZATION AND BIOLOGICAL IMPLICATIONS. J Clin Invest. 1972 Dec;51(12):3163–3172. doi: 10.1172/JCI107143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Martin K., Hruska K. Parathyroid hormone metabolism and its potential as a uremic toxin. Am J Physiol. 1980 Jul;239(1):F1–12. doi: 10.1152/ajprenal.1980.239.1.F1. [DOI] [PubMed] [Google Scholar]
- Smith R., Stern G. Myopathy, osteomalacia and hyperparathyroidism. Brain. 1967 Sep;90(3):593–602. doi: 10.1093/brain/90.3.593. [DOI] [PubMed] [Google Scholar]
- Snell K., Duff D. A. The release of alanine by rat diaphragm muscle in vitro. Biochem J. 1977 Feb 15;162(2):399–403. doi: 10.1042/bj1620399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Tischler M. E., Desautels M., Goldberg A. L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982 Feb 25;257(4):1613–1621. [PubMed] [Google Scholar]
- Watanabe A. M., Besch H. R., Jr Interaction between cyclic adenosine monophosphate and cyclic gunaosine monophosphate in guinea pig ventricular myocardium. Circ Res. 1975 Sep;37(3):309–317. doi: 10.1161/01.res.37.3.309. [DOI] [PubMed] [Google Scholar]
- Watanabe A. M., McConnaughey M. M., Strawbridge R. A., Fleming J. W., Jones L. R., Besch H. R., Jr Muscarinic cholinergic receptor modulation of beta-adrenergic receptor affinity for catecholamines. J Biol Chem. 1978 Jul 25;253(14):4833–4836. [PubMed] [Google Scholar]


